Novel Hybrid Nanoparticles: Synthesis, Functionalization, Characterization, and Their Application in the Uptake of Scandium (III)Ions from Aqueous Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Preparation of SiO2 Nanoparticles
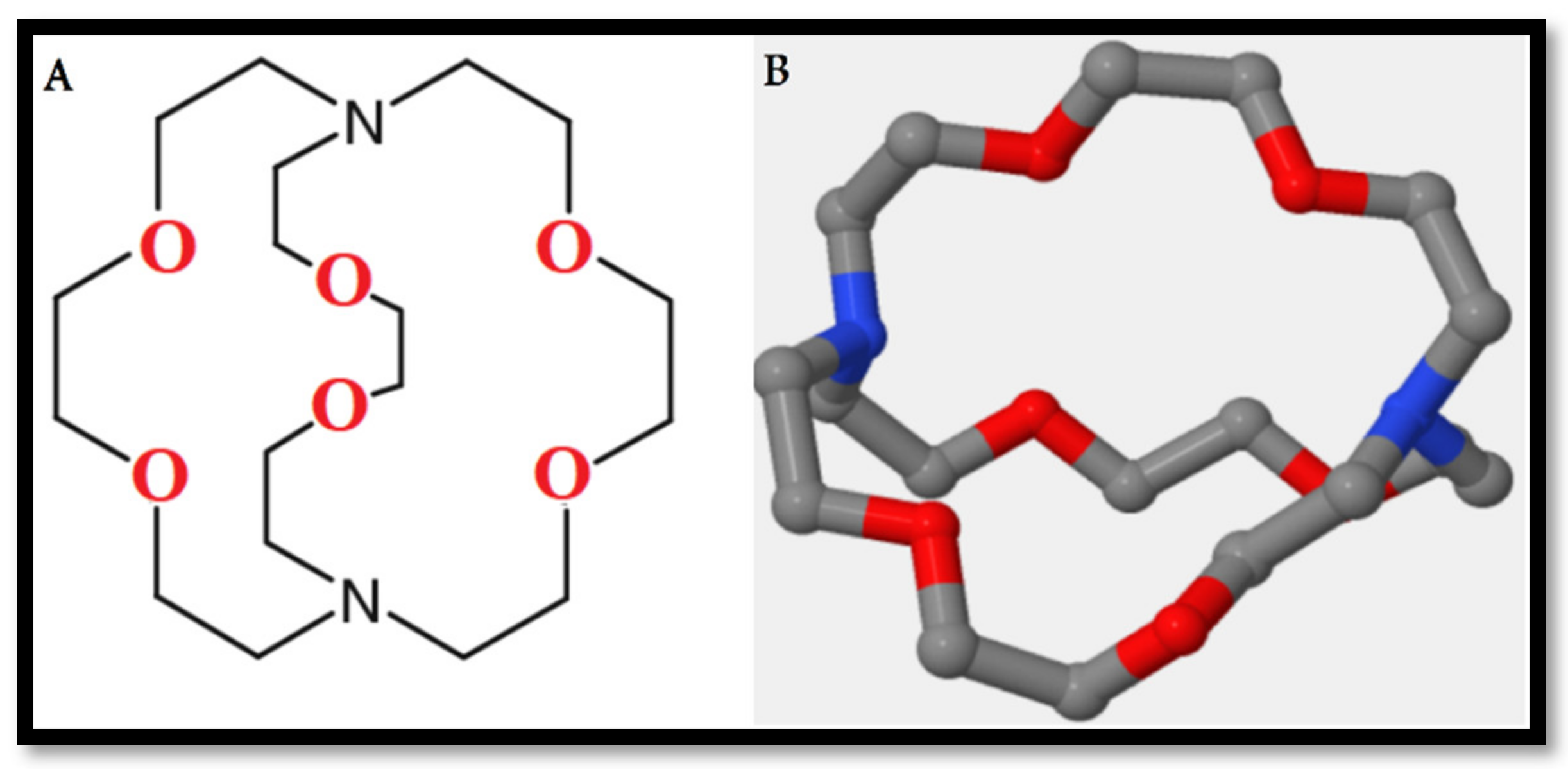
2.4. Immobilized Ligands
2.5. Extraction and Back Extraction Experiments
2.6. Analytical Procedures
3. Results and Discussion
3.1. Characterization
3.2. Mechanism of the Surface Modification
3.3. Extraction and Stripping/Back Extraction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adu-Wusu, K.; Hassan, N.M.; Nash, C.A.; Marra, J.C. Sorption of cesium from aqueous waste solution on a SuperLigÒ644 resin. J. Radioanal. Nucl. Chem. 2006, 267, 381–388. [Google Scholar] [CrossRef]
- Salman, S.R.; Petros, A.G.; Jalhoom, M.G. Spectroscopic Evidence of the Formation of New Kryptand K22C2. Spectrosc. Lett. 1992, 25, 163–174. [Google Scholar] [CrossRef]
- Banica, R.; Barvinschi, P.; Vaszilcsin, N.; Nyari, T. A comparative study of the electrochemical deposition of molybdenum oxides thin films on copper and platinum. J. Alloys Compd. 2009, 483, 402–405. [Google Scholar] [CrossRef]
- Devol, T.A.; Clements, J.P.; Farawila, A.; O’Hara, M.J.; Egorov, O.B.; Grate, J.W. Characterization and application of SuperLig® 620 solid phase extraction resin for automated process monitoring of 90Sr. J. Radioanal. Nucl. Chem. 2009, 282, 623–628. [Google Scholar] [CrossRef]
- Bradshaw, J.S.; Izatt, R.M.; Savage, P.B.; Bruening, R.L.; Krakowiak, K.E. The Design of Ion Selective Macrocycles and the Solid-Phase Extraction of Ions Using Molecular Recognition Technology: A Synopsis. Supramol. Chem. 2000, 12, 23–26. [Google Scholar] [CrossRef]
- Izatt, N.E.; Bruening, R.L.; Krakowiak, K.E.; Izatt, S.R. Contributions of Professor Reed M. Izatt to Molecular Recognition Technology: From Laboratory to Commercial Application. Ind. Eng. Chem. Res. 2000, 39, 3405–3411. [Google Scholar] [CrossRef]
- Izatt, N.E.; Izatt, S.R.; Bruening, R.L. Green procedure for the selective recovery of precious, specialty, and toxic metals from electronic wastes. In Proceedings of the 2012 Electronics Goes Green 2012+, Berlin, Germany, 9–12 September 2012. [Google Scholar]
- Hassan, N.M.; King, W.D.; McCabe, D.J.; Hamm, L.L.; Johnson, M.E. Superlig® 639 equilibrium sorption data for technetium from hanford tank waste supernates. Solvent Extr. Ion Exch. 2002, 20, 211–226. [Google Scholar] [CrossRef]
- Izatt, R.M. Review of Selective Ion Separations at BYU Using Liquid Membrane and Solid Phase Extraction Procedures. J. Incl. Phenom. Macrocycl. Chem. 1997, 29, 197–220. [Google Scholar] [CrossRef]
- Salman, A.D.; Tatjána, J.; Al-Mayyahi, M.A.; Ibrahim, R.I.; Abdullah, T.A.; Khader, E.H. Improvement of mechanical properties of Oil well cement by incorporate Nano-CaCO3 prepared from eggshell waste. IOP Conf. Ser. Mater. Sci. Eng. 2020, 765, 012006. [Google Scholar] [CrossRef]
- Izatt, S.; Izatt, N.; Bruening, R. Review of selective separations of cobalt, uranium, zinc, nickel and associated contaminants from various process streams. In Proceedings of the Sixth Southern African Base Metals Conference, Phalaborwa, South Africa, 18–20 July 2011. [Google Scholar]
- Zhang, P.; Inoue, K.; Tsuyama, H. Recovery of metal values from spent hydrodesulfurization catalysts by liquid-liquid extraction. Energy Fuels 1995, 9, 231–239. [Google Scholar] [CrossRef]
- Izatt, R.M.; Izatt, S.R.; Izatt, N.E.; Krakowiak, K.E.; Bruening, R.L.; Navarro, L. Industrial applications of molecular recognition technology to separations of platinum group metals and selective removal of metal impurities from process streams. Green Chem. 2015, 17, 2236–2245. [Google Scholar] [CrossRef]
- Habibi, S.; Jafari, A.; Fakhroueian, Z. Application of novel functionalized Al2O3/silica by organosiloxane and amine reagents for enhanced oil recovery. Appl. Nanosci. 2020, 10, 2085–2100. [Google Scholar] [CrossRef]
- Zhou, Z.-Y.; Tian, N.; Li, J.-T.; Broadwell, I.; Sun, S.-G. Nanomaterials of High Surface Energy with Exceptional Properties in Catalysis and Energy Storage. Chem. Soc. Rev. 2011, 40, 4167–4185. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.J.; Ibrahim, R.I.; Salman, A.D. Characterization of nano-silica prepared from local silica sand and its application in cement mortar using optimization technique. Adv. Powder Technol. 2015, 26, 1123–1133. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Liu, S.-W.; Lin, C.-M.; Chen, C.-H. Recognition of Potassium Ion in Water by 15-Crown-5 Functionalized Gold Nanoparticles. Anal. Chem. 2002, 74, 330–335. [Google Scholar] [CrossRef]
- Kucio, K.; Charmas, B.; Pasieczna-Patkowska, S.; Ziezio, M. Mechanochemical synthesis of nanophotocatalysts SiO2/TiO2/Fe2O3: Their structural, thermal and photocatalytic properties. Appl. Nanosci. 2020. [Google Scholar] [CrossRef]
- Anastas, P.T.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Zadra, J.B.; Engel, A.L.; Heinen, H.J. Process for Recovering Gold and Silver from Activated Carbon by Leaching and Electrolysis; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1952; Volume 4843. [Google Scholar]
- Bradshaw, J.S.; Bruening, R.L.; Krakowiak, K.E.; Tarbet, B.J.; Bruening, M.L.; Izatt, R.M.; Christensen, J.J. Preparation of silica gel-bound macrocycles and their cation-binding properties. J. Chem. Soc. Chem. Commun. 1988, 1988, 812–814. [Google Scholar] [CrossRef]
- Swain, S.S.; Unnikrishnan, L.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of surface-functionalized mesoporous graphene nanohybrid. Appl. Nanosci. 2019, 9, 1531–1552. [Google Scholar] [CrossRef]
- Chekhlov, A.N. Crystal Structure of (2.2.2-Cryptand)lithium Perchlorate. Russ. J. Co-ord. Chem. 2003, 29, 828–832. [Google Scholar] [CrossRef]
- Salman, A.D.; Jani, G.H.; Fatalla, A. Comparative Study of the Effect of Incorporating SiO2 Nano-Particles on Properties of Poly methyl Methacrylate Denture Bases. Biomed. Pharmacol. J. 2017, 10, 1525–1535. [Google Scholar] [CrossRef]
- Fenton, D.E.; Mercer, M.; Poonia, N.S.; Truter, M.R. Preparation and crystal structure of a binuclear complex of potassium with one molecule of cyclic polyether: Bis(potassium thiocyanate)dibenzo-24-crown-8. J. Chem. Soc. Chem. Commun. 1972, 1972, 66. [Google Scholar] [CrossRef]
- Jiao, D.; Zhao, N.; Scherman, O.A. A “green” method for isolation of cucurbit[7]uril via a solid state metathesis reaction. Chem. Commun. 2010, 46, 2007–2009. [Google Scholar] [CrossRef]
- Gongyi, G.; Yuli, C.; Yu, L. Solvent Extraction off Scandium from Wolframite Residue. JOM 1988, 40, 28–31. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- Kerkove, M.A.; Wood, T.D.; Sanders, P.G.; Kampe, S.L.; Swenson, D. The Diffusion Coefficient of Scandium in Dilute Aluminum-Scandium Alloys. Met. Mater. Trans. A 2014, 45, 3800–3805. [Google Scholar] [CrossRef]
- Hiraoka, M. Crown Compounds: Their Characteristics and Applications; Elsevier Science Ltd.: New York, NY, USA, 1982. [Google Scholar]
- Pedersen, C.J. Synthetic Multidentate Macrocyclic Compounds. In Synthetic Multidentate Macrocyclic Compounds; Christensen, J.J., Ed.; Elsevier: New York, NY, USA, 1978. [Google Scholar]
- Cooper, S.R. Crown Compounds: Toward Future Applications; Wiley-VCH: New York, NY, USA, 1992. [Google Scholar]
- Lindoy, L.F. The Chemistry of Macrocyclic Ligand Complexes; Cambridge University Press (CUP): Cambridge, UK, 1989. [Google Scholar]
- Abdurrahmanoglu, S.; Gündüz, C.; Çakır, Ü.; Bulut, M.; Çakir, Ü.; Çiçek, B. The synthesis and complexation study of some coumestan and coumestan analog derivatives of crown ethers using conductometry. Dye. Pigment. 2005, 65, 197–204. [Google Scholar] [CrossRef]
- Alivertis, D.; Paraskevopoulos, G.; Theodorou, V.; Skobridis, K. Dendritic effects of crown ether-functionalized dendrimers on the solvent extraction of metal ions. Tetrahedron Lett. 2009, 50, 6019–6021. [Google Scholar] [CrossRef]
- Çiçek, B.; Yıldız, A.; Çiçek, B. Synthesis, Metal Ion Complexation and Computational Studies of Thio Oxocrown Ethers. Molecules 2011, 16, 8670–8683. [Google Scholar] [CrossRef]
- Han, W.-S.; Lee, Y.-H.; Jung, K.-J.; Ly, S.-Y.; Hong, T.-K.; Kim, M.-H. Potassium ion-selective polyaniline solid-contact electrodes based on 4′,4″(5″)-di-tert-butyldibenzo-18-crown-6-ether ionophore. J. Anal. Chem. 2008, 63, 987–993. [Google Scholar] [CrossRef]
- Izatt, R.M.; Bradshaw, J.S.; Pawlak, K.; Bruening, R.L.; Tarbet, B.J. Thermodynamic and kinetic data for macrocycle interaction with neutral molecules. Chem. Rev. 1992, 92, 1261–1354. [Google Scholar] [CrossRef]
- Saleh, N.J.; Al-Zaidi, B.Y.S.; Sabbar, Z.M. A Comparative Study of Y Zeolite Catalysts Derived from Natural and Commercial Silica: Synthesis, Characterization, and Catalytic Performance. Arab. J. Sci. Eng. 2017, 43, 5819–5836. [Google Scholar] [CrossRef]
- Khayatian, G.; Karoonian, F.S. Conductance and Thermodynamic Study of the Complexation of Ammonium Ion with Different Crown Ethers in Binary Nonaqueous Solvents. J. Chin. Chem. Soc. 2008, 55, 377–384. [Google Scholar] [CrossRef]
- Deorkar, N.V.; Khopkar, S.M. Separation of Scandium Using Liquid–Liquid Extraction with Macrocyclic Polyether(s) from Picrate Media. Bull. Chem. Soc. Jpn. 1991, 64, 1962–1965. [Google Scholar] [CrossRef]
- Pozzi, G.; Quici, S.; Fish, R.H. Perfluorocarbon Soluble Crown Ethers as Phase Transfer Catalysts. Adv. Synth. Catal. 2008, 350, 2425–2436. [Google Scholar] [CrossRef]
- Rounaghi, G.; Mohajeri, M.; Tarahomi, S.; Rahmanian, R. Study of Complex Formation of Dibenzo-18-Crown-6 with Ce3+, Y3+, UO2+ and Sr2+ Cations in Acetonitrile–Dioxane Binary Solvent Mixtures. J. Solut. Chem. 2011, 40, 377–389. [Google Scholar] [CrossRef]
- Pal, J.; Deb, M.K.; Deshmukh, D.K. Microwave-assisted synthesis of silver nanoparticles using benzo-18-crown-6 as reducing and stabilizing agent. Appl. Nanosci. 2013, 4, 507–510. [Google Scholar] [CrossRef][Green Version]
- Shamsipur, M.; Pouretedal, H.R. Conductance Study of Complexation of Lead Ions by Several 18-Membered Crown Ethers in Acetonitrile-Dimethyl Sulfoxide Mixtures Between 25 and 55 °C. J. Solut. Chem. 1999, 28, 1187–1205. [Google Scholar] [CrossRef]
- Shim, J.; Jang, E.; Chung, K.-C. Lead Ion-Selective Polypyrrole Solid-Contact Electrode Based on Crown Ether. Anal. Lett. 2007, 40, 3038–3049. [Google Scholar] [CrossRef]
- Tu, C.; Surowiec, K.; Gega, J.; Purkiss, D.W.; Bartsch, R.A. Di-ionizable calix[4]arene-1,2-crown-5 and -crown-6 ethers in cone conformations: Synthesis and divalent metal ion extraction. Tetrahedron 2008, 64, 1187–1196. [Google Scholar] [CrossRef]
- Weber, E.; Toner, J.L.; Goldberg, I.; Vögtle, F.; Laidler, D.A.; Stoddart, J.F.; Bartsch, R.A.; Liotta, C.L. Crown ethers—Complexes and selectivity. In Crown Ethers and Analogs (1989); John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1989. [Google Scholar]
- Yongzhu, J.; Hirose, K.; Nakamura, T.; Nishioka, R.; Ueshige, T.; Tobe, Y. Preparation and evaluation of a chiral stationary phase covalently bound with a chiral pseudo-18-crown-6 ether having a phenolic hydroxy group for enantiomer separation of amino compounds. J. Chromatogr. A 2006, 1129, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Rounaghi, G.H.; Tarahomi, S.; Mohajeri, M. A conductometric study of complexation reaction between dibenzo-24-crown-8 with yttrium cation in some binary mixed non-aqueous solvents. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 319–325. [Google Scholar] [CrossRef]
- Smirnov, D.; Molchanova, T. The investigation of sulphuric acid sorption recovery of scandium and uranium from the red mud of alumina production. Hydrometallurgy 1997, 45, 249–259. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, R.; Li, Y.; Gao, B.; An, F.; Huang, X.; Zhang, Y.; Xu, Y. Binding and recognizing properties of ionic imprinted polymer towards Sc(III). Funct. Mater. 2014, 451, 87. [Google Scholar]
- Roosen, J.; Van Roosendael, S.; Borra, C.R.; Van Gerven, T.; Mullens, S.; Binnemans, K. Recovery of scandium from leachates of Greek bauxite residue by adsorption on functionalized chitosan–silica hybrid materials. Green Chem. 2016, 18, 2005–2013. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Sukhinina, N.S.; Masalov, V.M.; Emelchenko, G.A. Adsorption of lanthanides and scandium ions by silica sol-gel material doped with novel bifunctional ionic liquid, trioctylmethylammonium 1-phenyl-3-methyl-4-benzoyl-5-onate. J. Environ. Chem. Eng. 2016, 4, 3788–3796. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G.; Alevizos, G. Adsorption of Scandium and Neodymium on Biochar Derived after Low-Temperature Pyrolysis of Sawdust. Minerals 2017, 7, 200. [Google Scholar] [CrossRef]
- Ramasamy, D.L.; Puhakka, V.; Repo, E.; Khan, S.; Sillanpää, M. Coordination and silica surface chemistry of lanthanides (III), scandium (III) and yttrium (III) sorption on 1-(2-pyridylazo)-2-napththol (PAN) and acetylacetone (acac) immobilized gels. Chem. Eng. J. 2017, 324, 104–112. [Google Scholar] [CrossRef]
- Ramasamy, D.L.; Puhakka, V.; Repo, E.; Ben Hammouda, S.; Sillanpää, M. Two-stage selective recovery process of scandium from the group of rare earth elements in aqueous systems using activated carbon and silica composites: Dual applications by tailoring the ligand grafting approach. Chem. Eng. J. 2018, 341, 351–360. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, K.; Wu, Y.; Lei, Q.; Peng, C.; Chen, W. Separation and recovery of iron and scandium from acid leaching solution of red mud using D201 resin. J. Rare Earths 2020, 38, 1322–1329. [Google Scholar] [CrossRef]








| Sc, ppm Model Solution | Sc, ppm (Aqueous Phase) After Extraction | Extraction Capacity mg/g | Extraction% | Sc, ppm Concentration on the Solid Phase before Back Extraction/ Stripping | Sc, ppm Concentration in the Aqueous Phase after Back Extraction/ Stripping | Back Extraction/Stripping % |
|---|---|---|---|---|---|---|
| 15 | 0.5 | 14.5 | 96.7 | 14.5 | 14 | 96.5 |
| 25 | 1.5 | 23.5 | 94.0 | 23.5 | 23 | 97.8 |
| 50 | 1.7 | 48.3 | 96.6 | 48.3 | 45 | 93.1 |
| 75 | 14 | 61 | 81.3 | 61 | 58 | 95.0 |
| Component (m %) | SiO2 | Na2O3 | MgO | CaO | Fe2O3 | Al2O3 | K2O | SO3 |
|---|---|---|---|---|---|---|---|---|
| HCl (3N) (5 °C/min) | 97.5 | 0.23 | 0.15 | 0.08 | 0.22 | 0.9 | 0.11 | 1.1 |
| Nanosilica | 98.7 | 0.004 | 0.05 | 0.02 | 0.05 | 0.2 | 0.003 | 0.7 |
| Solid Phase | Year | References |
|---|---|---|
| Ampholyte resins, AFI-21 and AFI-22 | 1997 | [51] |
| Ionic imprinted polymer materials IIP-PEI/SiO2 | 2014 | [52] |
| Chitosan–silica hybrid materials DTPA–chitosan–silica and EGTA–chitosan–silica | 2016 | [53] |
| Silica sol-gel doped with ionic liquid | 2016 | [54] |
| Biochar | 2017 | [55] |
| Mesoporous silica-PAN | 2017 | [56] |
| Activated carbon and silica composites | 2018 | [57] |
| D201 resin | 2020 | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salman, A.D.; Juzsakova, T.; Jalhoom, M.G.; Le Phuoc, C.; Mohsen, S.; Adnan Abdullah, T.; Zsirka, B.; Cretescu, I.; Domokos, E.; Stan, C.D. Novel Hybrid Nanoparticles: Synthesis, Functionalization, Characterization, and Their Application in the Uptake of Scandium (III)Ions from Aqueous Media. Materials 2020, 13, 5727. https://doi.org/10.3390/ma13245727
Salman AD, Juzsakova T, Jalhoom MG, Le Phuoc C, Mohsen S, Adnan Abdullah T, Zsirka B, Cretescu I, Domokos E, Stan CD. Novel Hybrid Nanoparticles: Synthesis, Functionalization, Characterization, and Their Application in the Uptake of Scandium (III)Ions from Aqueous Media. Materials. 2020; 13(24):5727. https://doi.org/10.3390/ma13245727
Chicago/Turabian StyleSalman, Ali Dawood, Tatjána Juzsakova, Moayed G. Jalhoom, Cuong Le Phuoc, Saja Mohsen, Thamer Adnan Abdullah, Balázs Zsirka, Igor Cretescu, Endre Domokos, and Catalina Daniela Stan. 2020. "Novel Hybrid Nanoparticles: Synthesis, Functionalization, Characterization, and Their Application in the Uptake of Scandium (III)Ions from Aqueous Media" Materials 13, no. 24: 5727. https://doi.org/10.3390/ma13245727
APA StyleSalman, A. D., Juzsakova, T., Jalhoom, M. G., Le Phuoc, C., Mohsen, S., Adnan Abdullah, T., Zsirka, B., Cretescu, I., Domokos, E., & Stan, C. D. (2020). Novel Hybrid Nanoparticles: Synthesis, Functionalization, Characterization, and Their Application in the Uptake of Scandium (III)Ions from Aqueous Media. Materials, 13(24), 5727. https://doi.org/10.3390/ma13245727







