Low-Temperature Hydrothermal Treatment Surface Functionalization of the Ultrafine-Grained TiMo Alloys for Medical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Specimens
2.2. Alkaline Treatment
2.3. Hydrothermal Treatment
2.4. Materials Characterization
- (1)
- Base specimens:
- Ti(α)—ref. code 04-003-5042
- Ti(β)—Ti0.9Mo0.1—ref. code 04-018-6034
- (2)
- Alkali treatment:
- TiO—ref. code 01-086-2352
- Na2Ti2O4(OH)2—ref. code 00-057-0123
- Na0.55Mo2O4—ref. code 00-040-1022
- (3)
- Hydrothermal treatment:
- Ca10(PO4)6(OH)2—ref. code 01-080-3958
- Na2HPO4—ref. code 00-001-0997
- measuring liquid: glycerol,
- drop volume: 0.5 µL,
- dosing speed 0.2 mL/min,
- measuring time: 8 s,
- probing frequency for multiple measurements: 50 fps,
- base cut off: automatic/manual,
- CA fitting method: Young Laplace,
- measurements conditions: ambient 23 °C.
3. Results and Discussion
3.1. Base Materials—Ti-Mo Systems
3.2. Acid Etching
3.3. Alkali Treatment in NaOH Solution with Different Molar Concentration
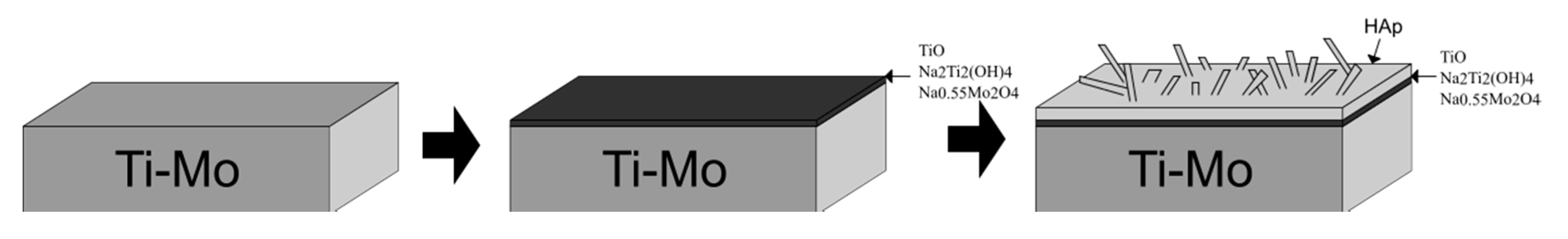
3.4. Hydrothermal Treatment in Ca-EDTA Solution
3.5. Wettability Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Marczewski, M.; Miklaszewski, A.; Jurczyk, M. Structure evolution analysis in ultrafine-grained Zr and Nb-based beta titanium alloys. J. Alloys Compd. 2018, 765, 459–469. [Google Scholar] [CrossRef]
- Sochacka, P.; Miklaszewski, A.; Jurczyk, M. Development of b -type Ti-x at. % Mo alloys by mechanical alloying and powder metallurgy: Phase evolution and mechanical properties. J. Alloys Compd. 2019, 776, 370–378. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, K.; He, S.; Wang, B.; Zhu, W.; Ren, F. Materials Science & Engineering C Fabrication of high strength, antibacterial and biocompatible Ti-5Mo-5Ag alloy for medical and surgical implant applications. Mater. Sci. Eng. C 2020, 106, 110165. [Google Scholar]
- Sochacka, P.; Miklaszewski, A.; Jurczyk, M. The influence of mo content on phase transformation in Ti-Mo alloys. Arch. Metall. Mater. 2017, 62, 2051–2056. [Google Scholar] [CrossRef]
- Mareci, D.; Solcan, C.; Mircea, F.; Carmen, L.; Fern, L. New Ti-6Al-2Nb-2Ta-1Mo alloy as implant biomaterial: In vitro corrosion and in vivo osseointegration evaluations. Mater. Chem. Phys. 2020, 240, 1–10. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Vicente, F.B.; Araújo, R.O.; Lourenço, M.L.; Kuroda, P.A.B.; Buzalaf, M.A.R.; Grandini, C.R. Effect of the substitutional elements on the microstructure of the Ti-15Mo-Zr and Ti-15Zr-Mo systems alloys. J. Mater. Res. Technol. 2015. [Google Scholar] [CrossRef]
- Martins, J.R.S., Jr.; Araujo, R.O.; Nogueira, R.A.; Grandini, C.R. Internal friction and microstructure of Ti and Ti-Mo alloys containing oxygen. Arch. Metall. Mater. 2016, 61, 25–30. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Wu, H.; Song, M.; Zhang, T.; Lan, X.; Yao, T. Materials Characterization Elastic modulus of phases in Ti–Mo alloys. Mater. Charact. 2015, 106, 302–307. [Google Scholar] [CrossRef]
- Cardoso, F.F.; Ferrandini, P.L.; Lopes, E.S.N.; Cremasco, A.; Caram, R. Ti-Mo alloys employed as biomaterials: Effects of composition and aging heat treatment on microstructure and mechanical behavior. J. Mech. Behav. Biomed. Mater. 2014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. International Journal of Adhesion & Adhesives Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar]
- Neves, G.A. A brief review on hydroxyapatite production and use in biomedicine. Ceramica 2019, 65, 282–302. [Google Scholar]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Kinetic Study on the Crystal Growth of Hydroxyapatite. Langmuir 2001, 17, 8092–8097. [Google Scholar] [CrossRef]
- Haider, A.; Gupta, K.C.; Kang, I.K. PLGA/nHA hybrid nanofiber scaffold as a nanocargo carrier of insulin for accelerating bone tissue regeneration. Nanoscale Res. Lett. 2014, 9, 1–12. [Google Scholar] [CrossRef]
- Gadow, R.; Killinger, A.; Stiegler, N. Hydroxyapatite coatings for biomedical applications deposited by different thermal spray techniques. Surf. Coatings Technol. 2010. [Google Scholar] [CrossRef]
- Cüneyt Tas, A. Synthesis of biomimetic Ca-hydroxyapatite powders at 37 °C in synthetic body fluids. Biomaterials 2000, 21, 1429–1438. [Google Scholar] [CrossRef]
- Sygnatowicz, M.; Tiwari, A. Controlled synthesis of hydroxyapatite-based coatings for biomedical application. Mater. Sci. Eng. C 2009, 29, 1071–1076. [Google Scholar] [CrossRef]
- Cui, F.Z.; Luo, Z.S.; Feng, Q.L. Highly adhesive hydroxyapatite coatings on titanium alloy formed by ion beam assisted deposition. J. Mater. Sci. Mater. Med. 1997, 8, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Kim, H.E.; Lee, I.S. Ion-beam-assisted deposition (IBAD) of hydroxyapatite coating layer on Ti-based metal substrate. Biomaterials 2000, 21, 469–473. [Google Scholar] [CrossRef]
- Gross, K.A.; Chai, C.S.; Kannangara, G.S.K.; Ben-Nissan, B.; Hanley, L. Thin hydroxyapatite coatings via sol-gel synthesis. J. Mater. Sci. Mater. Med. 1998, 9, 839–843. [Google Scholar] [CrossRef]
- Hu, X.; Shen, H.; Cheng, Y.; Xiong, X.; Wang, S.; Fang, J.; Wei, S. One-step modification of nano-hydroxyapatite coating on titanium surface by hydrothermal method. Surf. Coatings Technol. 2010, 205, 2000–2006. [Google Scholar] [CrossRef]
- Faghihi-Sani, M.A.; Arbabi, A.; Mehdinezhad-Roshan, A. Crystallization of hydroxyapatite during hydrothermal treatment on amorphous calcium phosphate layer coated by PEO technique. Ceram. Int. 2013, 39, 1793–1798. [Google Scholar] [CrossRef]
- Kim, C.; Kendall, M.R.; Miller, M.A.; Long, C.L.; Larson, P.R.; Humphrey, M.B.; Madden, A.S.; Tas, A.C. Comparison of titanium soaked in 5 M NaOH or 5 M KOH solutions. Mater. Sci. Eng. C 2013, 33, 327–339. [Google Scholar] [CrossRef]
- Hsu, H.; Wu, S.; Hsu, S.; Hsu, C.; Ho, W. Bone-like nano-hydroxyapatite coating on low-modulus Ti–5Nb–5Mo alloy using hydrothermal and post-heat treatments. Thin Solid Films 2019, 687, 137463. [Google Scholar] [CrossRef]
- Baddeley, M. Material Fundamentals and Clinical Performance of Plasma-Sprayed Hydroxyapatite Coatings: A Review. Keynes Post Keynes. Polit. Econ. 1999, 570–592. [Google Scholar]
- Song, H.J.; Kim, J.W.; Kook, M.S.; Moon, W.J.; Park, Y.J. Fabrication of hydroxyapatite and TiO 2 nanorods on microarc-oxidized titanium surface using hydrothermal treatment. Appl. Surf. Sci. 2010, 256, 7056–7061. [Google Scholar] [CrossRef]
- Titanium, P. Characterization of Nano-Scale Hydroxyapatite Coating Synthesized from Eggshells Through Hydrothermal Reaction on Commercially Pure Titanium. Coatings 2020, 10, 112. [Google Scholar]
- Suchanek, K.; Bartkowiak, A.; Gdowik, A.; Perzanowski, M.; Kąc, S.; Szaraniec, B.; Suchanek, M.; Marszałek, M. Crystalline hydroxyapatite coatings synthesized under hydrothermal conditions on modified titanium substrates. Mater. Sci. Eng. C 2015, 51, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.-F.; Lai, C.-H.; Hsu, H.-C.; Wu, S.-C. Surface modification of a low-modulus Ti-7.5Mo alloy treated with aqueous NaOH. Surf. Coat. Technol. 2009, 203, 3142–3150. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Salam Hamdy, A.; Khalil, K.A.; Lim, J.H. A novel simple one-step air jet spinning approach for deposition of poly(vinyl acetate)/hydroxyapatite composite nanofibers on Ti implants. Mater. Sci. Eng. C 2015, 49, 681–690. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Arce, H.; Montero, M.L.; Aenz, A.S.; Casta, V.M. Effect of pH and temperature on the formation of hydroxyapatite at low temperatures by decomposition of a Ca-EDTA complex. Polyhedron 2004, 23, 1897–1901. [Google Scholar] [CrossRef]
- Lak, A.; Mazloumi, M.; Mohajerani, M.; Kajbafvala, A.; Zanganeh, S.; Arami, H.; Sadrnezhaad, S.K. Self-assembly of dandelion-like hydroxyapatite nanostructures via hydrothermal method. J. Am. Ceram. Soc. 2008, 91, 3292–3297. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]








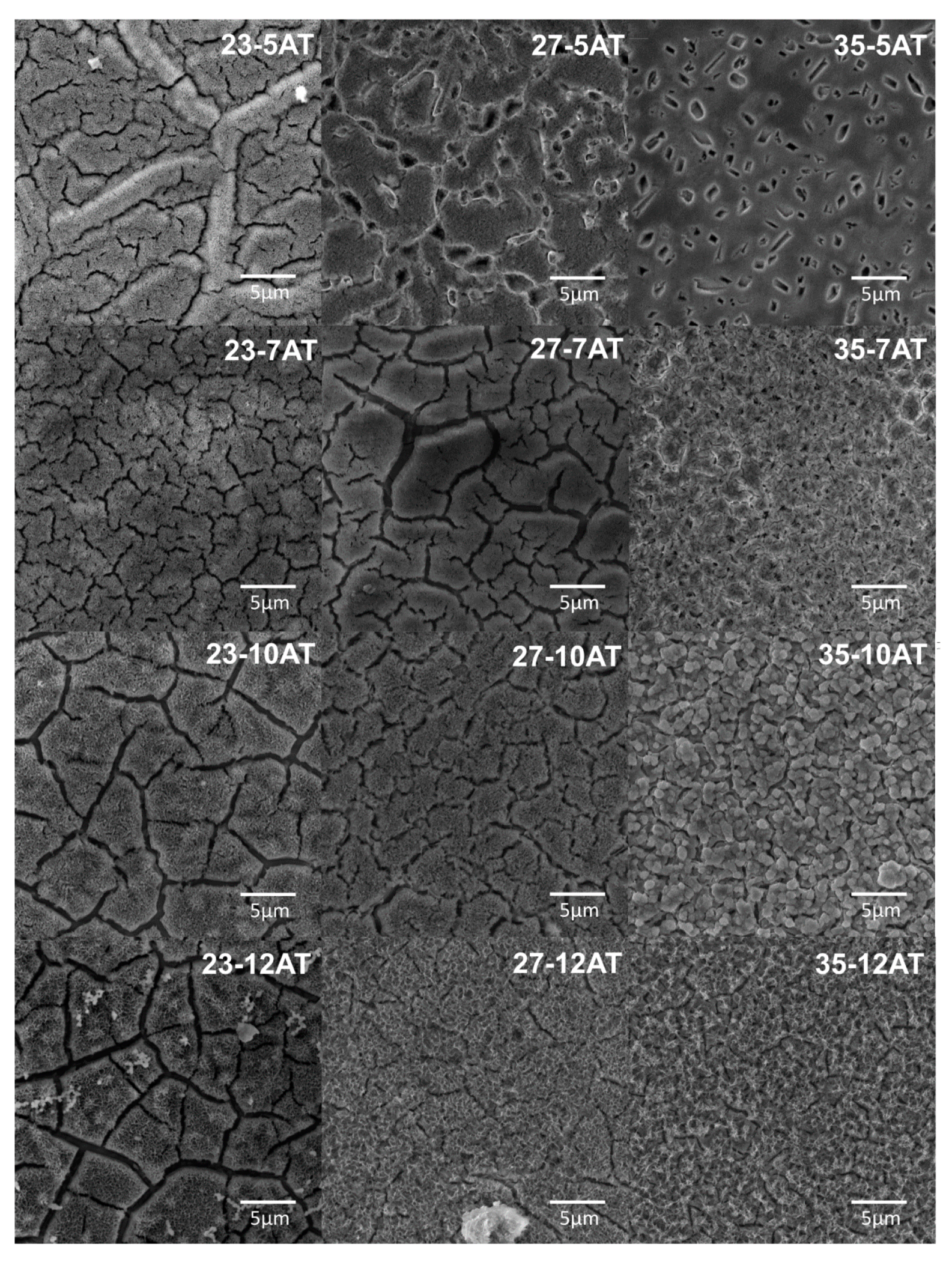


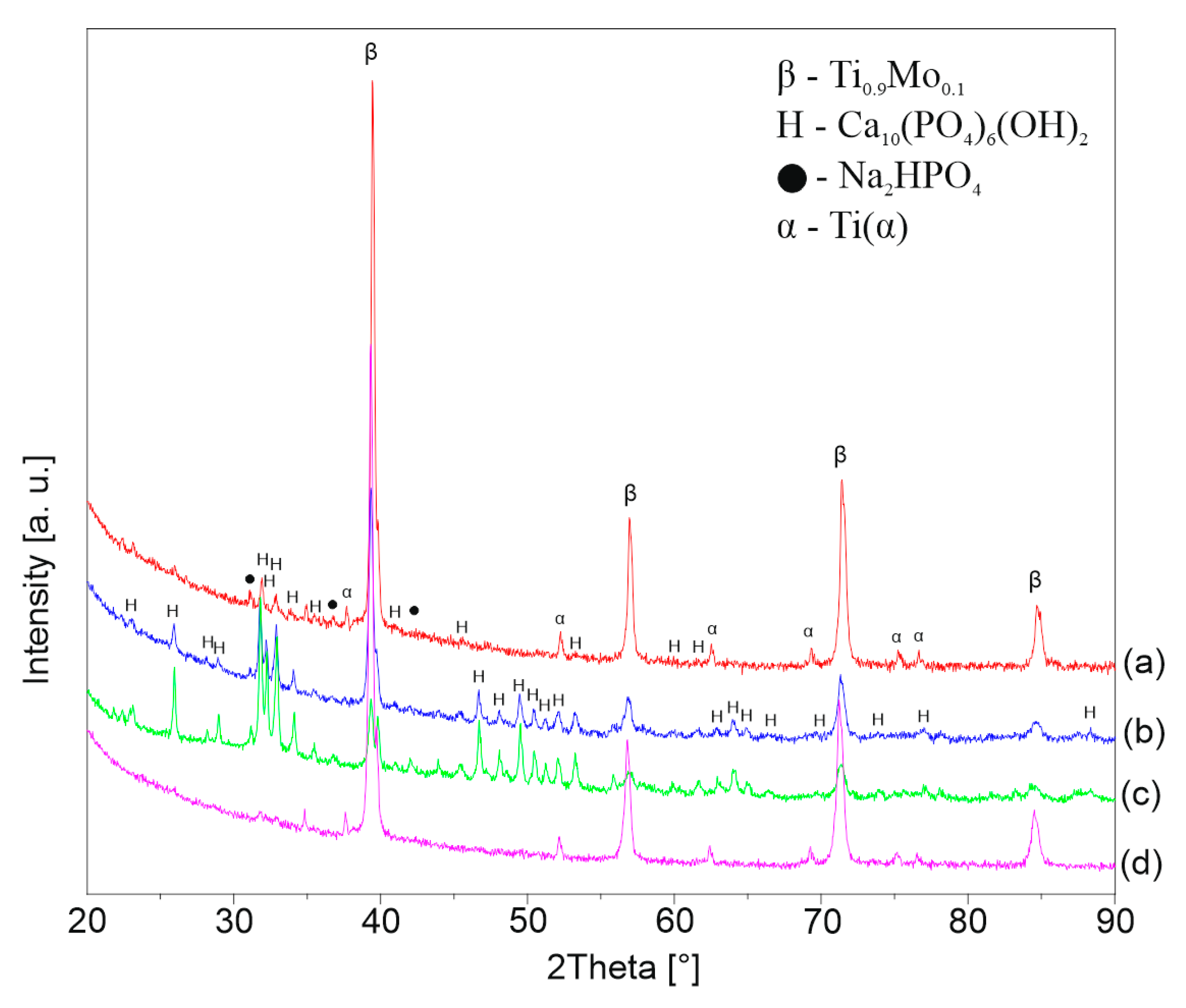

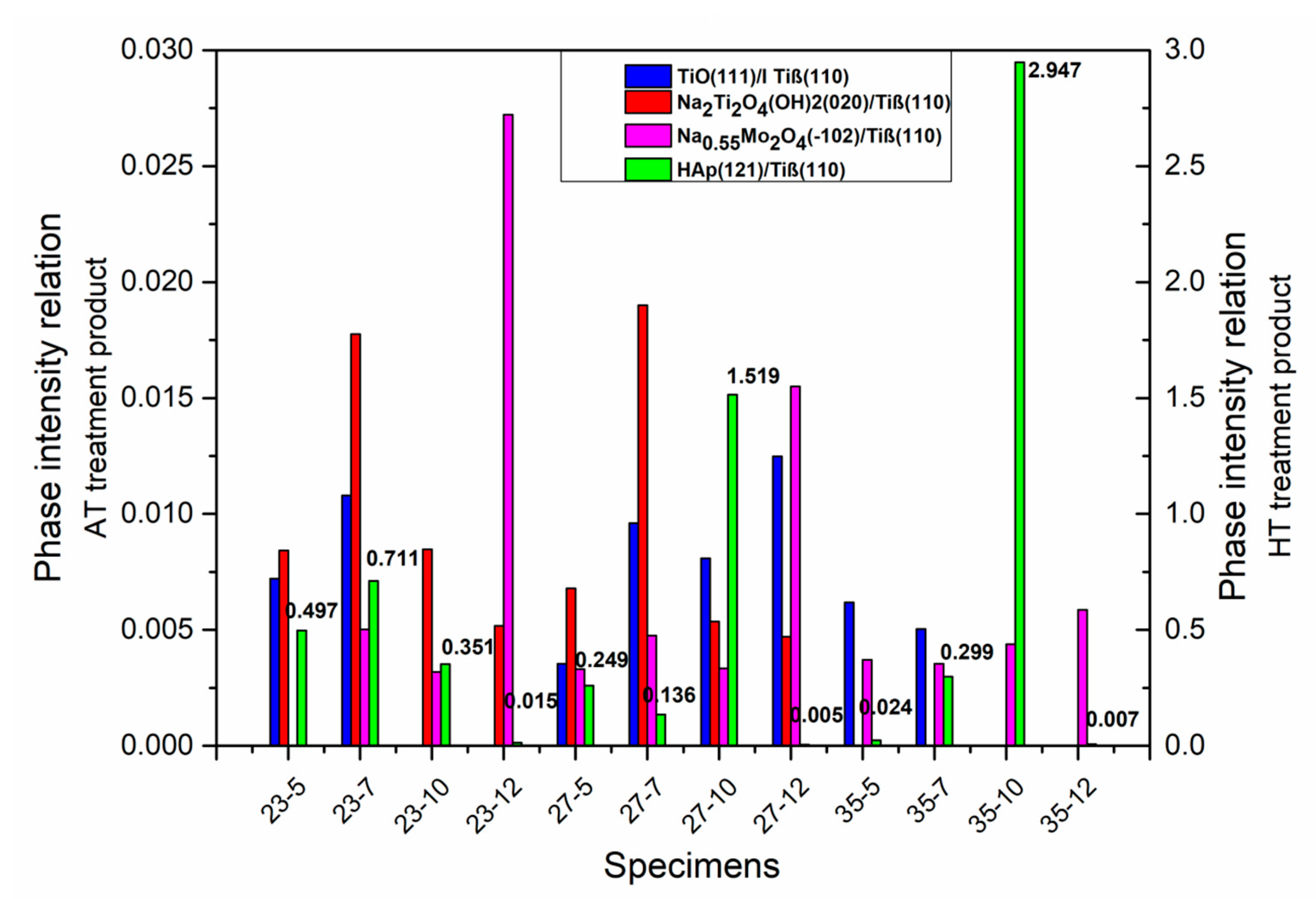

| Sample/Molar Concentration of NaOH | 5 M | 7 M | 10 M | 12 M |
|---|---|---|---|---|
| Ti23Mo | 23-5AT | 23-7AT | 23-10AT | 23-12AT |
| Ti27Mo | 27-5AT | 27-7AT | 27-10AT | 27-12AT |
| Ti35Mo | 35-5AT | 35-7AT | 35-10AT | 35-12AT |
| Sample/Molar Concentration of NaOH | 5 M | 7 M | 10 M | 12 M |
|---|---|---|---|---|
| Ti23Mo | 23-5HT | 23-7HT | 23-10HT | 23-12HT |
| Ti27Mo | 27-5HT | 27-7HT | 27-10HT | 27-12HT |
| Ti35Mo | 35-5HT | 35-7HT | 35-10HT | 35-12HT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piechowiak, D.; Miklaszewski, A.; Jurczyk, M. Low-Temperature Hydrothermal Treatment Surface Functionalization of the Ultrafine-Grained TiMo Alloys for Medical Applications. Materials 2020, 13, 5763. https://doi.org/10.3390/ma13245763
Piechowiak D, Miklaszewski A, Jurczyk M. Low-Temperature Hydrothermal Treatment Surface Functionalization of the Ultrafine-Grained TiMo Alloys for Medical Applications. Materials. 2020; 13(24):5763. https://doi.org/10.3390/ma13245763
Chicago/Turabian StylePiechowiak, Daria, Andrzej Miklaszewski, and Mieczysław Jurczyk. 2020. "Low-Temperature Hydrothermal Treatment Surface Functionalization of the Ultrafine-Grained TiMo Alloys for Medical Applications" Materials 13, no. 24: 5763. https://doi.org/10.3390/ma13245763
APA StylePiechowiak, D., Miklaszewski, A., & Jurczyk, M. (2020). Low-Temperature Hydrothermal Treatment Surface Functionalization of the Ultrafine-Grained TiMo Alloys for Medical Applications. Materials, 13(24), 5763. https://doi.org/10.3390/ma13245763






