Electronic Structure and Surface Properties of Copper Thiocyanate: A Promising Hole Transport Material for Organic Photovoltaic Cells
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Crystal Structure β-CuSCN
3.2. Electronic Properties of β-CuSCN
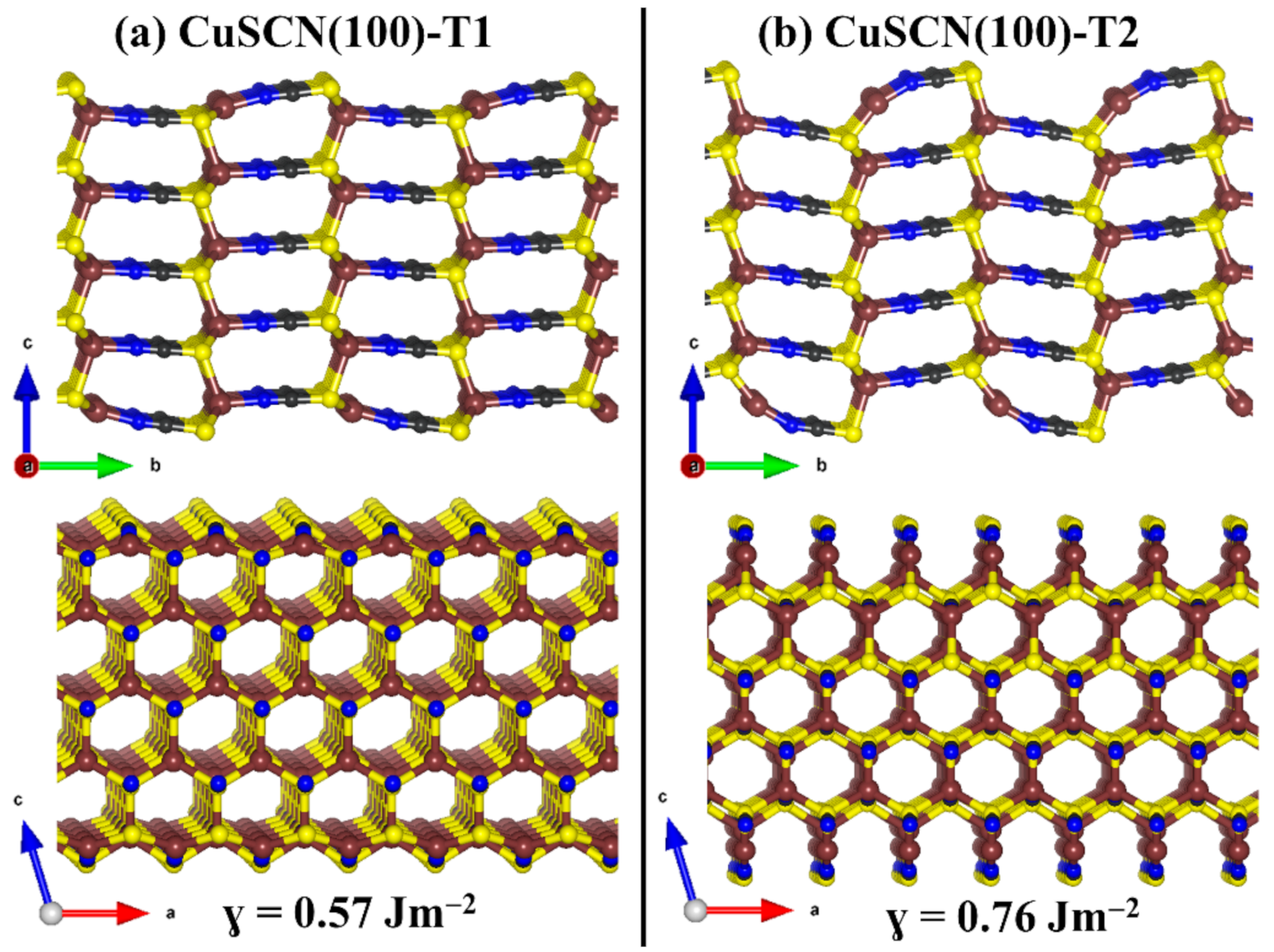
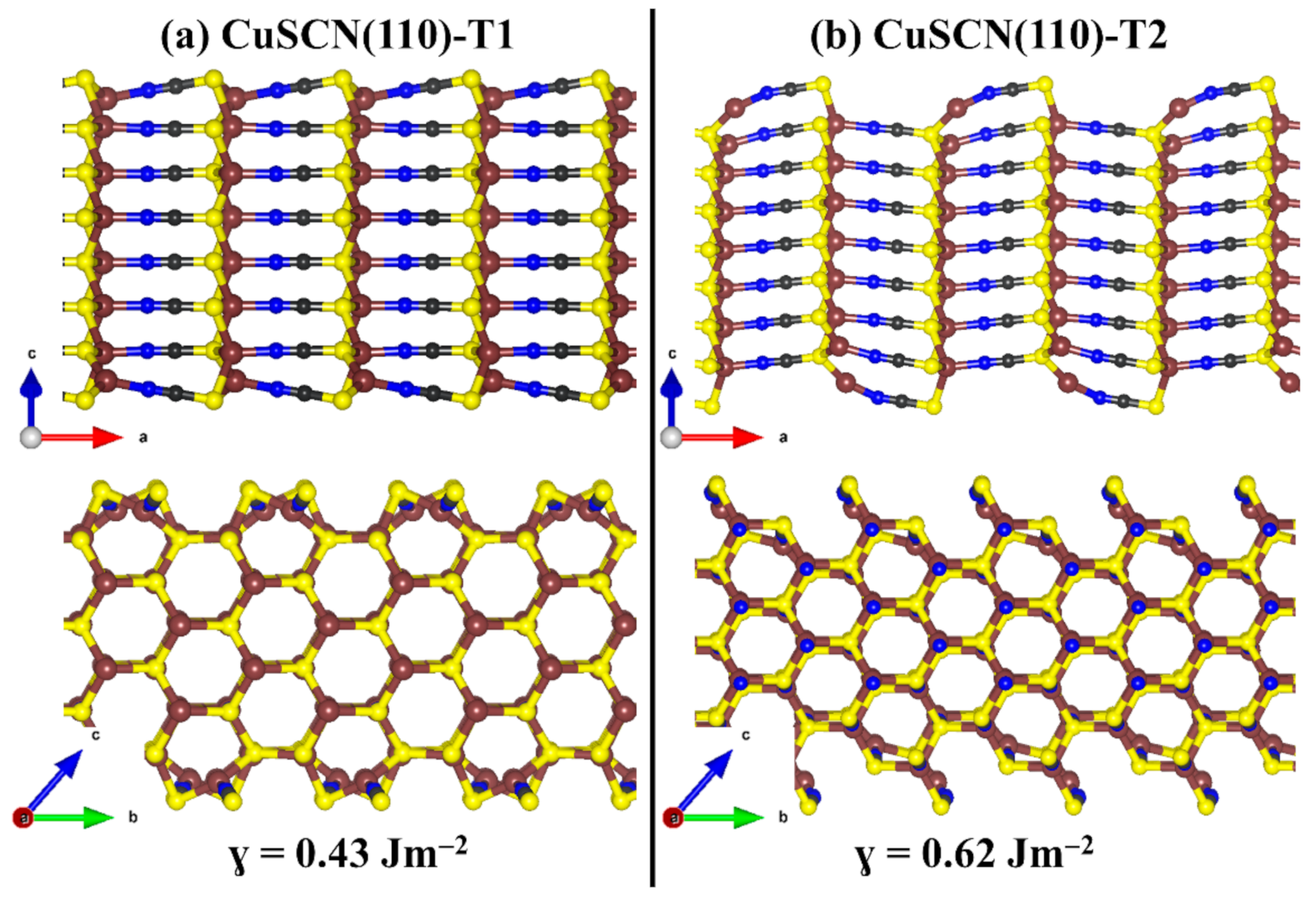
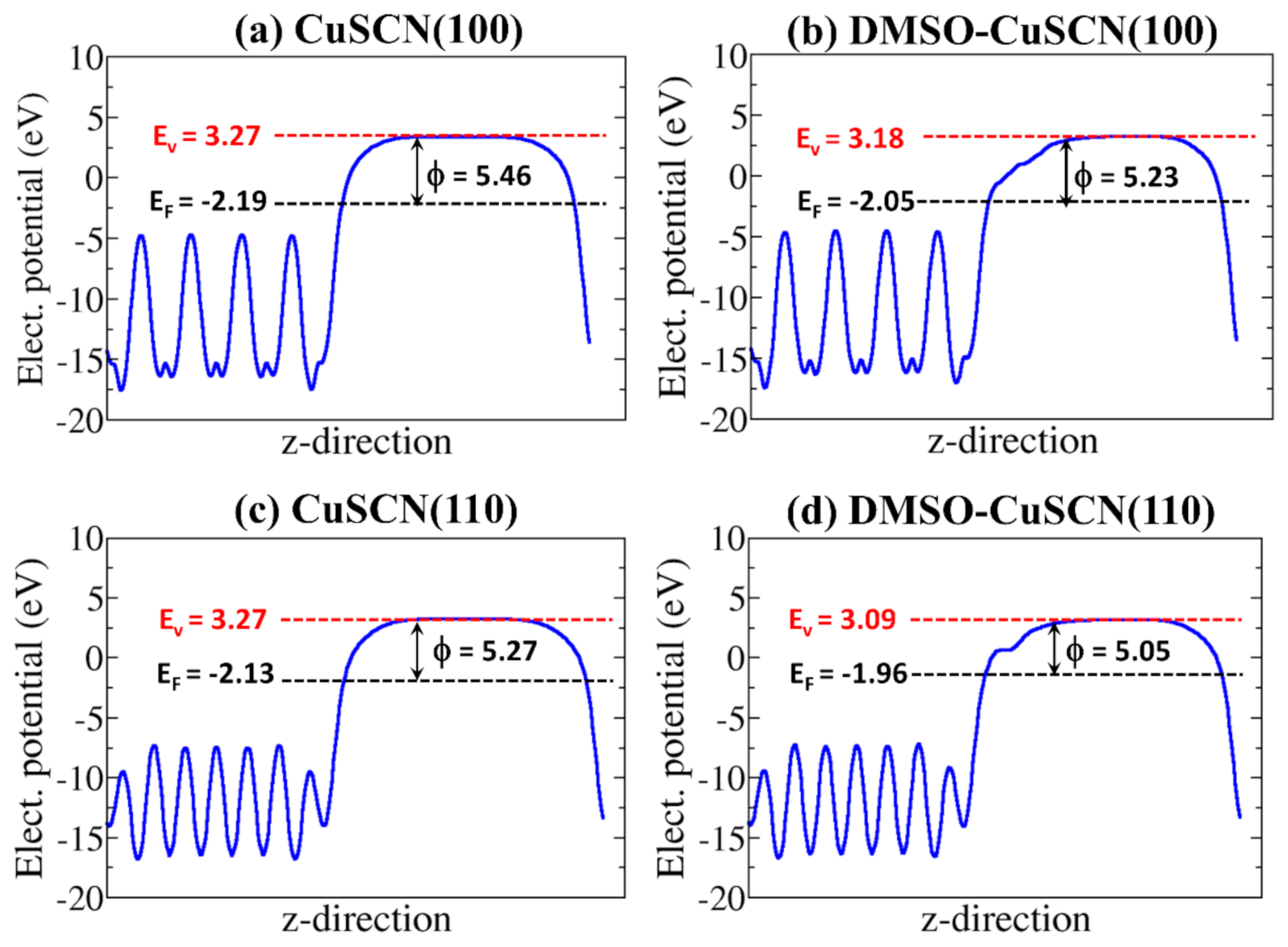
3.3. Surface Properties
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sidney, E.C.; Evan, N.; Michael, C.D.; Daniel, R.G. Colloidal nanocrystals of lead-free double-perovskite (elpasolite) semiconductors: Synthesis and anion exchange to access new materials. Nano Lett. 2018, 18, 1118–1123. [Google Scholar]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [PubMed]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.-S.; Chang, J.A.; Lee, Y.H.; Kim, H.J.; Sarkar, A.; Nazeeruddin, M.K. Efficient inorganic–organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 2013, 7, 486–491. [Google Scholar]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar]
- National Renewable Energy Laboratory Service. Photovoltaic Research: Efficiency Chart. Available online: https://www.nrel.gov/pv/assets/images/efficiency-chart.png (accessed on 17 December 2020).
- Gao, L.; Yang, G. Organic-Inorganic Halide Perovskites: From Crystallization of Polycrystalline Films to Solar Cell Applications. Sol. RRL 2020, 4, 1900200. [Google Scholar]
- O’Regan, B.; Lenzmann, F.; Muis, R.; Wienke, J. A solid-state dye-sensitized solar cell fabricated with pressure-treated P25-TiO2 and CuSCN: Analysis of pore filling and IV characteristics. Chem. Mater. 2002, 14, 5023–5029. [Google Scholar]
- Yang, Y.; Hoang, M.T.; Yao, D.; Pham, N.D.; Tiong, V.T.; Wang, X.; Wang, H. Spiro-OMeTAD or CuSCN as a preferable hole transport material for carbon-based planar perovskite solar cells. Mater. Chem. A 2020, 8, 12723–12734. [Google Scholar]
- Ponseca, C.S.; Hutter, E.M.; Piatkowski, P.; Cohen, B.; Pascher, T.; Douhal, A.; Yartsev, A.; Sundström, V.; Savenije, T.J. Mechanism of Charge Transfer and Recombination Dynamics in Organo Metal Halide Perovskites and Organic Electrodes, PCBM, and Spiro-OMeTAD: Role of Dark Carriers. J. Am. Chem. Soc. 2015, 137, 16043–16048. [Google Scholar]
- Abrusci, A.; Stranks, S.D.; Docampo, P.; Yip, H.; Jen, A.K.-Y.; Snaith, H.J. High-Performance Perovskite-Polymer Hybrid Solar Cells via Electronic Coupling with Fullerene Monolayers. Nano Lett. 2013, 13, 3124–3128. [Google Scholar]
- Shariatinia, Z. Recent progress in development of diverse kinds of hole transport materials for the perovskite solar cells: A review. Renew. Sustain. Energy Rev. 2020, 119, 109608. [Google Scholar]
- Bi, D.; Tress, W.; Dar, M.I.; Gao, P.; Luo, J.; Renevier, C.; Schenk, K.; Abate, A.; Giordano, F.; Correa Baena, J.-P.; et al. Efficient Luminescent Solar Cells Based on Tailored Mixed-Cation Perovskites. Sci. Adv. 2016, 2, 1501170. [Google Scholar]
- Abate, A.; Hollman, D.J.; Teuscher, J.; Pathak, S.; Avolio, R.; D’Errico, G.; Vitiello, G.; Fantacci, S.; Snaith, H.J. Protic Ionic Liquids as P-Dopant for Organic Hole Transporting Materials and Their Application in High Efficiency Hybrid Solar Cells. J. Am. Chem. Soc. 2013, 135, 13538–13548. [Google Scholar] [PubMed]
- Reza, K.M.; Gurung, A.; Bahrami, B.; Mabrouk, S.; Elbohy, H.; Pathak, R.; Chen, K.; Chowdhury, A.H.; Rahman, M.T.; Letourneau, S.; et al. Tailored PEDOT:PSS hole transport layer for higher performance in perovskite solar cells: Enhancement of electrical and optical properties with improved morphology. J. Energy Chem. 2020, 44, 41–50. [Google Scholar]
- Syed, A.A.; Poon, C.Y.; Li, H.W.; Zhu, F. A sodium citrate-modified-PEDOT:PSS hole transporting layer for performance enhancement in inverted planar perovskite solar cells. J. Mater. Chem. C 2019, 7, 5260–5266. [Google Scholar]
- Huang, D.; Goh, T.; Kong, J.; Zheng, Y.; Zhao, S.; Xu, Z.; Taylor, A.D. Perovskite solar cells with a DMSO-treated PEDOT:PSS hole transport layer exhibit higher photovoltaic performance and enhanced durability. Nanoscale 2017, 9, 4236–4243. [Google Scholar] [PubMed]
- Yaacobi-Gross, N.; Treat, N.D.; Pattanasattayavong, P.; Faber, H.; Perumal, A.K.; Stingelin, N.; Bradley, D.D.C.; Stavrinou, P.N.; Heeney, M.; Anthopoulos, T.D. High-Efficiency Organic Photovoltaic Cells Based on the Solution-Processable Hole Transporting Interlayer Copper Thiocyanate (CuSCN) as a Replacement for PEDOT:PSS. Adv. Energy Mater. 2015, 5, 1401529. [Google Scholar]
- Huang, J.; Miller, P.F.; Wilson, J.S.; de Mello, A.J.; de Mello, J.C.; Bradley, D.D.C. Investigation of the Effects of Doping and Post-Deposition Treatments on the Conductivity, Morphology, and Work Function of Poly(3,4-ethylenedioxythiophene)/Poly(styrene sulfonate) Films. Adv. Funct. Mater. 2005, 15, 290–296. [Google Scholar]
- Nardes, A.M.; Kemerink, M.; de Kok, M.M.; Vinken, E.; Maturova, K.; Janssen, R.A. Conductivity, work function, and environmental stability of PEDOT:PSS thin films treated with sorbitol. Org. Electron. 2008, 9, 727–734. [Google Scholar]
- Im, S.G.; Gleason, K.K.; Olivetti, E.A. Doping level and work function control in oxidative chemical vapor deposited poly (3,4-ethylenedioxythiophene). Appl. Phys. Lett. 2007, 90, 152112. [Google Scholar]
- Arora, N.; Dar, M.I.; Hinderhofer, A.; Pellet, N.; Schreiber, F.; Zakeeruddin, S.M.; Grätzel, M. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%. Science 2017, 358, 768–771. [Google Scholar]
- Ni, Y.; Jin, Z.; Yu, K.; Fu, Y.; Liu, T.; Wang, T. Electrochemical deposition characteristics of p-CuSCN on n-ZnO rod arrays films. Electrochim. Acta 2008, 53, 6048–6054. [Google Scholar] [CrossRef]
- O’Regan, B.; Schwartz, D.T. Efficient photo-hole injection from adsorbed cyanine dyes into electrodeposited copper (I) thiocyanate thin films. Chem. Mater. 1995, 7, 1349–1354. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; Kang, B.; Wang, P.; Qiu, Y. Review of Recent Progress in Solid-State Dye-Sensitized Solar Cells. Sol. Energy Mater. Sol. Cell 2006, 90, 549–573. [Google Scholar] [CrossRef]
- Kumara, G.R.R.A.; Konno, A.; Senadeera, G.K.R.; Jayaweera, P.V.V.; De Silva, D.B.R.A.; Tennakone, K. Dye-Sensitized Solar Cell with the Hole Collector p-CuSCN Deposited from a Solution in n-Propyl Sulphide. Sol. Energy Mater. Sol. Cells 2001, 69, 195–199. [Google Scholar] [CrossRef]
- Miller, K.M.; McCullough, S.M.; Lepekhina, E.A.; Thibau, I.J.; Pike, R.D.; Li, X.; Killarney, J.P.; Patterson, H.H. Copper(I) thiocyanate-amine networks: Synthesis, structure, and luminescence behavior. Inorg. Chem. 2011, 50, 7239–7249. [Google Scholar] [CrossRef]
- Qin, P.; Tanaka, S.; Ito, S.; Tetreault, N.; Manabe, K.; Nishino, H.; Nazeeruddin, M.K.; Grätzel, M. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat. Commun. 2014, 5, 3834. [Google Scholar] [CrossRef] [Green Version]
- Sankapal, B.R.; Goncalves, E.; Ennaoui, A.; Lux-Steiner, M.C. Wide band gap p-type windows by CBD and SILAR methods. Thin Solid Films 2004, 451, 128–132. [Google Scholar] [CrossRef]
- O’Regan, B.; Schwartz, D.T.; Zakeeruddin, S.M.; Grätzel, M. Electrodeposited Nanocomposite n–p Heterojunctions for Solid-State Dye-Sensitized Photovoltaics. Adv. Mater. 2000, 12, 1263–1267. [Google Scholar] [CrossRef]
- Ni, Y.; Jin, Z.G.; Fu, Y. lectrodeposition of p-Type CuSCN Thin Films by a New Aqueous Electrolyte with Triethanolamine Chelation. J. Am. Ceram. Soc. 2007, 90, 2966–2973. [Google Scholar] [CrossRef]
- Chavhan, S.D.; Ou, T.H.; Jiang, M.-R.; Wang, C.-W.; Jou, J.-H. Enabling High-Efficiency Organic Light-Emitting Diode with Trifunctional Solution-Processable Copper(I) Thiocyanate. J. Phys. Chem. C 2018, 122, 18836–18840. [Google Scholar] [CrossRef]
- Perumal, A.; Faber, H.; Yaacobi-Gross, N.; Pattanasattayavong, P.; Burgess, C.; Jha, S.; McLachlan, M.A.; Stavrinou, P.N.; Anthopoulos, T.D.; Bradley, D.D.C. High-efficiency, solution-processed, multilayer phosphorescent organic light-emitting diodes with a copper thiocyanate hole-injection/hole-transport layer. Adv. Mater. 2015, 27, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyatt-Moon, G.; Georgiadou, D.G.; Semple, J.; Anthopoulos, T.D. Deep ultraviolet copper (I) thiocyanate (CuSCN) photodetectors based on coplanar nanogap electrodes fabricated via adhesion lithography. ACS Appl. Mater. Interfaces 2017, 9, 41965–41972. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Dou, J.; Wang, M.; Li, J.; Huang, J.; Shao, J. Flexible all-inorganic photoconductor detectors based on perovskite/hole-conducting layer heterostructures. J. Mater. Chem. C 2018, 6, 6739–6746. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Li, M.G.; Wang, H.L.; Zhang, W.; Chen, R.F.; Huang, W. Applications of novel hole-transporting material copper(I) thiocyanate (CuSCN) in optoelectronic devices. Prog. Chem. 2018, 30, 785–796. [Google Scholar]
- Jaffe, J.E.; Kaspar, T.C.; Droubay, T.C.; Varga, T.; Bowden, M.E.; Exarhos, G.J. Electronic and Defect Structures of CuSCN. J. Phys. Chem. C. 2010, 114, 9111–9117. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B. Condens. Matter Mater. Phys. 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P. Projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B. 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Watson, G.W.; Kelsey, E.T.; de Leeuw, N.H.; Harris, D.J.; Parker, S.C. Atomistic simulation of dislocations, surfaces and interfaces in MgO. J. Chem. Soc. Faraday Trans. 1996, 92, 433–438. [Google Scholar] [CrossRef]
- Walsh, A.; Butler, K.T. Prediction of Electron Energies in Metal Oxides. Acc. Chem. Res. 2014, 47, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Dzade, N.Y.; Gao, L.; Scanlon, D.O.; Öztürk, Z.; Hollingsworth, N.; Weckhuysen, B.M.; Hensen, E.J.M.; de Leeuw, N.H.; Hofmann, J.P. Enhanced Photoresponse of FeS2 Films—The Role of Marcasite–Pyrite Phase Junctions. J. Adv. Mat. 2017, 28, 9602–9607. [Google Scholar] [CrossRef]
- Makov, G.; Payne, M.C. Periodic boundary conditions in ab initio calculations. Phys. Rev. B Condens. Matter Mater. Phys. 1995, 51, 4014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, A.J.; Ganose, A.M.; Regoutz, A.; Egdell, R.G.; Scanlon, D.O. Galore: Broadening and Weighting for Simulation of Photoelectron Spectroscopy. J. Open Source Softw. 2018, 3, 773. [Google Scholar] [CrossRef]
- Gelius, U.; Siegbahn, K. ESCA studies of molecular core and valence levels in the gas phase. Gen. Discuss. Faraday Soc. 1972, 54, 257–268. [Google Scholar] [CrossRef]
- Gelius, U. Recent progress in ESCA studies of gases. J. Electron Spectrosc. Relat. Phenom. 1974, 5, 985–1057. [Google Scholar] [CrossRef]
- Scofield, J.H. Theoretical Photoionization cross Sections from 1 to 1500 KeV. Available online: https://www.osti.gov/biblio/4545040 (accessed on 17 December 2020).
- Oropeza, F.E.; Dzade, N.Y.; Pons-Martí, A.; Yang, Z.; Zhang, K.H.L.; de Leeuw, N.H.; Hensen, E.J.M.; Hofmann, J.P. Electronic Structure and Interface Energetics of CuBi2O4 Photoelectrodes. J. Phys. Chem. C 2020, 124, 22416–22425. [Google Scholar] [CrossRef]
- Smith, D.L.; Saunders, V.I. Preparation and structure refinement of the 2H polytype of β-copper(I) thiocyanate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1982, 38, 907–909. [Google Scholar] [CrossRef]
- Chen, K.J.; Laurent, A.D.; Boucher, F.; Odobel, F.; Jacquemin, D. Determining the most promising anchors for CuSCN: Ab initio insights towards p-type DSSCs. J. Mater. Chem. A 2016, 4, 2217. [Google Scholar] [CrossRef]
- Ji, W.; Yue, G.-Q.; Ke, F.-S.; Wu, S.; Zhao, H.-B.; Chen, L.-Y.; Wang, S.-Y.; Jia, Y. Electronic structures and optical properties of CuSCN with Cu vacancies. J. Korean Phys. Soc. 2012, 60, 1253–1257. [Google Scholar] [CrossRef]
- Tennakone, K.; Jayatissa, A.H.; Fernando, C.A.N.; Wickramanayake, S.; Punchihewa, S.; Weerasena, L.K.; Premasiri, W.D.R. Semiconducting and Photoelectroehemical Properties of n- and p-Type P-CuCNS. Phys. Status Solidi 1987, 103, 491–497. [Google Scholar] [CrossRef]
- Wijeyasinghe, N.; Regoutz, A.; Eisner, F.; Du, T.; Tsetseris, L.; Lin, Y.-H.; Faber, H.; Pattanasattayavong, P.; Li, J.; Yan, F.; et al. Copper(I) Thiocyanate (CuSCN) Hole-Transport Layers Processed from Aqueous Precursor Solutions and Their Application in Thin-Film Transistors and Highly Efficient Organic and Organometal Halide Perovskite Solar Cells. Adv. Funct. Mater. 2017, 27, 1701818. [Google Scholar] [CrossRef]
- Wu, W.; Jin, Z.; Hua, Z.; Fu, Y.; Qiu, J. A study on solution deposited CuSCN thin films: Structural, electrochemical, optical properties. Electrochim. Acta 2005, 50, 2343–2349. [Google Scholar] [CrossRef]
- Baig, S.; Hendsbee, A.D.; Kumar, P.; Ahmed, S.; Li, Y. Yttrium-doped CuSCN thin film transistor: Synthesis and optoelectronic characterization study. Mater. Chem. C 2019, 7, 14543–14554. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Zhou, Y.; Li, W.; Lee, Y.; Kanda, H.; Gao, X.X.; Hu, R.; Brooks, K.G.; Zia, R.; et al. The Synergism of DMSO and Diethyl Ether for Highly Reproducible and Efficient MA0.5FA0.5PbI3 Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 2001300. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y. Understanding Molecular Adsorption on CuSCN Surfaces toward Perovskite Solar Cell Applications. J. Phys. Chem. C 2019, 123, 26785–26793. [Google Scholar] [CrossRef]
- Cheng, J.; Xie, F.; Liu, Y.; Sha, W.E.I.; Li, X.; Yang, Y.; Choy, W.C.H. Efficient hole transport layers with widely tunable work function for deep HOMO level organic solar cells. J. Mater. Chem. A 2015, 3, 23955–23963. [Google Scholar] [CrossRef]
- Singh-Miller, N.E.; Marzari, N. Surface energies, work functions, and surface relaxations of low-index metallic surfaces from first principles. Phys. Rev. B Condens. Matter Mater. Phys. 2009, 80, 235407. [Google Scholar] [CrossRef] [Green Version]
- Smoluchowski, R. Anisotropy of the Electronic Work Function of Metals. Phys. Rev. 1941, 60, 661. [Google Scholar] [CrossRef]
- Dzade, N.Y. First-Principles Insights into the Interface Chemistry between 4-Aminothiophenol and Zinc Phosphide (Zn3P2) Nanoparticles. ACS Omega 2020, 5, 1025–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzade, N.Y.; de Leeuw, N.H. Periodic DFT+U investigation of the bulk and surface properties of marcasite (FeS2). Phys. Chem. Chem. Phys. 2017, 19, 27478–27488. [Google Scholar] [CrossRef]
- Rondiya, S.R.; Karbhal, I.; Jadhav, C.D.; Nasane, M.P.; Davies, T.E.; Shelke, M.V.; Jadkar, S.R.; Chavan, P.G.; Dzade, N.Y. Uncovering the origin of enhanced field emission properties of rGO-MnO2 heterostructures: A synergistic experimental and computational investigation. RSC Adv. 2020, 10, 25988–25998. [Google Scholar] [CrossRef]
- Dzade, N.Y.; Roldan, A.; de Leeuw, N.H. Surface and shape modification of mackinawite (FeS) nanocrystals by cysteine adsorption: A first-principles DFT-D2 study. Phys. Chem. Chem. Phys. 2016, 18, 32007–32020. [Google Scholar] [CrossRef] [Green Version]
- Wijeyasinghe, N.; Anthopoulos, T. Copper(I) thiocyanate (CuSCN) as a hole-transport material for large-area opto/electronics. Semicond. Sci. Technol. 2015, 30, 104002. [Google Scholar] [CrossRef]
- Wijesekara, A.; Varagnolo, S.; Dabera, G.D.M.R.; Marshall, K.P.; Pereira, H.J.; Hatton, R.A. Assessing the suitability of copper thiocyanate as a hole-transport layer in inverted CsSnI3 perovskite photovoltaics. Sci. Rep. 2018, 8, 15722. [Google Scholar] [CrossRef] [Green Version]
- Dzikri, I.; Hariadi, M.; Purnamaningsih, R.W.; Poespawati, N.R. Analysis of the role of hole transport layer materials to the performance of perovskite solar cell. E3S Web Conf. 2018, 67, 01021. [Google Scholar] [CrossRef]
- Kim, M.; Park, S.; Jeong, J.; Shin, D.; Kim, J.; Ryu, S.H.; Kim, K.S.; Lee, H.; Yi, Y. Band-Tail Transport of CuSCN: Origin of Hole Extraction Enhancement in Organic Photovoltaics. J. Phys. Chem. Lett. 2016, 7, 2856–2861. [Google Scholar] [CrossRef]
- Deng, Q.; Li, Y.; Chen, L.; Wang, S.; Wang, G.; Sheng, Y.; Shao, G. The effects of electron and hole transport layer with the electrode work function on perovskite solar cells. Mod. Phys. Lett. B. 2016, 30, 1650341. [Google Scholar] [CrossRef]
- Chen, S.; Manders, J.R.; Tsang, S.-W.; So, F. Metal oxides for interface engineering in polymer solar cells. J. Mater. Chem. 2012, 22, 24202–24212. [Google Scholar] [CrossRef]




| Exp. | GGA + D3 | GGA | GGA + D2 | HSE06 | GGA | |
|---|---|---|---|---|---|---|
| Reference | [53] | This Work | [36] | [54] | [54] | [55] |
| a (Å) | 3.850 | 3.828 | 3.781 | 3.832 | 3.960 | 3.857 |
| c (Å) | 10.938 | 10.970 | 10.987 | 11.023 | 11.055 | 10.979 |
| c/a | 2.841 | 2.865 | 2.906 | 2.876 | 2.791 | 2.847 |
| Direction | m*h (me) | m*e (me) |
|---|---|---|
| Γ−M | 0.1756 | 0.0066 |
| M−K | 0.0189 | 0.0379 |
| K−Γ | 0.0146 | 0.0049 |
| Γ−A | 0.0037 | 0.4921 |
| A−L | 0.0350 | 0.0049 |
| L−H | 0.0068 | 0.0793 |
| H−K | 0.0079 | 0.00131 |
| Parameter | DMSO (Gas-Phase) | CuSCN (100) | CuSCN (110) |
|---|---|---|---|
| Eads (eV) | −1.12 | −0.91 | |
| ∆q (e−) | 0.08 | 0.04 | |
| dCu–O (Å) | 2.126 | 2.191 | |
| dS–O (Å) | 1.520 | 1.525 | 1.519 |
| dS–C (Å) | 1.789 | 1.807 | 1.808 |
| α(OSC) (°) | 121.7 | 104.2 (106.0) | 104.7 (105.4) |
| α(CSC) (°) | 116.6 | 98.7 | 98.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odeke, B.A.; Chung, G.D.; Fajemisin, J.A.; Suraj, K.S.; Tonui, D.K.; Tobi, A.R.; Bewaale, T.C.; Ajibola, J.A.; Dzade, N.Y. Electronic Structure and Surface Properties of Copper Thiocyanate: A Promising Hole Transport Material for Organic Photovoltaic Cells. Materials 2020, 13, 5765. https://doi.org/10.3390/ma13245765
Odeke BA, Chung GD, Fajemisin JA, Suraj KS, Tonui DK, Tobi AR, Bewaale TC, Ajibola JA, Dzade NY. Electronic Structure and Surface Properties of Copper Thiocyanate: A Promising Hole Transport Material for Organic Photovoltaic Cells. Materials. 2020; 13(24):5765. https://doi.org/10.3390/ma13245765
Chicago/Turabian StyleOdeke, Bonaventure A., Gyang D. Chung, Jesutofunmi A. Fajemisin, Kabir S. Suraj, Denis K. Tonui, Ayinla R. Tobi, Thomas C. Bewaale, Jamiu A. Ajibola, and Nelson Y. Dzade. 2020. "Electronic Structure and Surface Properties of Copper Thiocyanate: A Promising Hole Transport Material for Organic Photovoltaic Cells" Materials 13, no. 24: 5765. https://doi.org/10.3390/ma13245765








