Electromagnetic Shielding Performance of Different Metallic Coatings Deposited by Arc Thermal Spray Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Process of Coatings
2.2. Characterization of Coatings
2.3. Conductivity Measurement of Coatings
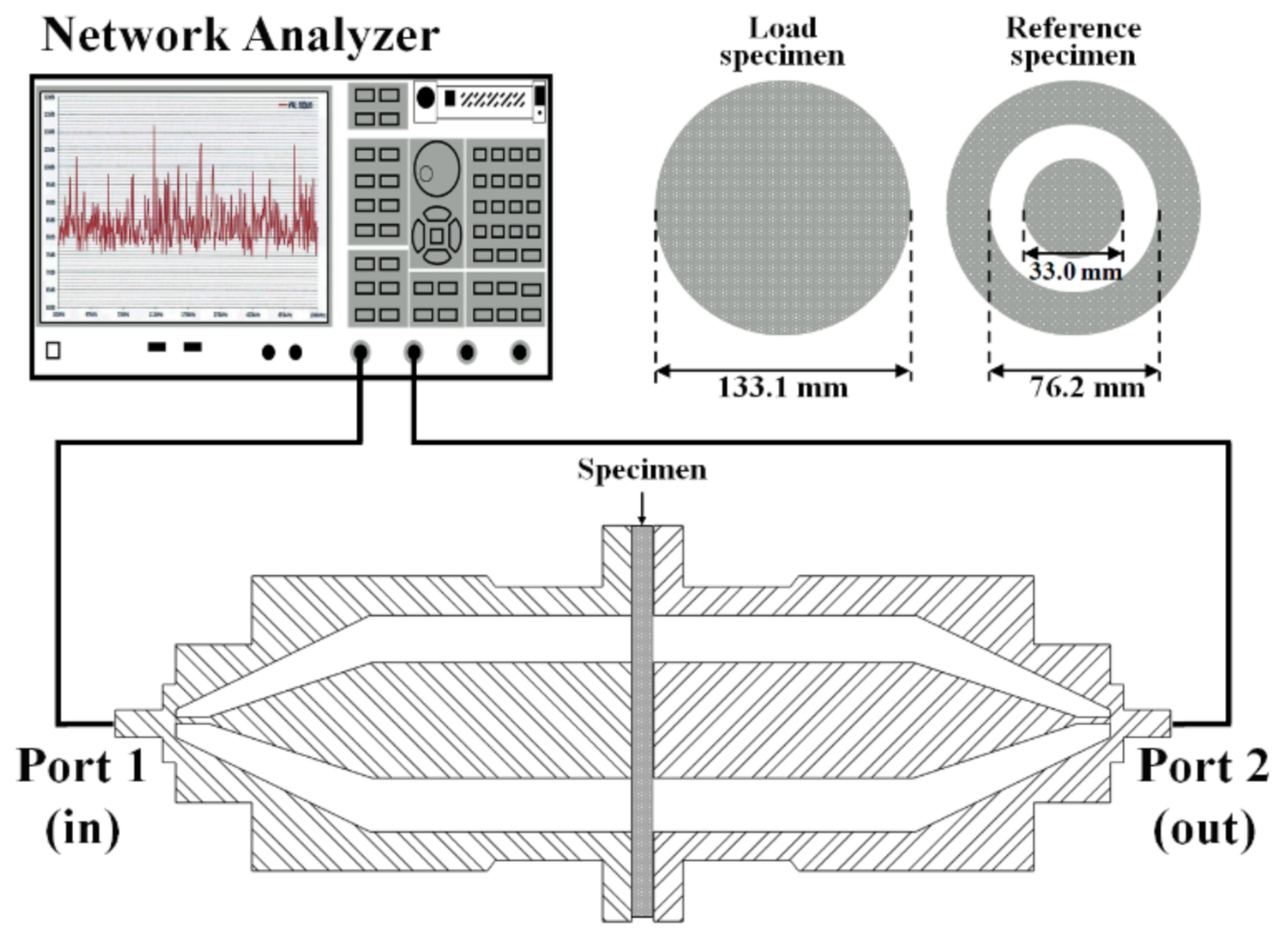
2.4. EMI Shielding Evaluation of Coatings
3. Results and Discussion
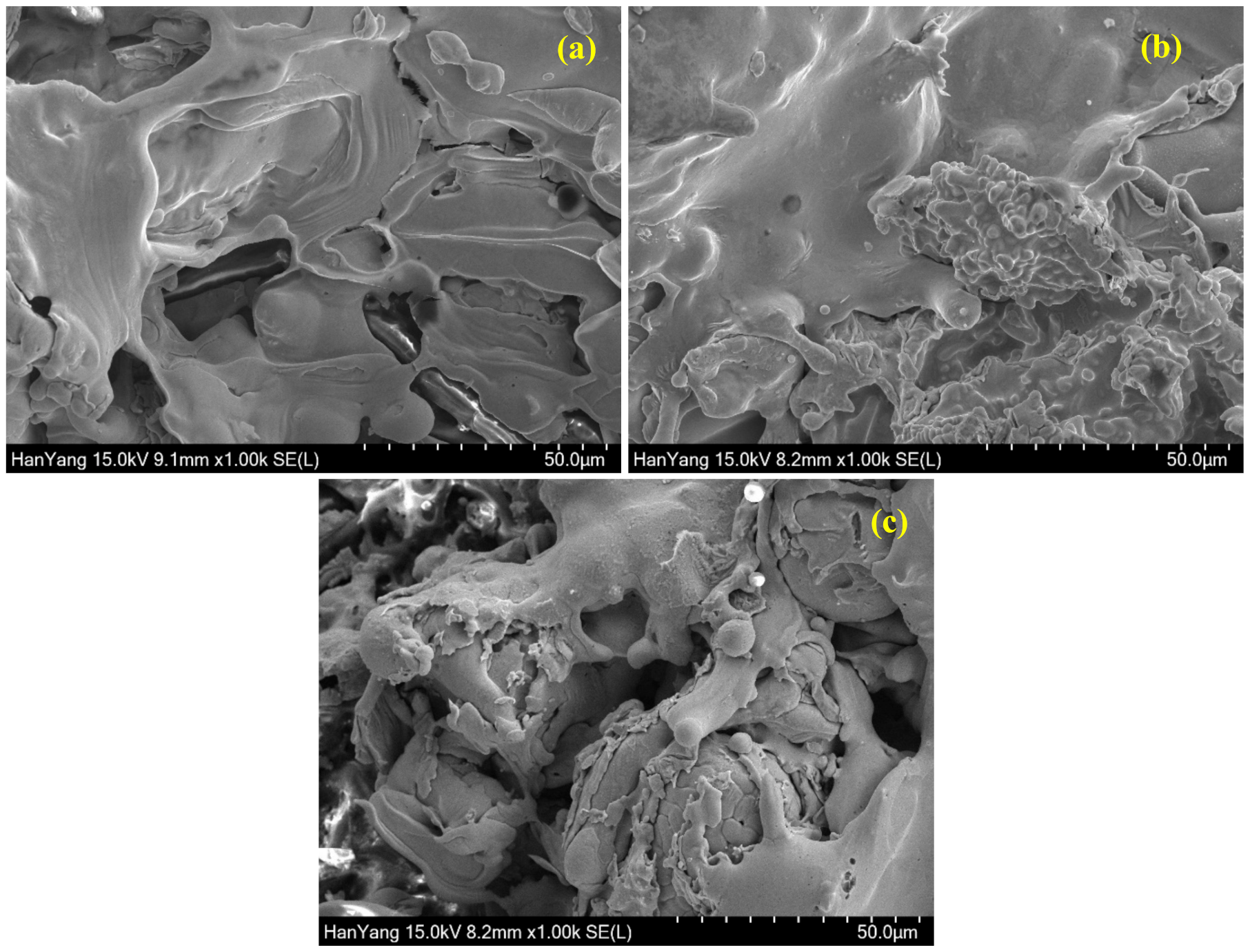
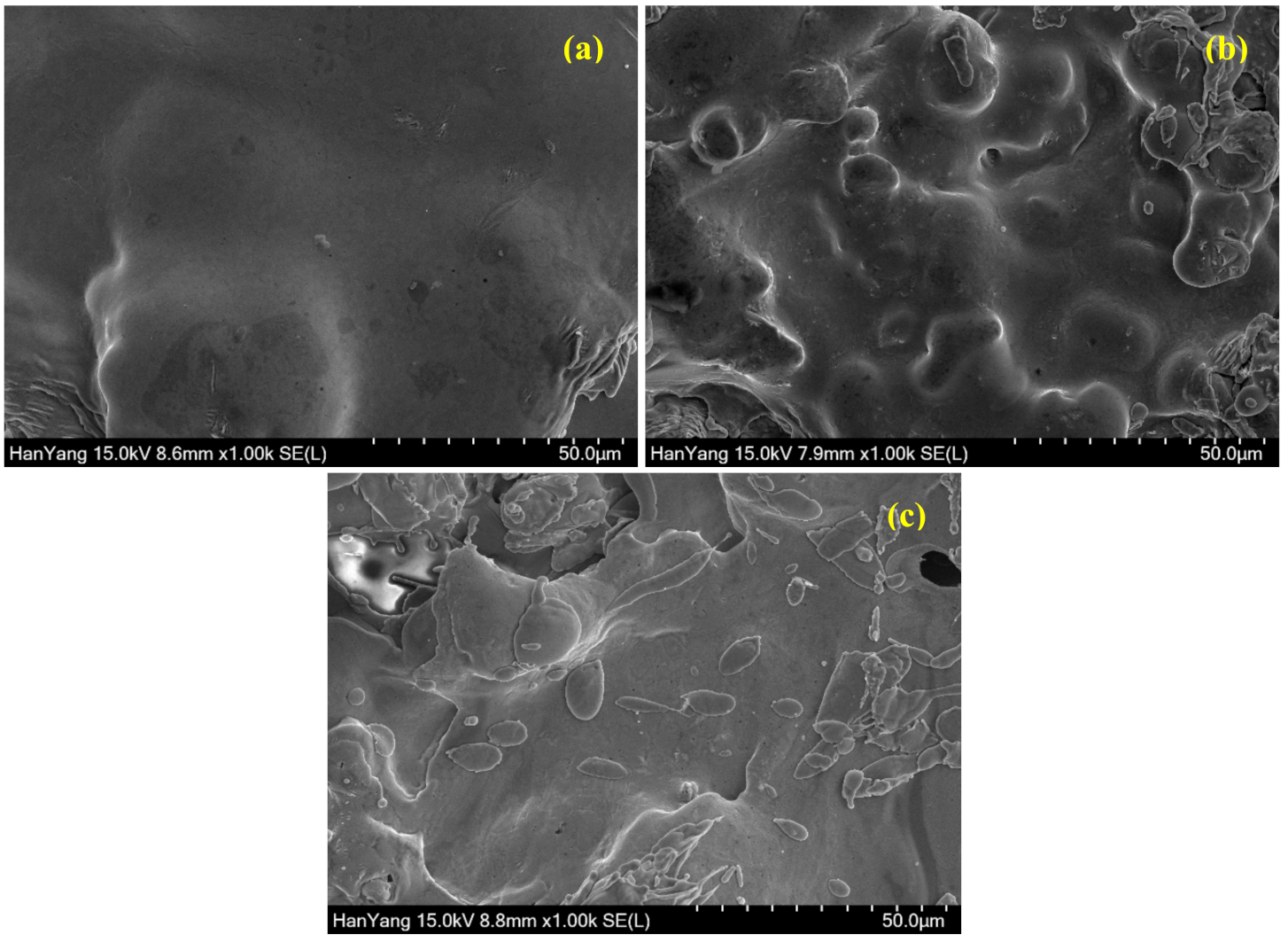
3.1. SEM of Coatings
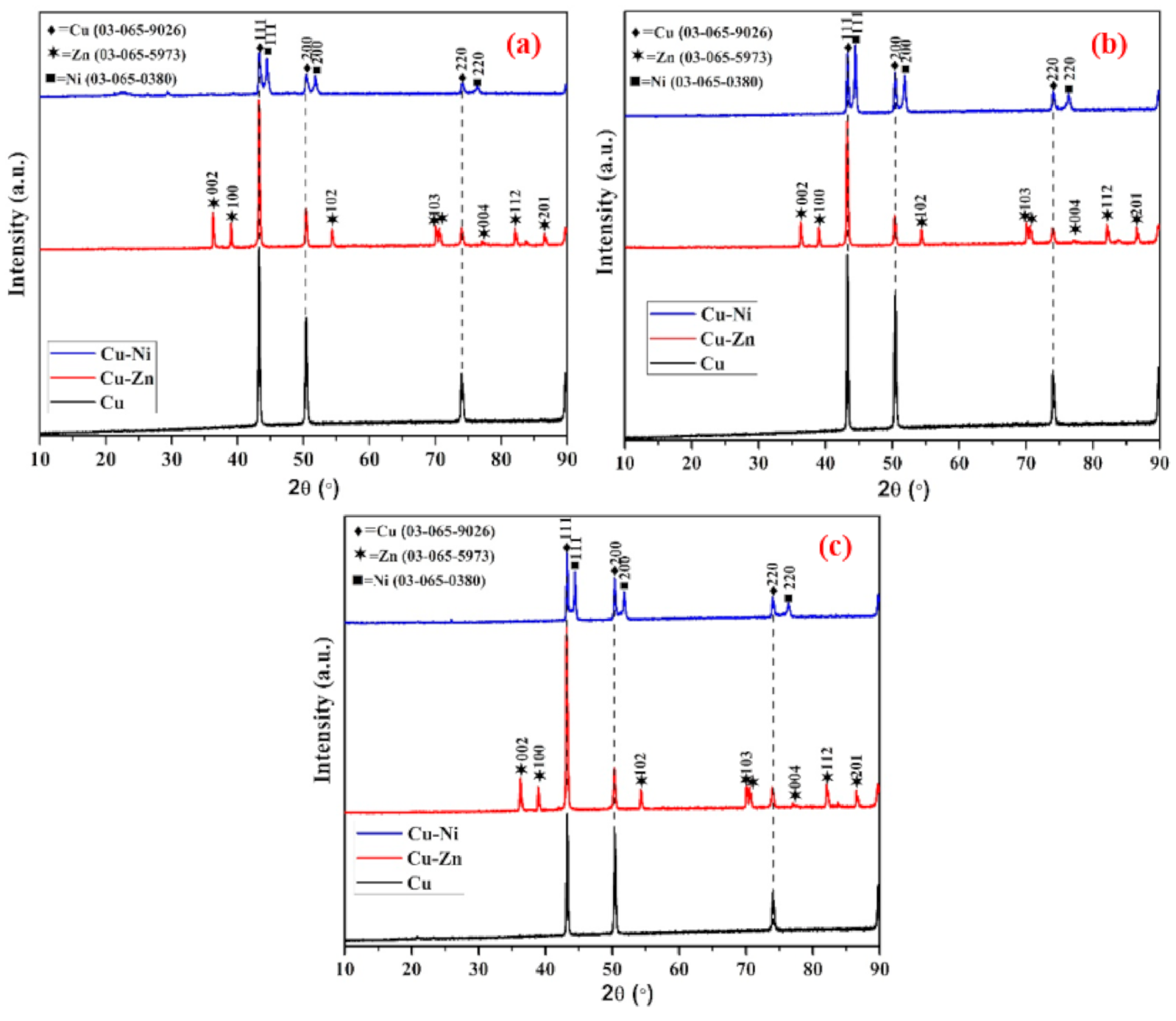
3.2. XRD of Coating
3.3. Electrical Conductivity of Coating
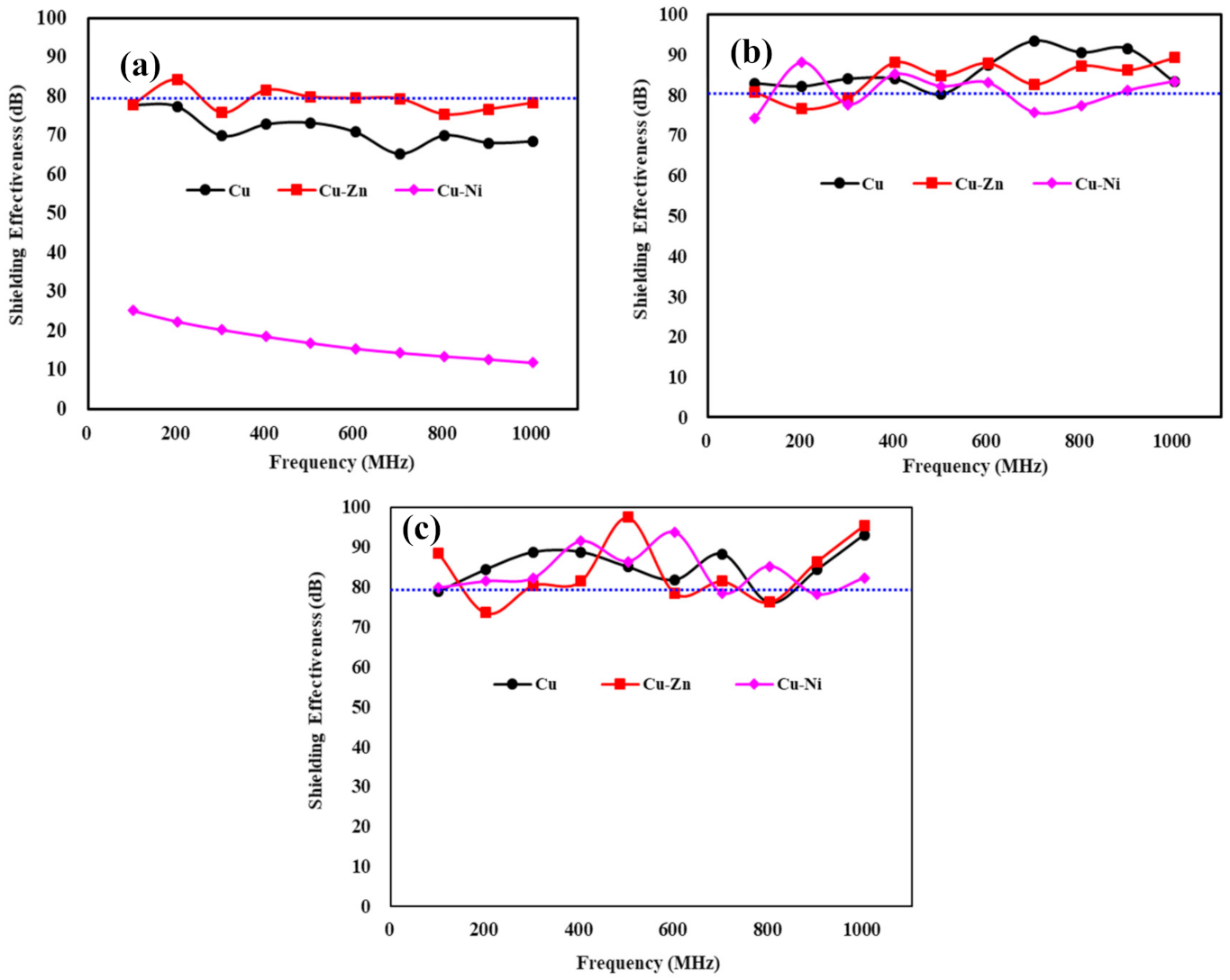
3.4. Shielding Effectiveness Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Zhou, H.; Zhuang, J.; Liu, Q. Influence of spatial configurations on electromagnetic interference shielding of ordered mesoporous carbon/ordered mesoporous silica/silica composites. Sci. Rep. 2013, 3, 3252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gut, J. The Swiss EMP concept of general defense. IEEE Antennas Propag. Soc. Newsl. 1987, 29, 4–10. [Google Scholar] [CrossRef]
- Fugetsu, B.; Sano, E.; Sunada, M.; Sambongi, Y.; Shibuya, T.; Wang, X.; Hiraki, T. Electrical conductivity and electromagnetic interference shielding efficiency of carbon nanotube/cellulose composite paper. Carbon 2008, 46, 1256–1258. [Google Scholar] [CrossRef]
- Brillaud, E.; Piotrowski, A.; de Seze, R. Effect of an acute 900 MHz GSM exposure on glia in the rat brain: A time-dependent study. Toxicology 2007, 238, 23–33. [Google Scholar] [CrossRef]
- Xu, X.B.; Li, Z.-M.; Shi, L.; Bian, X.-C.; Xiang, Z.-D. Ultralight conductive carbon-nanotube-polymer composite. Small 2007, 3, 408–411. [Google Scholar] [CrossRef]
- Nam, I.W.; Kim, H.K.; Lee, H.K. Influence of silica fume additions on electromagnetic interference shielding effectiveness of multi-walled carbon nanotube/cement composites. Constr. Build. Mater. 2012, 30, 480–487. [Google Scholar] [CrossRef]
- Chung, D.-D.-L. Electromagnetic interference shielding effectiveness of carbon materials. Carbon 2001, 39, 279–285. [Google Scholar] [CrossRef]
- Kim, H.-K.; Nam, I.-W.; Lee, H.-K. Enhanced effect of carbon nanotube on mechanical and electrical properties of cement composites by incorporation of silica fume. Compos. Struct. 2014, 107, 60–69. [Google Scholar] [CrossRef]
- Khushnood, R.-A.; Ahmad, S.; Savi, P.; Tulliani, J.-M.; Giorcelli, M.; Ferro, G.-A. Improvement in electromagnetic interference shielding effectiveness of cement composites using carbonaceous nano/micro inerts. Constr. Build. Mater. 2015, 85, 208–216. [Google Scholar] [CrossRef]
- Osawa, Z.; Kuwabara, S. Thermal stability of the shielding effectiveness of composites to electromagnetic interference. Effects of matrix polymers and surface treatment of fillers. Polym. Degrad. Stab. 1992, 35, 33–43. [Google Scholar] [CrossRef]
- Park, J.; Hu, X.; Torfeh, M.; Okoroanyanwu, U.; Arbabi, A.; Watkins, J.J. Exceptional electromagnetic shielding efficiency of silver coated carbon fiber fabrics via a roll-to-roll spray coating process. J. Mater. Chem. C 2020, 8, 11070–11078. [Google Scholar] [CrossRef]
- MIL-STD-188-125-1, High-Altitude Electromagnetic Pulse(HEMP) Protection for Ground-Based C4I Facilities Performing Critical, Time-Urgent Missions Part 1 Fixed Facilities; Department of Defense Interface Standard, Department of Defense: Alexandria, VA, USA, 1998.
- IEEE Std 299. IEEE Standard Method for Measuring the Shielding Effectiveness of ELECTROMAGNETIC Shielding Enclosures; IEEE: Piscataway, NJ, USA, 2006; p. 50. [Google Scholar]
- Savage, E.; Gilbert, J.; Radasky, W. The Early-Time (E1) High-Altitude Electromagnetic Pulse (HEMP) and Its Impact on the US Power Grid. Report Meta-R-320 for Oak Ridge National Laboratory. 2010. Available online: https://www.eiscouncil.org/App_Data/Upload/9b03e596-19c8-49bd-8d4e-a8863b6ff9a0.pdf (accessed on 9 November 2020).
- Gurevich, V. Protection of Substation Critical Equipment against Intentional Electromagnetic Threats; Wiley Blackwell: Hoboken, NJ, USA, 2017. [Google Scholar]
- Foster, J.-S.; Gjelde, E.; Graham, W.-R.; Hermann, R.-J.; Kluepfel, H.-M.; Lawson, R.-L.; Soper, G.-K.; Wood, L.-L.; Woodard, J.-B. Report of the Commission to assess the Threat to the United States from Electromagnetic Pulse (emp) Attack: Critical National Infrastructures. 2008. Available online: http://www.empcommission.org/docs/A2473-EMP_Commission-7MB.pdf (accessed on 9 November 2020).
- Hung, F.-S.; Hung, F.-Y.; Chiang, C.-M. Crystallization and annealing effects of sputtered tin alloy films on electromagnetic interference shielding. Appl. Surf. Sci. 2011, 257, 3733–3738. [Google Scholar] [CrossRef]
- Guan, H.; Liu, S.; Duan, Y.; Cheng, J. Cement based electromagnetic shielding and absorbing building materials. Cem. Concr. Compos. 2006, 28, 468–474. [Google Scholar] [CrossRef]
- Song, K.; Pan, F.S.; Chen, X.H.; Zhang, Z.H.; Tang, A.T.; She, J.; Yu, Z.W.; Pan, H.C.; Xu, X.Y. Effect of texture on the electromagnetic shielding property of magnesium alloy. Mater. Lett. 2015, 157, 73–76. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, X.-H.; Song, K.; Liu, C.-Q.; Dai, Y.; Zhao, D.; Pan, F.-S. Effect of Alloying Element on Electromagnetic Interference Shielding Effectiveness of Binary Magnesium Alloys. Acta Metall. Sin. (Engl. Lett.) 2019, 32, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Pandey, R.; Tekumalla, S.; Gupta, M. Enhanced (X-band) microwave shielding properties of pure magnesium by addition of diamagnetic titanium micro-particulates. J. Alloys Compd. 2019, 770, 473–482. [Google Scholar] [CrossRef]
- Chen, X.-H.; Liu, L.-Z.; Liu, J.; Pan, F.-S. Enhanced electromagnetic interference shielding of Mg-Zn-Zr alloy by Ce addition. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 492–498. [Google Scholar] [CrossRef]
- Gupta, A.K.; Bafna, M.; Srivastava, S.; Khanna, R.K.; Vijay, Y.K. Study of electromagnetic shielding effectiveness of metal oxide polymer composite in their bulk and layered forms. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Yuan, S.M.; Ma, C.C.; Chuang, C.Y.; Yu, K.C.; Yen, W.S.; Yang, C.C.; Win, M.H. Effect of processing method on the shielding effectiveness of electromagnetic interference of MWCNT-PMMA. Compos. Sci. Technol. 2008, 68, 963–968. [Google Scholar] [CrossRef]
- Panwar, V.; Kang, B.; Park, J.-O.; Park, S.; Mehra, R.-M. Study of dielectric properties of styrene-acrylonitrile graphite sheets composites in low and high frequency region. Eur. Polym. J. 2009, 45, 1777–1784. [Google Scholar] [CrossRef]
- Kim, J.-T.; Park, C.-W.; Kim, B.-J. A study on synergetic EMI shielding behaviors of Ni-Co alloy-coated carbon fibers-reinforced composites. Synth. Met. 2017, 223, 212–217. [Google Scholar] [CrossRef]
- Nasouri, K.; Shoushtari, A.-M. Designing, modeling and manufacturing of lightweight carbon nanotubes/polymer composite nanofibers for electromagnetic interference shielding application. Compos. Sci. Technol. 2017, 145, 46–54. [Google Scholar] [CrossRef]
- Thomassin, J.-M.; Jérôme, C.; Pardoen, T.; Bailly, C.; Huynen, I.; Detrembleur, C. Polymer/carbon based composites as electromagnetic interference (EMI) shielding materials. Mater. Sci. Eng. R Rep. 2013, 74, 211–232. [Google Scholar] [CrossRef]
- Varela-Rizo, H.; de Oca, G.-H.; Rodriguez-Pastor, I.; Monti, M.; Terenzi, A.; Martin-Gullon, I. Analysis of the electrical and rheological behavior of different processed CNF/PMMA nanocomposites. Compos. Sci. Technol. 2012, 72, 218–224. [Google Scholar] [CrossRef]
- Al-Saleh, M.-H.; Gelves, G.-A.; Sundararaj, U. Copper nanowire/polystyrene nanocomposites: Lower percolation threshold and higher EMI shielding. Compos. Part A Appl. Sci. Manuf. 2011, 42, 92–97. [Google Scholar] [CrossRef]
- Al-Saleh, M.-H.; Sundararaj, U. Electromagnetic interference shielding mechanisms of CNT/polymer composites. Carbon 2009, 47, 1738–1746. [Google Scholar] [CrossRef]
- Wang, R.-X.; Tao, X.-M.; Wang, Y.; Wang, G.-F.; Shang, S.-M. Microstructures and electrical conductance of silver nanocrystalline thin films on flexible polymer substrates. Surf. Coat. Technol. 2010, 204, 1206–1210. [Google Scholar] [CrossRef]
- Guo, R.-H.; Jiang, S.-Q.; Yuen, C.-W.-M.; Ng, M.-C.-F. Effect of copper content on the properties of Ni–Cu–P plated polyester fabric. J. Appl. Electrochem. 2009, 39, 907–912. [Google Scholar] [CrossRef]
- Yip, J.; Jiang, S.; Wong, C. Characterization of metallic textiles deposited by magnetron sputtering and traditional metallic treatments. Surf. Coat. Technol. 2009, 204, 380–385. [Google Scholar] [CrossRef]
- Wei, Q.; Xiao, X.; Hou, D.; Ye, H.; Huang, F. Characterization of nonwoven material functionalized by sputter coating of copper. Surf. Coat. Technol. 2008, 202, 2535–2539. [Google Scholar] [CrossRef]
- Bula, K.; Koprowska, J.; Janukiewicz, J. Application of cathode sputtering for obtaining ultra-thin metallic coatings on textile products. FIBRES Text. In East. Eur. 2006, 14, 75–79. [Google Scholar]
- Jiang, S.-Q.; Newton, E.; Yuen, C.-W.-M.; Kan, C.-W. Chemical silver plating on cotton and polyester fabrics and its application on fabric design. Text. Res. J. 2006, 76, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Yuen, C.-W.-M.; Jiang, S.-Q.; Kan, C.-W.; Tung, W.-S. Influence of surface treatment on the electroless nickel plating of textile fabric. Appl. Surf. Sci. 2007, 253, 5250–5257. [Google Scholar] [CrossRef]
- Savage, H.-S.; McCormack, R.-G. Arc-Sprayed Coatings for Electromagnetic Pulse Protection: Assessment of Physical and Electrical Properties (No. CERL-TR-M-89/15); Construction Engineering Research Lab (Army): Champaign, IL, USA, 1989. [Google Scholar]
- Choe, H.-B.; Lee, H.-S.; Shin, J.-H. Experimental study on the electrochemical anti-corrosion properties of steel structures applying the arc thermal metal spraying method. Materials 2014, 7, 7722–7736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-S.; Choe, H.-B.; Baek, I.-Y.; Singh, J.-K.; Ismail, M.-A. Study on the Shielding Effectiveness of an Arc Thermal Metal Spraying Method against an Electromagnetic Pulse. Materials 2017, 10, 1155. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-S.; Park, J.-H.; Singh, J.-K.; Choi, H.-J.; Mandal, S.; Jang, J.-M.; Yang, H.-M. Electromagnetic Shielding Performance of Carbon Black Mixed Concrete with Zn–Al Metal Thermal Spray Coating. Materials 2020, 13, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.; Pan, F.; Chen, X.; Tang, A.; Pan, H.; Luo, S. Effect of Zn content on electromagnetic interference shielding effectiveness of Mg–Zn alloys. Mater. Res. Innov. 2014, 18, S4-193–S4-197. [Google Scholar] [CrossRef]
- Song, M.-K.; Hong, S.-M.; Park, J.-M. Coatings material for shielding of electromagnetic wave. Polym. Sci. Technol. 2001, 12, 689–697. [Google Scholar]
- Grimberg, R. Electromagnetic metamaterials. Mater. Sci. Eng. B 2013, 178, 1285–1295. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Abel, E.-W. Effect of Cu content in electroless Ni–Cu–P–PTFE composite coatings on their anti-corrosion properties. Mater. Chem. Phys. 2004, 87, 332–335. [Google Scholar] [CrossRef]
- Yao, W.-L.; Xiong, G.; Yang, Y.; Huang, H.-Q.; Zhou, Y.-F. Effect of silica fume and colloidal graphite additions on the EMI shielding effectiveness of nickel fiber cement based composites. Constr. Build. Mater. 2017, 150, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.-X.; Guo, R.-H. Electromagnetic shielding and corrosion resistance of electroless Ni-P/Cu-Ni multilayer plated polyester fabric. Surf. Coat. Technol. 2011, 205, 4274–4279. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, J.H.; Singh, J.K.; Ismail, M.A. Protection of reinforced concrete structures of waste water treatment reservoirs with stainless steel coating using arc thermal spraying technique in acidified water. Materials 2016, 9, 753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-S.; Singh, J.-K. Influence of calcium nitrate on morphology and corrosion characteristics of ammonium phosphate treated Aluminum coating deposited by arc thermal spraying process. Corros. Sci. 2019, 146, 254–268. [Google Scholar] [CrossRef]
- Lee, H.-S.; Park, J.-H.; Singh, J.-K.; Ismail, M.-A. Deposition of coating to protect waste water reservoir in acidic solution by arc thermal spray process. Adv. Mater. Sci. Eng. 2018, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-S.; Kwon, S.-J.; Singh, J.-K.; Ismail, M.-A. Influence of Zn and Mg alloying on the corrosion resistance properties of Al coating applied by arc thermal spray process in simulated weather solution. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 591–603. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Singh, J.-K.; Lee, H.-S. Ozone resistance, water permeability, and concrete adhesion of metallic films sprayed on a concrete structure for advanced water purification. Coatings 2017, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-S.; Singh, J.-K.; Ismail, M.-A. An effective and novel pore sealing agent to enhance the corrosion resistance performance of Al coating in artificial ocean water. Sci. Rep. 2017, 7, 41935. [Google Scholar] [CrossRef]
- Lee, H.-S.; Singh, J.-K.; Park, J.-H. Pore blocking characteristics of corrosion products formed on Aluminum coating produced by arc thermal metal spray process in 3.5 wt.% NaCl solution. Constr. Build. Mater. 2016, 113, 905–916. [Google Scholar] [CrossRef]
- Lee, H.-S.; Singh, J.-K.; Ismail, M.-A.; Bhattacharya, C. Corrosion resistance properties of Aluminum coating applied by arc thermal metal spray in SAE J2334 solution with exposure periods. Metals 2016, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- ASTM D4935, Standard Test Method for Measuring the Electromagnetic Shielding Effectiveness of Planar Materials; American Society of Testing Materials: West Conshohocken, PA, USA, 2018.
- Munalli, D.; Dimitrakis, G.; Chronopoulos, D.; Greedy, S.; Long, A. Electromagnetic shielding effectiveness of carbon fibre reinforced Composites. Compos. Part B Eng. 2019, 173, 106906. [Google Scholar] [CrossRef]
- Tailor, S.; Modi, A.; Modi, S.-C. Thermally Sprayed Thin Copper Coatings by W-HVOF. J. Therm. Spray Technol. 2019, 28, 273–282. [Google Scholar] [CrossRef]
- Tailor, S.; Vashishtha, N.; Modi, A.; Modi, S.-C. An Investigation on Splat and Flattening Behavior of Thermally Sprayed Copper on A Rough Surface: A New Approach. J. Therm. Spray Eng. 2020, 2, 37–42. [Google Scholar]
- Lee, H.-S.; Singh, J.-K.; Ismail, M.-A.; Bhattacharya, C.; Seikh, A.-H.; Alharthi, N.; Hussain, R.-R. Corrosion mechanism and kinetics of Al-Zn coating deposited by arc thermal spraying process in saline solution at prolong exposure periods. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bonabi, S.-F.; Ashrafizadeh, F.; Sanati, A.; Nahvi, S.-M. Structure and Corrosion Behavior of Arc-Sprayed Zn-Al Coatings on Ductile Iron Substrate. J. Therm. Spray Technol. 2018, 27, 524–537. [Google Scholar] [CrossRef]
- Jang, J.-M.; Lee, H.-S. A Study on the Electrical Conductivity and Electromagnetic Pulse Shielding Characteristics of Metal Sprayed Coating. In Proceedings of the Korean Institute of Building Construction Conference 2020; The Korean Institute of Building Construction: Seoul, Korea, 2020; pp. 8–9. [Google Scholar]
- Lu, N.-N.; Wang, X.-J.; Meng, L.-L.; Ding, C.; Liu, W.-Q.; Shi, H.-L.; Hu, X.-S.; Wu, K. Electromagnetic interference shielding effectiveness of magnesium alloy-fly ash composites. J. Alloys Compd. 2015, 650, 871–877. [Google Scholar] [CrossRef]
- Schulz, R.-B.; Plantz, V.-C.; Brush, D.-R. Shielding theory and practice. IEEE Trans. Electromagn. Compat. 1988, 30, 187–201. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, G.; Huang, Y.; Ma, Y.; Du, F.; Li, F.; Guo, T.; Chen, Y. Reflection and absorption contributions to the electromagnetic interference shielding of single-walled carbon nanotube/polyurethane composites. Carbon 2007, 45, 821–827. [Google Scholar] [CrossRef]
- Saini, P.; Choudhary, V.; Singh, B.; Mathur, R.; Dhawan, S. Polyaniline-MWCNT nanocomposites for microwave absorption and EMI shielding. Mater. Chem. Phys. 2009, 113, 919–926. [Google Scholar] [CrossRef]
- Tai, M.-F.; Kok, S.-L.; Mukai, K. EMI Shielding Performance by Metal Plating on Mold Compound. In Proceedings of the 37th International Electronics Manufacturing Technology (IEMT) Conference, George Town, Malaysia, 22–26 September 2016; pp. 1–4. [Google Scholar]
- Simon, R.-M. EMI Shielding through conductive plastics. Polym. Plast. Technol. Eng. 1981, 17, 1–10. [Google Scholar] [CrossRef]
- Lu, G.; Li, X.; Jiang, H. Electrical and shielding properties of ABS resin filled with nickel-coated carbon fibers. Compos. Sci. Technol. 1996, 56, 193–200. [Google Scholar] [CrossRef]
- Ninghi, Y.H.; Du, F.; Xiaolin, X.H.; Gao, H.; Ma, Y.; Li, F.; Chen, Y.; Eklind, P.C. Electromagnetic interference (EMI) shielding of single walled carbon nanotube epoxy composites. Nanotubes 2006, 6, 1141–1145. [Google Scholar]


| Coating | For Deposition of Coating | Thickness (µm) of Coating | |
|---|---|---|---|
| Wire-1 | Wire-2 | ||
| Cu | Cu | Cu | 100 |
| 200 | |||
| 500 | |||
| Cu-Zn | Cu | Zn | 100 |
| 200 | |||
| 500 | |||
| Cu-Ni | Cu | Ni | 100 |
| 200 | |||
| 500 | |||
| Coatings | Thickness (µm) | Porosity (%) | Elements (wt.%) | ||||
|---|---|---|---|---|---|---|---|
| Outer/Top Surface | Cross Section | Cu | Zn | Ni | O | ||
| Cu | 100 | 38 | 34 | 99.78 | - | - | 0.22 |
| 200 | 12 | 12 | 99.57 | - | - | 0.43 | |
| 500 | 7 | 7 | 99.60 | - | - | 0.40 | |
| Cu-Zn | 100 | 21 | 21 | 32.52 | 66.34 | - | 1.14 |
| 200 | 13 | 14 | 30.43 | 68.59 | - | 0.98 | |
| 500 | 8 | 8 | 31.23 | 67.93 | - | 0.85 | |
| Cu-Ni | 100 | 48 | 49 | 49.62 | - | 47.40 | 2.98 |
| 200 | 27 | 27 | 33.76 | - | 64.97 | 1.27 | |
| 500 | 23 | 23 | 18.91 | - | 80.54 | 0.55 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.-M.; Lee, H.-S.; Singh, J.K. Electromagnetic Shielding Performance of Different Metallic Coatings Deposited by Arc Thermal Spray Process. Materials 2020, 13, 5776. https://doi.org/10.3390/ma13245776
Jang J-M, Lee H-S, Singh JK. Electromagnetic Shielding Performance of Different Metallic Coatings Deposited by Arc Thermal Spray Process. Materials. 2020; 13(24):5776. https://doi.org/10.3390/ma13245776
Chicago/Turabian StyleJang, Jong-Min, Han-Seung Lee, and Jitendra Kumar Singh. 2020. "Electromagnetic Shielding Performance of Different Metallic Coatings Deposited by Arc Thermal Spray Process" Materials 13, no. 24: 5776. https://doi.org/10.3390/ma13245776





