The Functional Properties of Mg–Zn–X Biodegradable Magnesium Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alloy Compositions and Processing
2.2. Microstructure Characterisation
2.3. Mechanical Testing
2.4. Corrosion Testing
2.5. In Vitro Toxicity Testing
3. Results
3.1. Microstructure
3.2. Mechanical Properties
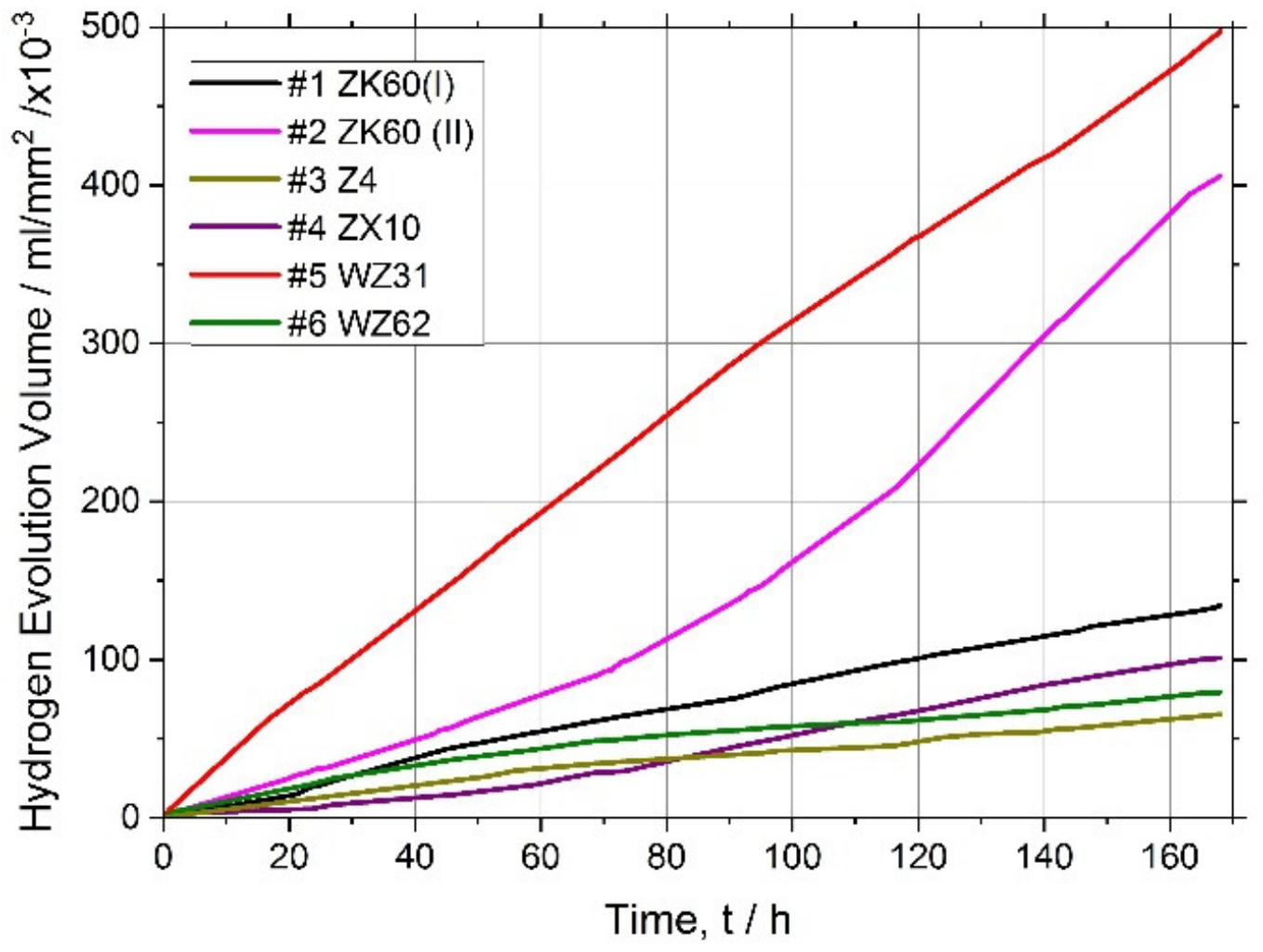
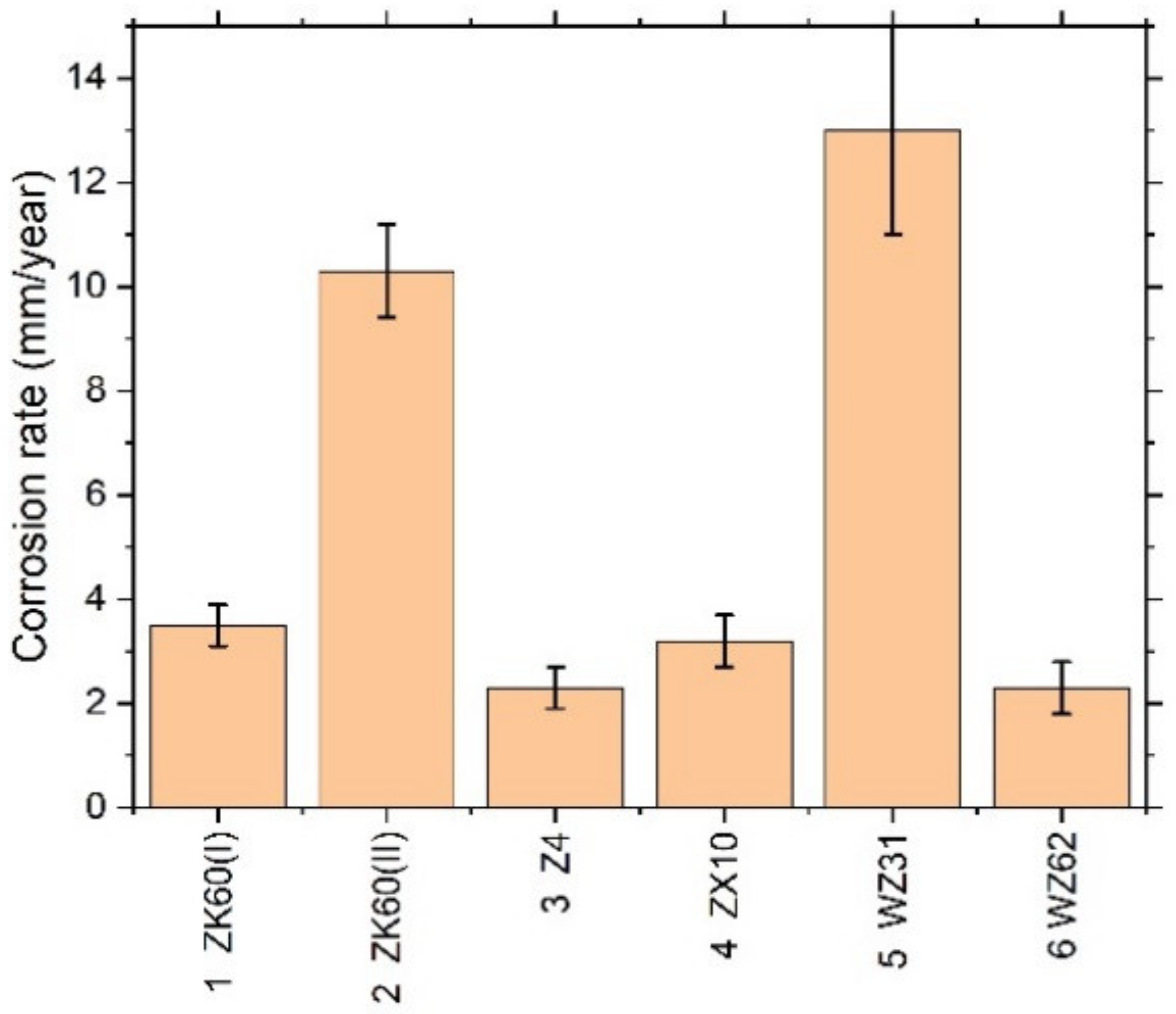
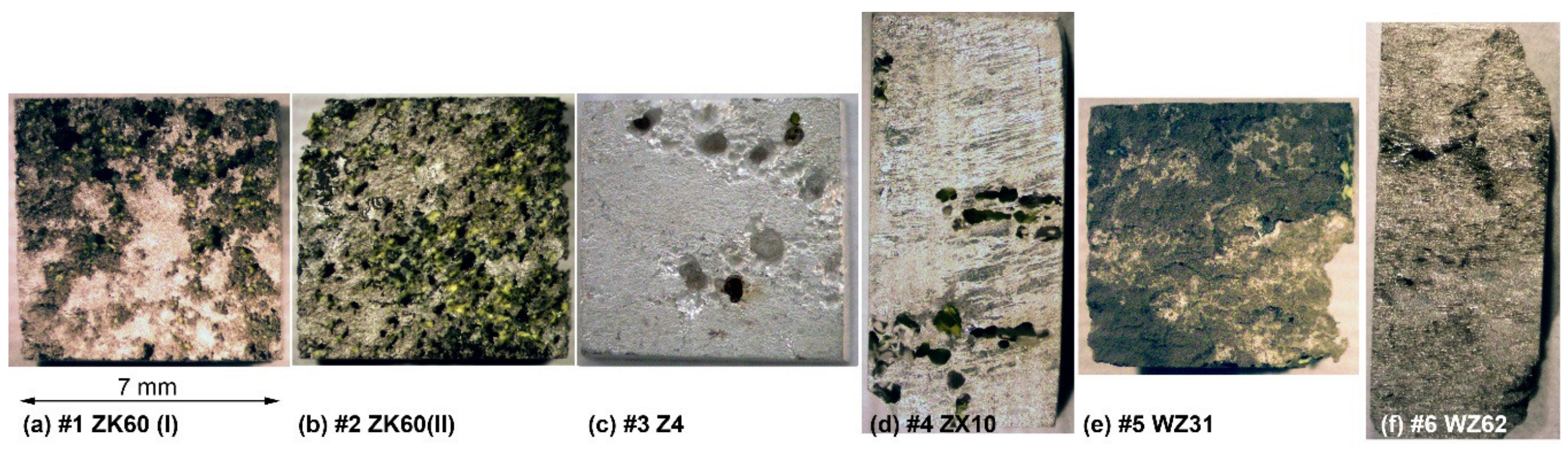
3.3. Corrosion Resistance
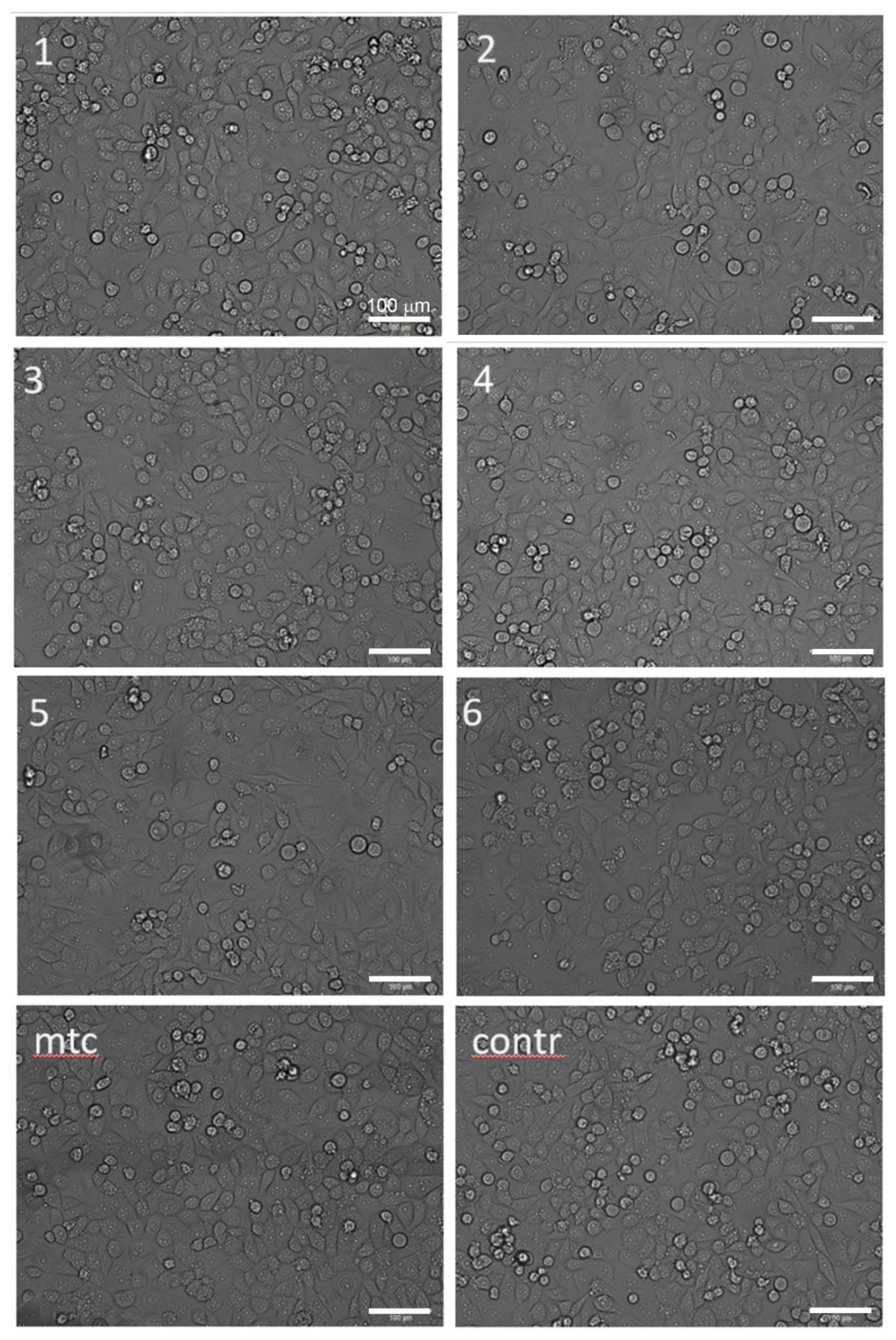
3.4. Cytotoxicity Test
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feng, G.; Qin, C.; Yi, X.; Xia, J.; Chen, J.; Chen, X.; Chen, T.; Jiang, X. Effect of novel bioresorbable scaffold composed of poly-L-lactic acid and amorphous calcium phosphate nanoparticles on inflammation and calcification of surrounding tissues after implantation. J. Mater. Sci. Mater. Med. 2018, 29, 112. [Google Scholar] [CrossRef] [PubMed]
- Gritsch, L.; Lovell, C.; Goldmann, W.H.; Boccaccini, A.R. Do bioresorbable polyesters have antimicrobial properties? J. Mater. Sci. Mater. Med. 2018, 29, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babker, A.; Sotnik, S.; Lyashenko, V. Polymeric Materials in Medicine. Sch. J. Appl. Med Sci. 2018, 6, 148–153. [Google Scholar] [CrossRef]
- Kaliyadan, A.; Siu, H.; Fischman, D.L.; Ruggiero, N.J.; Jasti, B.; Walinsky, P.; Ogilby, J.D.; Savage, M.P. “Very” very late stent thrombosis: Acute myocardial infarction from drug-eluting stent thrombosis more than 5 years after implantation. J. Invasive Cardiol. 2014, 26, 413–416. [Google Scholar]
- Okura, H.; Takagi, T.; Yoshida, K. Therapies targeting inflammation after stent implantation. Curr. Vasc. Pharmacol. 2013, 11, 399–406. [Google Scholar] [CrossRef]
- Alexy, R.D.; Levi, D.S. Materials and manufacturing technologies available for production of a pediatric bioabsorbable stent. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Pliam, M.B.; Zapolanski, A.; Anastassiou, P.; Ryan, C.J.; Manila, L.L.; Shaw, R.E.; Pira, B.-K. Influence of prior coronary stenting on the immediate and mid-term outcome of isolated coronary artery bypass surgery. Innovations 2007, 2, 217–225. [Google Scholar] [CrossRef]
- Erne, P.; Schier, M.; Resink, T.J. The Road to Bioabsorbable Stents: Reaching Clinical Reality? Cardiovasc. Interv. Radiol. 2006, 29, 11–16. [Google Scholar] [CrossRef]
- Peuster, M.; Wohlsein, P.; Brugmann, M.; Ehlerding, M.; Seidler, K.; Fink, C.; Brauer, H.; Fischer, A.; Hausdorf, G. A novel approach to temporary stenting: Degradable cardiovascular stents produced from corrodible metal—Results 6–18 months after implantation into New Zealand white rabbits. Heart 2001, 86, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018, 15. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Erinc, M.; Sillekens, W.H.; Mannens, R.G.T.M.; Werkhoven, R.J. Applicability of existing magnesium alloys as biomedical implant materials. In Proceedings of the Magnesium Technology 2009, San Francisco, CA, USA, 15–19 February 2009; pp. 209–214. [Google Scholar]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persaud-Sharma, D.; McGoron, A. Biodegradable Magnesium Alloys: A Review of Material Development and Applications. J. Biomim. Biomater. Tissue Eng. 2012, 12, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Yang, C.; Lin, S.; Yu, Q.; Wang, G. Biodegradable stents for coronary artery disease treatment: Recent advances and future perspectives. Mater. Sci. Eng. C 2018, 91, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, J.; Becker, M.; Martinelli, E.; Weinberg, A.M.; Mingler, B.; Kilian, H.; Pogatscher, S.; Uggowitzer, P.J.; Löffler, J.F. High-Strength Low-Alloy (HSLA) Mg–Zn–Ca Alloys with Excellent Biodegradation Performance. JOM 2014, 66, 566–572. [Google Scholar] [CrossRef]
- Hofstetter, J.; Martinelli, E.; Pogatscher, S.; Schmutz, P.; Povoden-Karadeniz, E.; Weinberg, A.M.; Uggowitzer, P.J.; Löffler, J.F. Influence of trace impurities on the in vitro and in vivo degradation of biodegradable Mg-5Zn-0.3Ca alloys. Acta Biomater. 2015, 23, 347–353. [Google Scholar] [CrossRef]
- Chen, J.; Tan, L.; Yang, K. Effect of heat treatment on mechanical and biodegradable properties of an extruded ZK60 alloy. Bioact. Mater. 2017, 2, 19–26. [Google Scholar] [CrossRef]
- Qiang, G.; Mostaed, E.; Zanella, C.; Zhentao, Y.; Vedani, M. Ultra-Fine Grained Degradable Magnesium for Biomedical Applications. Rare Met. Mater. Eng. 2014, 43, 2561–2566. [Google Scholar] [CrossRef]
- Hänzi, A.C.; Sologubenko, A.S.; Uggowitzer, P.J. Design strategy for new biodegradable Mg-Y-Zn alloys for medical applications. Int. J. Mater. Res. 2009, 100, 1127–1136. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Kim, J.I.; Kim, D.Y.; Kang, H.J.; Lee, B.; Lee, K.W.; Kim, M.S. Biodegradable stent. J. Biomed. Sci. Eng. 2012, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Im, S.H.; Jung, Y.; Kim, S.H. Current status and future direction of biodegradable metallic and polymeric vascular scaffolds for next-generation stents. Acta Biomater. 2017, 60, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Shen, L.; Chen, J.; Zhang, X.; Kwak, M.; Wu, Y.; Fan, R.; Zhang, L.; Pei, J.; Yuan, G.; et al. A promising biodegradable magnesium alloy suitable for clinical vascular stent application. Sci. Rep. 2017, 7, 46343. [Google Scholar] [CrossRef] [PubMed]
- Asqardoust, S.; Zarei Hanzaki, A.; Abedi, H.R.; Krajnak, T.; Minárik, P. Enhancing the strength and ductility in accumulative back extruded WE43 magnesium alloy through achieving bimodal grain size distribution and texture weakening. Mater. Sci. Eng. A 2017, 698, 218–229. [Google Scholar] [CrossRef]
- Martynenko, N.S.; Lukyanova, E.A.; Serebryany, V.N.; Gorshenkov, M.V.; Shchetinin, I.V.; Raab, G.I.; Dobatkin, S.V.; Estrin, Y. Increasing strength and ductility of magnesium alloy WE43 by equal-channel angular pressing. Mater. Sci. Eng. A 2018, 712, 625–629. [Google Scholar] [CrossRef]
- Naujokat, H.; Gülses, A.; Wiltfang, J.; Açil, Y. Effects of degradable osteosynthesis plates of MgYREZr alloy on cell function of human osteoblasts, fibroblasts and osteosarcoma cells. J. Mater. Sci. Mater. Med. 2017, 28, 126. [Google Scholar] [CrossRef]
- Abe, E.; Kawamura, Y.; Hayashi, K.; Inoue, A. Long-period ordered structure in a high-strength nanocrystalline Mg-1at% Zn-2at% Y alloy studied by atomic-resolution Z-contrast STEM. Acta Mater. 2002, 50, 3845–3857. [Google Scholar] [CrossRef]
- Estrin, Y.; Vinogradov, A. Extreme grain refinement by severe plastic deformation: A wealth of challenging science. Acta Mater. 2013, 61, 782–817. [Google Scholar] [CrossRef]
- Vinogradov, A.; Orlov, D.; Estrin, Y. Improvement of fatigue strength of a Mg–Zn–Zr alloy by integrated extrusion and equal-channel angular pressing. Scr. Mater. 2012, 67, 209–212. [Google Scholar] [CrossRef]
- Vinogradov, A.; Vasilev, E.; Kopylov, V.I.; Linderov, M.; Brilevesky, A.; Merson, D. High Performance Fine-Grained Biodegradable Mg-Zn-Ca Alloys Processed by Severe Plastic Deformation. Metals 2019, 9, 186. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Estrin, Y.; Fu, H.; Song, G.; Zúberová, Z. The Effect of Pre-Processing and Grain Structure on the Bio-Corrosion and Fatigue Resistance of Magnesium Alloy AZ31. Adv. Eng. Mater. 2007, 9, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Martynenko, N.; Lukyanova, E.; Serebryany, V.; Prosvirnin, D.; Terentiev, V.; Raab, G.; Dobatkin, S.; Estrin, Y. Effect of equal channel angular pressing on structure, texture, mechanical and in-service properties of a biodegradable magnesium alloy. Mater. Lett. 2019, 238, 218–221. [Google Scholar] [CrossRef]
- Lukyanova, E.; Anisimova, N.; Martynenko, N.; Kiselevsky, M.; Dobatkin, S.; Estrin, Y. Features of in vitro and in vivo behaviour of magnesium alloy WE43. Mater. Lett. 2018, 215, 308–311. [Google Scholar] [CrossRef]
- Lukyanova, E.A.; Martynenko, N.S.; Shakhova, I.; Belyakov, A.N.; Rokhlin, L.L.; Dobatkin, S.V.; Estrin, Y.Z. Strengthening of age-hardenable WE43 magnesium alloy processed by high pressure torsion. Mater. Lett. 2016, 170, 5–9. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zehetbauer, M.J.; Zhu, Y.T. Producing bulk ultrafine-grained materials by severe plastic deformation. JOM 2006, 58, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Zhilyaev, A.P.; Langdon, T.G. Using high-pressure torsion for metal processing: Fundamentals and applications. Prog. Mater. Sci. 2008, 53, 893–979. [Google Scholar] [CrossRef]
- Azushima, A.; Kopp, R.; Korhonen, A.; Yang, D.Y.; Micari, F.; Lahoti, G.D.; Groche, P.; Yanagimoto, J.; Tsuji, N.; Rosochowski, A.; et al. Severe plastic deformation (SPD) processes for metals. CIRP Ann.-Manuf. Technol. 2008, 57, 716–735. [Google Scholar] [CrossRef]
- Vinogradov, A.; Estrin, Y. Analytical and numerical approaches to modelling severe plastic deformation. Prog. Mater. Sci. 2018, 95, 172–242. [Google Scholar] [CrossRef]
- Orlov, D.; Ralston, K.D.; Birbilis, N.; Estrin, Y. Enhanced corrosion resistance of Mg alloy ZK60 after processing by integrated extrusion and equal channel angular pressing. Acta Mater. 2011, 59, 6176–6186. [Google Scholar] [CrossRef]
- Orlov, D.; Raab, G.; Lamark, T.T.; Popov, M.; Estrin, Y. Improvement of mechanical properties of magnesium alloy ZK60 by integrated extrusion and equal channel angular pressing. Acta Mater. 2011, 59, 375–385. [Google Scholar] [CrossRef]
- Minárik, P.; Zemková, M.; Král, R.; Mhaede, M.; Wagner, L.; Hadzima, B. Effect of microstructure on the corrosion resistance of the AE42 magnesium alloy processed by rotary swaging. Acta Phys. Pol. A 2015, 128, 805–807. [Google Scholar] [CrossRef]
- Markushev, M.V.; Nugmanov, D.R.; Sitdikov, O.; Vinogradov, A. Structure, texture and strength of Mg-5.8Zn-0.65Zr alloy after hot-to-warm multi-step isothermal forging and isothermal rolling to large strains. Mater. Sci. Eng. A 2018, 709, 330–338. [Google Scholar] [CrossRef]
- Seipp, S.; Wagner, M.F.X.; Hockauf, K.; Schneider, I.; Meyer, L.W.; Hockauf, M. Microstructure, crystallographic texture and mechanical properties of the magnesium alloy AZ31B after different routes of thermo-mechanical processing. Int. J. Plast. 2012, 35, 155–166. [Google Scholar] [CrossRef]
- Vinogradov, A.; Mimaki, T.; Hashimoto, S.; Valiev, R. On the corrosion behaviour of ultra-fine grain copper. Scr. Mater. 1999, 41, 319–326. [Google Scholar] [CrossRef]
- Liao, J.; Hotta, M.; Yamamoto, N. Corrosion behavior of fine-grained AZ31B magnesium alloy. Corros. Sci. 2012, 61, 208–214. [Google Scholar] [CrossRef]
- Ullmann, B.; Reifenrath, J.; Seitz, J.-M.; Bormann, D.; Meyer-Lindenberg, A. Influence of the grain size on the in vivo degradation behaviour of the magnesium alloy LAE442. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2013, 227, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Aung, N.N.; Zhou, W. Effect of grain size and twins on corrosion behaviour of AZ31B magnesium alloy. Corros. Sci. 2010, 52, 589–594. [Google Scholar] [CrossRef]
- Zeng, R.; Kainer, K.U.; Blawert, C.; Dietzel, W. Corrosion of an extruded magnesium alloy ZK60 component—The role of microstructural features. J. Alloy. Compd. 2011, 509, 4462–4469. [Google Scholar] [CrossRef]
- Song, G.-L.; Xu, Z. Effect of microstructure evolution on corrosion of different crystal surfaces of AZ31 Mg alloy in a chloride containing solution. Corros. Sci. 2012, 54, 97–105. [Google Scholar] [CrossRef]
- Pu, Z.; Song, G.L.; Yang, S.; Outeiro, J.C.; Dillon, O.W.; Puleo, D.A.; Jawahir, I.S. Grain refined and basal textured surface produced by burnishing for improved corrosion performance of AZ31B Mg alloy. Corros. Sci. 2012, 57, 192–201. [Google Scholar] [CrossRef]
- Gu, X.N.; Li, N.; Zheng, Y.F.; Kang, F.; Wang, J.T.; Ruan, L. In vitro study on equal channel angular pressing AZ31 magnesium alloy with and without back pressure. Mater. Sci. Eng. B 2011, 176, 1802–1806. [Google Scholar] [CrossRef]
- Ratna Sunil, B.; Sampath Kumar, T.S.; Chakkingal, U.; Nandakumar, V.; Doble, M.; Devi Prasad, V.; Raghunath, M. In vitro and in vivo studies of biodegradable fine grained AZ31 magnesium alloy produced by equal channel angular pressing. Mater. Sci. Eng. C 2016, 59, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Nugmanov, D.; Sitdikov, O.; Markushev, M. Grain refinement in the magnesium alloy ZK60 during multi-step isothermal forging. Mater. Sci. Forum 2015, 830–831, 7–10. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; StJohn, D. An Hydrogen Evolution Method for the Estimation of the Corrosion Rate of Magnesium Alloys. In Essential Readings in Magnesium Technology; Mathaudhu, S.N., Luo, A.A., Neelameggham, N.R., Nyberg, E.A., Sillekens, W.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 565–572. [Google Scholar]
- Nidadavolu, E.P.S.; Feyerabend, F.; Ebel, T.; Willumeit-Römer, R.; Dahms, M. On the Determination of Magnesium Degradation Rates under Physiological Conditions. Materials 2016, 9, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- ISO 10993-5:2009 Biological Evaluation of Medical Devices-Part 5: Tests for in Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009.
- Kaibyshev, R. 5-Dynamic recrystallization in magnesium alloys. In Advances in Wrought Magnesium Alloys; Woodhead Publishing: Cambridge, UK, 2012; pp. 186–225. [Google Scholar]
- Galiyev, A.; Kaibyshev, R.; Gottstein, G. Correlation of plastic deformation and dynamic recrystallization in magnesium alloy ZK60. Acta Mater. 2001, 49, 1199–1207. [Google Scholar] [CrossRef]
- Al-Samman, T.; Gottstein, G. Dynamic recrystallization during high temperature deformation of magnesium. Mater. Sci. Eng. A 2008, 490, 411–420. [Google Scholar] [CrossRef]
- Polmear, I.J.; StJohn, D.; Nie, J.-F.; Qian, M.; Knovel (Firm). Light Alloys: Metallurgy of the Light Metals, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2017; p. 1. [Google Scholar]
- Kang, S.B.; Cho, J.H.; Kim, H.W.; Jin, Y.M. Effect of heat treatment on microstructure and mechanical properties in ZK60 alloy sheet. Mater. Sci. Forum 2008, 567–568, 361–364. [Google Scholar]
- Agnew, S.R.; Horton, J.A.; Lillo, T.M.; Brown, D.W. Enhanced ductility in strongly textured magnesium produced by equal channel angular processing. Scr. Mater. 2004, 50, 377–381. [Google Scholar] [CrossRef]
- Vinogradov, A.; Serebryany, V.N.; Dobatkin, S.V. Tailoring Microstructure and Properties of Fine Grained Magnesium Alloys by Severe Plastic Deformation. Adv. Eng. Mater. 2018, 20. [Google Scholar] [CrossRef]
- McNulty, R.E.; Hanawalt, J.D. Some Corrosion Characteristics of High Purity Magnesium Alloys. Trans. Electrochem. Soc. 1942, 81, 423–433. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding Magnesium Corrosion—A Framework for Improved Alloy Performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Lespagnol, J.; Birbilis, N.; Staiger, M.P. A survey of bio-corrosion rates of magnesium alloys. Corros. Sci. 2010, 52, 287–291. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Li, Y.; Zhao, J.; Qin, L.; Lai, Y. Corrosion and biocompatibility improvement of magnesium-based alloys as bone implant materials: A review. Regen. Biomater. 2017, 4, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Atrens, A.; Song, G.-L.; Cao, F.; Shi, Z.; Bowen, P.K. Advances in Mg corrosion and research suggestions. J. Magnes. Alloy. 2013, 1, 177–200. [Google Scholar] [CrossRef] [Green Version]
- Zainal Abidin, N.I.; Rolfe, B.; Owen, H.; Malisano, J.; Martin, D.; Hofstetter, J.; Uggowitzer, P.J.; Atrens, A. The in vivo and in vitro corrosion of high-purity magnesium and magnesium alloys WZ21 and AZ91. Corros. Sci. 2013, 75, 354–366. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Xia, K.; Wang, J.T.; Wu, X.; Chen, G.; Gurvan, M. Equal channel angular pressing of magnesium alloy AZ31. Mater. Sci. Eng. A 2005, 410–411, 324–327. [Google Scholar] [CrossRef]
- Gzyl, M.; Rosochowski, A.; Pesci, R.; Olejnik, L.; Yakushina, E.; Wood, P. Mechanical properties and microstructure of AZ31B magnesium alloy processed by I-ECAP. Metall. Mater. Trans. A 2014, 45, 1609–1620. [Google Scholar] [CrossRef] [Green Version]
- Hofstetter, J.; Rüedi, S.; Baumgartner, I.; Kilian, H.; Mingler, B.; Povoden-Karadeniz, E.; Pogatscher, S.; Uggowitzer, P.J.; Löffler, J.F. Processing and microstructure–property relations of high-strength low-alloy (HSLA) Mg–Zn–Ca alloys. Acta Mater. 2015, 98, 423–432. [Google Scholar] [CrossRef]
- Bian, D.; Zhou, W.; Liu, Y.; Li, N.; Zheng, Y.; Sun, Z. Fatigue behaviors of HP-Mg, Mg–Ca and Mg–Zn–Ca biodegradable metals in air and simulated body fluid. Acta Biomater. 2016, 41, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Prosenc, M.H.; Wolff, M.; Hort, N.; Willumeit, R.; Feyerabend, F. Interference of magnesium corrosion with tetrazolium-based cytotoxicity assays. Acta Biomater. 2010, 6, 1813–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, D.; Yun, Y.; Tan, Z.; Dong, Z.; Schulz, M.J. In vivo and in vitro degradation behavior of magnesium alloys as biomaterials. J. Mater. Sci. Technol. 2012, 28, 261–267. [Google Scholar] [CrossRef]
- Sanchez, A.H.M.; Luthringer, B.J.C.; Feyerabend, F.; Willumeit, R. Mg and Mg alloys: How comparable are in vitro and in vivo corrosion rates? A review. Acta Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| N | Alloy (wt.%) | Mg | Zn | Ca | Zr | Y | Al | Fe | Cu | Ni | Si | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ZK60(I) | Mg-6Zn-0.5Zr | 94.52 | 5.02 | 0.0003 | 0.44 | 0.006 | 0.0036 | 0.0032 | <0.0001 | <0.0001 | <0.0001 |
| 2 | ZK60(II) | Mg-6Zn-0.5Zr | 94.3 | 5.09 | 0.0003 | 0.52 | 0.007 | 0.0030 | 0.0036 | <0.0001 | <0.0001 | 0.0002 |
| 3 | Z4 | Mg-4Zn | 96.18 | 3.79 | 0.018 | 0.002 | 0.005 | 0.0096 | 0.0059 | <0.0001 | 0.0004 | <0.0001 |
| 4 | ZX10 | Mg-0.9Zn-0.2Ca | 98.90 | 0.85 | 0.218 | <0.0001 | <0.001 | 0.0085 | 0.0072 | 0.0008 | 0.0014 | 0.007 |
| 5 | WZ31 | Mg-1Zn-2.8Y | 96.40 | 1.08 | 0.0006 | 0.002 | 2.07 | 0.005 | 0.02 | <0.0001 | 0.0057 | 0.24 |
| 6 | WZ62 | Mg-2.0Zn-5.7Y | 92.24 | 2.0 | 0.003 | 0.032 | 5.72 | 0.046 | 0.0004 | 0.0012 | <0.0001 | 0.007 |
| № | Alloy | Composition (wt. %) | Processing/Temperature | Equivalent Imposed Strain | Grain Size, D/μm | Yield Stress, σ0.2/MPa | Ultimate Strength, σUTS/MPa | Elongation at Break εf (%) | Corrosion Rate in SBF, mm/Year |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ZK60 (I) | Mg-6Zn-0.5Zr | E + ECAP/350 °C | 3.4 + 2.0 | 7.7 ± 0.5 | 293 + 5 | 330 ± 8 | 16.2 ± 1.7 | 3.5 ± 0.4 |
| 2 | ZK60 (II) | Mg-6Zn-0.5Zr | Two-step MIF/400 °С + 300 °С | 4.2 + 3.0 | 5.0 ± 0.3 | 205 + 7 | 328 ± 4 | 30.3 ± 3.3 | 10.3 ± 0.9 |
| 3 | Z4 | Mg-4Zn | ECAP/415 °C | 1.15 | 126 ± 86 | 126 + 3 | 250 ± 2 | 18.5 ± 2.2 | 2.3 ± 0.4 |
| 4 | ZX10 | Mg-1Zn-0.1Ca | E/325 °C | 1.6 | 2.1 + 0.4 * | 230 + 4 | 285 ± 3 | 13.5 ± 2.2 | 3.2 |
| 5 | WZ31 | Mg-1Zn-2.9Y | ECAP/425 °C | 2.30 | 2.0 ± 1.6 | 277 + 4 | 318 ± 3 | 15.3 ± 3.6 | 13 ± 2 |
| 6 | WZ62 | Mg-2Zn-5.7Y | E/350 °C | 1.6 | 8.8 ± 4.2 | 364 + 5 | 430 ± 2 | 4.6 ± 2.8 | 2.3 |
| Dilution of the Extract, Times | Specimen * | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| ZK60 (I) | ZK60 (II) | Z4 | ZX10 | WZ31 | WZ62 | |
| 0 | 90.1 ± 10.4 | 48.1 ± 1.4 | 96.3 ± 2.6 | 95.4 ± 7.2 | 81.4 ± 13.6 | 100.8 ± 13.0 |
| 2 | 99.0 ± 5.1 | 84.5 ± 2.7 | 93.3 ± 8.7 | 97.6 ± 8.4 | 87.9 ± 10.1 | 95.2 ± 2.5 |
| 4 | 107.7 ± 13.1 | 90.2 ± 6.7 | 93.3 ± 3.9 | 99.1 ± 6.2 | 91.3 ± 1.4 | 102.0 ± 11.1 |
| 6 | 93.9 ± 1.7 | 100.8 ± 4.7 | 93.0 ± 2.3 | 94.5 ± 3.5 | 91.6 ± 13.7 | 96.0 ± 3.6 |
| 8 | 95.7 ± 15.0 | 95.3 ± 0.8 | 92.9 ± 1.3 | 96.8 ± 5.5 | 97.4 ± 5.0 | 103.4 ± 9.2 |
| 10 | 96.3 ± 12.0 | 96.5 ± 4.7 | 103.2 ± 1.8 | 97.7 ± 5.4 | 97.2 ± 15.3 | 98.3 ± 6.0 |
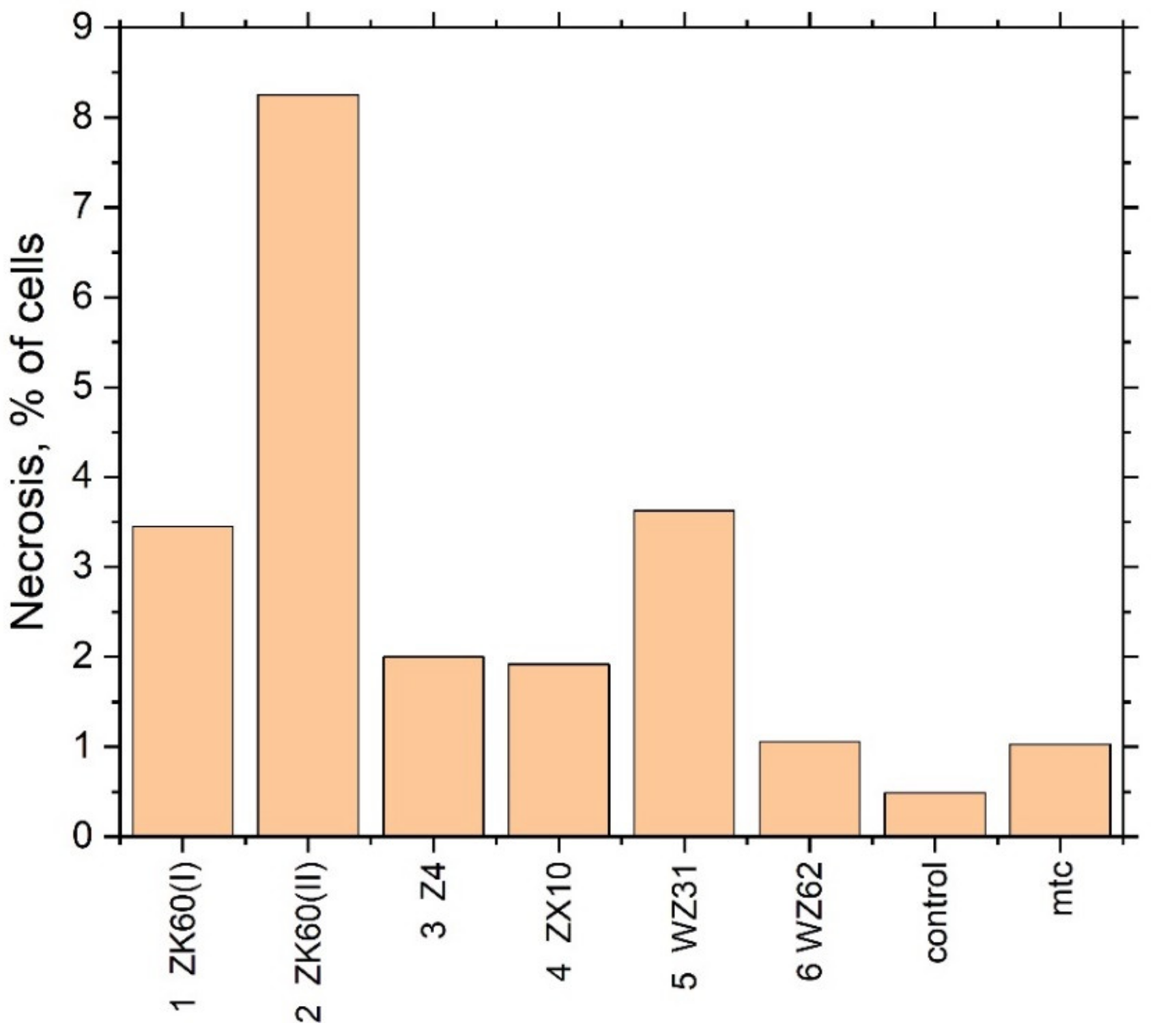
| Sample | Necrosis of Cells, % | рН of Culture Medium | |
|---|---|---|---|
| Control | 0.491 ± 0.005 | 7.77 | |
| Mitomycin | 1.03 ± 0.01 | - | |
| 1 | ZX60 (I) | 3.45 ± 0.03 | 8.06 |
| 2 | ZK60 (II) | 8.25 ± 0.09 | 8.25 |
| 3 | Z4 | 2.00 ± 0.02 | 8.11 |
| 4 | ZX10 | 1.92 ± 0.02 | 8.17 |
| 5 | WZ31 | 3.63 ± 0.04 | 8.25 |
| 6 | WZ62 | 1.06 ± 0.01 | 8.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merson, D.; Brilevsky, A.; Myagkikh, P.; Tarkova, A.; Prokhorikhin, A.; Kretov, E.; Frolova, T.; Vinogradov, A. The Functional Properties of Mg–Zn–X Biodegradable Magnesium Alloys. Materials 2020, 13, 544. https://doi.org/10.3390/ma13030544
Merson D, Brilevsky A, Myagkikh P, Tarkova A, Prokhorikhin A, Kretov E, Frolova T, Vinogradov A. The Functional Properties of Mg–Zn–X Biodegradable Magnesium Alloys. Materials. 2020; 13(3):544. https://doi.org/10.3390/ma13030544
Chicago/Turabian StyleMerson, Dmitry, Alexander Brilevsky, Pavel Myagkikh, Alexandra Tarkova, Alexei Prokhorikhin, Evgeny Kretov, Tatiana Frolova, and Alexei Vinogradov. 2020. "The Functional Properties of Mg–Zn–X Biodegradable Magnesium Alloys" Materials 13, no. 3: 544. https://doi.org/10.3390/ma13030544
APA StyleMerson, D., Brilevsky, A., Myagkikh, P., Tarkova, A., Prokhorikhin, A., Kretov, E., Frolova, T., & Vinogradov, A. (2020). The Functional Properties of Mg–Zn–X Biodegradable Magnesium Alloys. Materials, 13(3), 544. https://doi.org/10.3390/ma13030544






