Electrophoretic Deposition of Layer-by-Layer Unsheathed Carbon Nanotubes—A Step Towards Steerable Surface Roughness and Wettability
Abstract
1. Introduction
2. Materials and Methods
3. Results
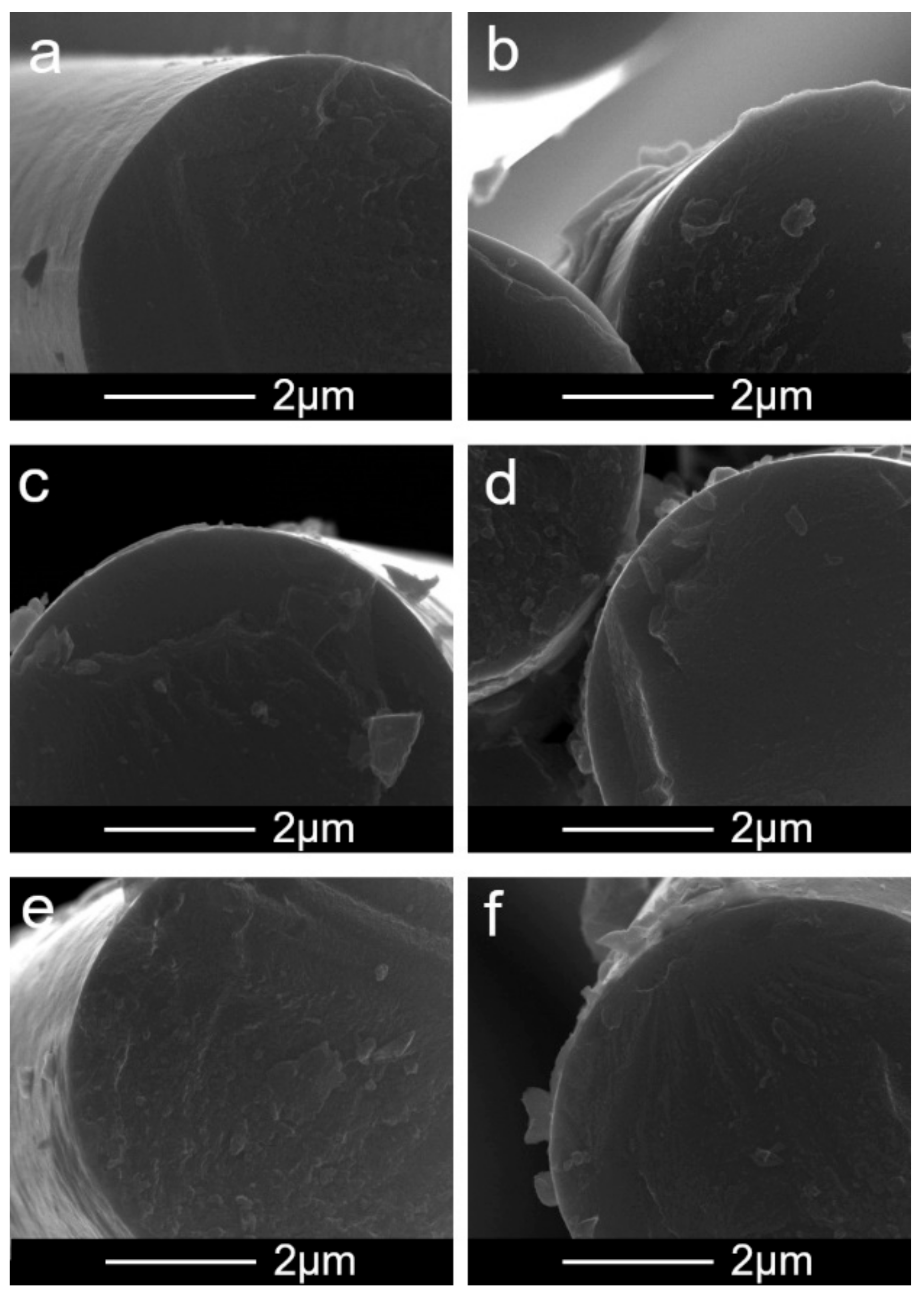
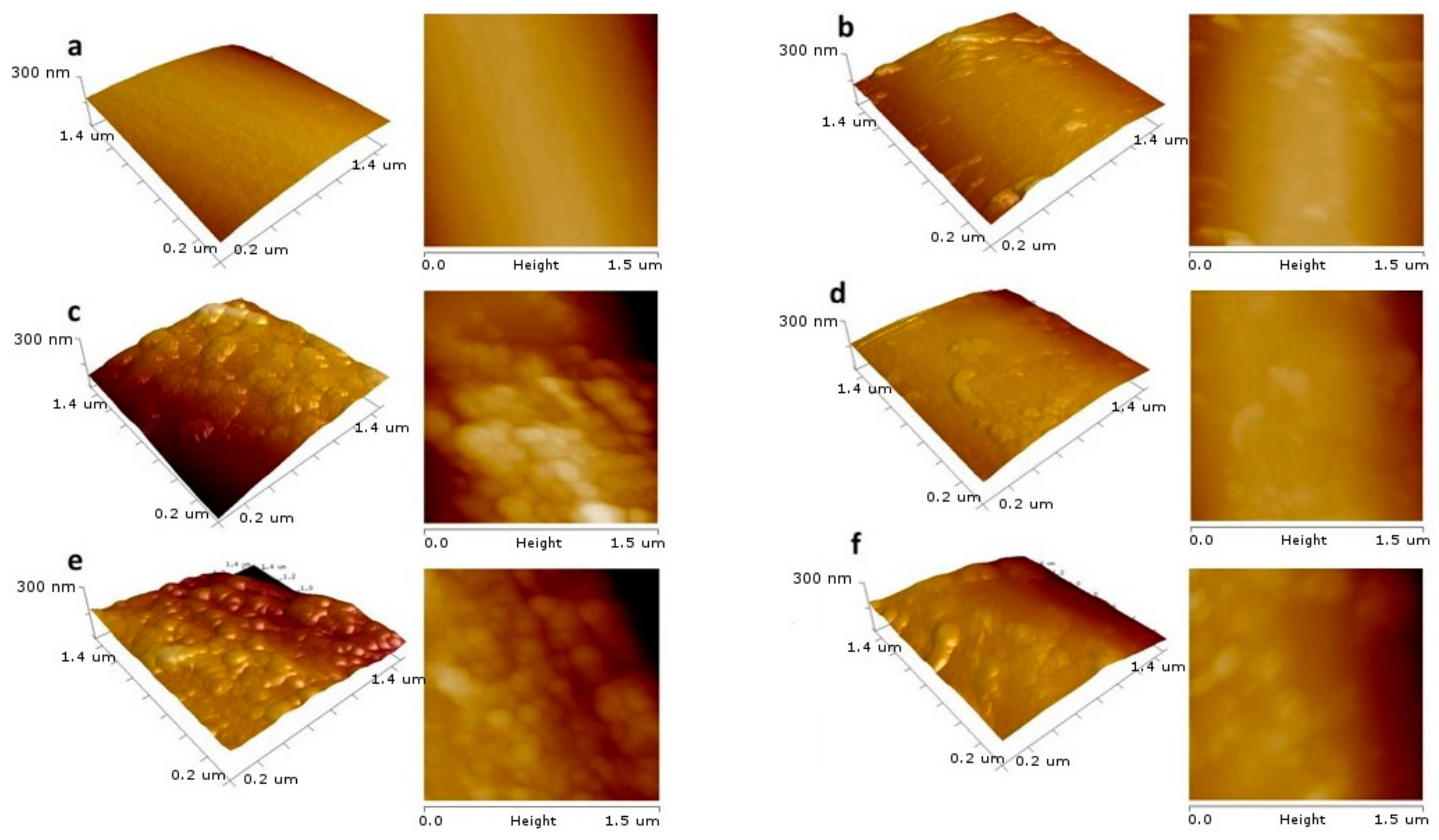
3.1. Characteristics of CNTs before the EPD Process
3.2. Characteristics of CNTs after the EPD Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Du, C.; Heldbrant, D.; Pan, N. Preparation and preliminary property study of carbon nanotubes films by electrophoretic deposition. Mater. Lett. 2002, 57, 434–438. [Google Scholar] [CrossRef]
- Schaefer, J.D.; Rodriguez, A.J.; Guzman, M.E.; Lim, C.S.; Minaie, B. Effects of electrophoretically deposited carbon nanofibers on the interface of single carbon fibers embedded in epoxy matrix. Carbon 2011, 49, 2750–2759. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Cho, J.; Roether, J.A.; Thomas, B.J.C.; Minay, E.J.; Shaffer, M.S.P. Electrophoretic deposition of carbon nanotubes. Carbon 2006, 44, 3149–3160. [Google Scholar] [CrossRef]
- Cho, J.; Konopka, K.; Rożniatowski, K.; Garcı’a-Lecina, E.; Shaffer, M.S.P.; Boccaccini, A.R. Characterisation of carbon nanotube films deposited by electrophoretic deposition. Carbon 2009, 47, 58–67. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Sharma, S.P. Study on the performance of different nano-species used for surface modification of carbon fiber for interface strengthening. Compos. Pt. A 2019, 125, 105509. [Google Scholar] [CrossRef]
- Guo, J.; Lu, C.; An, F.; He, S. Preparation and characterization of carbon nanotubes/carbon fiber hybrid material by ultrasonically assisted electrophoretic deposition. Mater. Lett. 2012, 66, 382–384. [Google Scholar] [CrossRef]
- Kim, H.; Ohb, E.; Hahn, H.T.; Lee, K.H. Enhancement of fracture toughness of hierarchical carbon fiber composites via improved adhesion between carbon nanotubes and carbon fibers. Compos. Pt. A 2015, 71, 72–83. [Google Scholar] [CrossRef]
- Tamrakar, S.; An, Q.; Thostenson, E.T.; Rider, A.N.; Haque, B.Z.G.; Gillespie, J.W. Tailoring Interfacial Properties by Controlling Carbon Nanotube Coating Thickness on Glass Fibers Using Electrophoretic Deposition. ACS Appl. Mater. Interf. 2016, 8, 1501–1510. [Google Scholar] [CrossRef]
- Haghbin, A.; Liaghat, G.H.; Arabi, A.M.; Hadavinia, H.; Pol, M.H. Investigations on electrophoretic deposition of carbon nanotubes on glass textures to improve polymeric composites interface. Compos. Sci. Technol. 2018, 155, 197–204. [Google Scholar] [CrossRef]
- An, Q.; Tamrakar, S.; Gillespie, J.W.; Rider, A.N.; Thostenson, E.T. Tailored glass fiber interphases via electrophoretic deposition of carbon nanotubes: Fiber and interphase characterization. Compos. Sci. Technol. 2018, 166, 131–139. [Google Scholar] [CrossRef]
- Guignier, C.; Bueno, M.A.; Camillieri, B.; Tourlonias, M.; Durand, B. Tribological behaviour and wear of carbon nanotubes grafted on carbon fibres. Compos. Pt. A 2015, 71, 168–175. [Google Scholar] [CrossRef]
- Qingliang, S.; Hejun, L.; Fengling, Z.; Qiang, S.; Qiangang, F. Electrophoretic deposition of carbon nanotubes for improved ablation resistance of carbon/carbon composites. Corros. Sci. 2018, 132, 204–213. [Google Scholar] [CrossRef]
- Deng, C.; Jiang, I.; Liu, F.; Fang, L.; Wang, J.; Li, D.; Wu, J. Influence of carbon nanotubes coatings onto carbon fiber by oxidative treatments combined with electrophoretic deposition on interfacial properties of carbon fiber composite. Appl. Surf. Sci. 2015, 357, 1274–1280. [Google Scholar] [CrossRef]
- Li, Q.; Church, J.S.; Naebe, M.; Fox, B.L. Interfacial characterization and reinforcing mechanism of novel carbon nanotube–Carbon fibre hybrid composites. Carbon 2016, 109, 74–86. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, C.; Su, Y.; Guo, Q.; Liu, F.; Deng, C.; Yao, X.; Zhou, L. Influence of carbon nanotube coatings on carbon fiber by ultrasonically assisted electrophoretic deposition on its composite interfacial property. Polymers 2016, 8, 302. [Google Scholar] [CrossRef]
- Liu, Y.T.; Yao, T.T.; Zhang, W.S.; Wu, G.P. Laser welding of carbon nanotube networks on carbon fibers from ultrasonic-directed assembly. Mater. Lett. 2019, 236, 244–247. [Google Scholar] [CrossRef]
- Goyal, A.; Liu, S.; Iqbal, Z.; Fetter, L.A.; Farrow, R.C. Directed self-assembly of individual vertically aligned carbon nanotubes. J. Vac. Sci. Technol. 2008, B 26, 2524–2528. [Google Scholar] [CrossRef]
- Jung, S.M.; Jung, H.Y.; Suh, J.S. Horizontally aligned carbon nanotube field emitters fabricated on ITO glass substrates. Carbon 2008, 46, 1973–1977. [Google Scholar] [CrossRef]
- Rigueur, J.L.; Hasan, S.A.; Mahajan, S.V.; Dickerson, J.H. Buckypaper fabrication by liberation of electrophoretically deposited carbon nanotubes. Carbon 2010, 48, 4090–4099. [Google Scholar] [CrossRef]
- Biswas, J.; Rottman-Yang, J.S.; Gonzalo-Juan, I.; Dickerson, J.H. Freestanding carbon nanotube films fabricated by post-electrophoretic deposition electrochemical separation. J. Electrochem. Soc. 2012, 159, K103–K106. [Google Scholar] [CrossRef]
- Mei, H.; Xu, Y.; Sun, Y.; Bai, Q.; Cheng, L. Carbon nanotube buckypaper-reinforced SiCN ceramic matrix composites of superior electrical conductivity. J. Eur. Ceramic Soc. 2016, 36, 1893–1898. [Google Scholar] [CrossRef]
- Xiao, A.W.; Evers, K.; Tkaczyk, M.; Jones, R.S.; Saxby, C.; Dragnevski, K.; Grobert, N. Electrophoretic fabrication of robust carbon nanotube “Buckyfilms” for flexible electronics. ACS Appl. Nano Mater. 2019, 2, 5190–5199. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, L.; Zhang, J.; Zhang, L.; Yao, N.; Zhang, B. Electron field emission properties of carbon nanotubes-deposited flexible film. Appl. Surf. Sci. 2005, 251, 258–261. [Google Scholar] [CrossRef]
- Zhao, H.; Song, H.; Li, Z.; Yuan, G.; Jin, X. Electrophoretic deposition and field emission properties of patterned carbon nanotubes. Appl. Surf. Sci. 2005, 251, 242–244. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, Y.C.; Wang, T.; Kuo, C.T. Feasibility study of high performance field emitter pattern with the horizontally oriented carbon nanotubes by electrophoresis. Diamond Relat. Mater. 2009, 18, 520–523. [Google Scholar] [CrossRef]
- Dinh, T.M.; Pech, D.; Brunet, M.; Achour, A. High resolution electrochemical micro-capacitors based on oxidized multi-walled carbon nanotubes. J. Phys. Conf. Ser. 2013, 476, 012106. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Yuan, D. Preparation of solid-phase microextraction fiber coated with single-walled carbon nanotubes by electrophoretic deposition and its application in extracting phenols from aqueous samples. J. Chromatogr. A 2009, 1216, 1305–1311. [Google Scholar] [CrossRef]
- Qian, W.; Cao, M.; Xie, F.; Dong, C. Thermo-electrochemical cells based on carbon nanotube electrodes by electrophoretic deposition. Nano-Micro Lett. 2016, 8, 240–246. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, H. Fabrication of patterned single-walled carbon nanotube films using electrophoretic deposition. Ultramicroscopy 2008, 108, 1005–1008. [Google Scholar] [CrossRef]
- Ogihara, H.; Fukasawa, M.; Saji, T. Fabrication of patterned carbon nanotube thin films using electrophoretic deposition and ultrasonic radiation. Carbon 2011, 49, 4595–4607. [Google Scholar] [CrossRef]
- Mazurenko, I.; Etienne, M.; Tananaiko, O.; Urbanova, V.; Zaitsev, V.; Walcarius, A. Electrophoretic deposition of macroporous carbon nanotube assemblies for electrochemical applications. Carbon 2013, 53, 302–312. [Google Scholar] [CrossRef]
- Song, Q.; Li, K.Z.; Li, H.L.; Li, H.J.; Ren, C. Grafting straight carbon nanotubes radially onto carbon fibers and their effect on the mechanical properties of carbon/carbon composites. Carbon 2012, 50, 3943–3960. [Google Scholar] [CrossRef]
- Zhu, G.; Pan, L.; Lu, T.; Liu, X.; Lv, T.; Xu, T.; Sun, Z. Electrophoretic deposition of carbon nanotubes films as counter electrodes of dye-sensitized solar cells. Electrochim. Acta 2011, 56, 10288–10291. [Google Scholar] [CrossRef]
- Chen, Z.; Boström, T. Electrophoretically deposited carbon nanotube spectrally selective solar absorbers. Solar Energy Mater. Solar Cells 2016, 144, 678–683. [Google Scholar] [CrossRef]
- Ma, J.; Tang, J.; Cheng, Q.; Zhang, H.; Shinya, N.; Qin, L.C. Carbon composite microelectrodes fabricated by electrophoretic deposition. J. Nanosci. Nanotechnol. 2012, 12, 1972–1978. [Google Scholar] [CrossRef]
- Hekmat, F.; Sohrabi, B.; Rahmanifar, M.S.; Jalali, A. Electrophoretic deposition of multi-walled carbon nanotubes onporous anodic aluminum oxide using ionic liquid as a dispersing agent. Appl. Surf. Sci. 2015, 341, 109–119. [Google Scholar] [CrossRef]
- Sebt Ahmadi, S.S.; Riahifar, R.; Raissi, B.; Sahba Yaghmaee, M.; Cornelis Metselaar, H.S.; Javaheria, M. Electrophoretic Deposition of MWCNT on a PTFE Layer for Making Working Electrode of Oxygen Sensor. J. Electrochem. Soc. 2017, 164, B506–B512. [Google Scholar] [CrossRef]
- Wu, D.C.; Shen, L.; Low, J.E.; Wong, S.Y.; Li, X.; Tjiu, W.C.; Liu, Y.; He, C.B. Multi-walled carbon nanotube/polyimide composite film fabricated through electrophoretic deposition. Polymer 2010, 51, 2155–2160. [Google Scholar] [CrossRef]
- Dhand, C.; Arya, S.K.; Singh, S.P.; Singh, B.P.; Datta, M.; Malhotra, B.D. Preparation of polyaniline/multiwalled carbon nanotube composite by novel electrophoretic route. Carbon 2008, 46, 1727–1735. [Google Scholar] [CrossRef]
- Rosenkranz, A.; Freeman, L.; Fleischmann, S.; Lasserre, F.; Fainman, Y.; Talke, F.E. Tip-enhanced Raman spectroscopy studies of nanodiamonds and carbon onions. Carbon 2018, 132, 495–502. [Google Scholar] [CrossRef]
- Kim, B.J.; Park, J.S.; Hwang, Y.J.; Park, J.S. Characteristics of silver meshes coated with carbon nanotubes via spray-coating and electrophoretic deposition for touch screen panels. Thin Solid Films 2015, 596, 68–71. [Google Scholar] [CrossRef]
- Schäfer, C.; Reinert, L.; MacLucas, T.; Grützmacher, P.; Merz, R.; Mücklich, F.; Suarez, S. Influence of Surface Design on the Solid Lubricity of Carbon Nanotubes-Coated Steel Surfaces. Tribol. Lett. 2018, 66, 89. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Huang, J.; Fan, Y.; Cui, F.Z. Biomedical investigation of CNT based coatings. Surf. Coat. Technol. 2011, 206, 759–766. [Google Scholar] [CrossRef]
- Fraczek-Szczypta, A.; Jantas, D.; Ciepiela, F.; Grzonka, J.; Bernasik, A.; Marzec, M. Carbon nanomaterials coatings—Properties and influence on nerve cells response. Diamond Relat. Mater. 2018, 84, 127–140. [Google Scholar] [CrossRef]
- Thomas, B.J.C.; Boccaccini, A.R.; Shaffer, M.S.P. Multi-walled carbon nanotube coatings using electrophoretic deposition (EPD). J. Am. Ceram. Soc. 2005, 88, 980–982. [Google Scholar] [CrossRef]
- Farrokhi-Rada, M.; Fateh, A.; Shahrabi, T. Electrophoretic deposition of vancomycin loaded halloysite nanotubeschitosan nanocomposite coatings. Surf. Coat. Technol. 2018, 349, 144–156. [Google Scholar] [CrossRef]
- Diba, M.; Fam, D.W.H.; Boccaccini, A.R.; Shaffer, M.S.P. Electrophoretic deposition of graphene-related materials: A review of the fundamentals. Progr. Mater. Sci. 2016, 82, 83–117. [Google Scholar] [CrossRef]
- Bakhoum, E.G.; Cheng, M.H.M. Electrophoretic coating of carbon nanotubes for high energy-density capacitor applications. J. Appl. Phys. 2009, 105, 104314. [Google Scholar] [CrossRef]
- Benko, A.; Nocuń, M.; Berent, K.; Gajewska, M.; Klita, Ł.; Wyrwa, J.; Błażewicz, M. Diluent changes the physicochemical and electrochemical properties of the electrophoretically-deposited layers of carbon nanotubes. Appl. Surf. Sci. 2017, 403, 206–217. [Google Scholar] [CrossRef]
- Duchamp, M.; Lee, K.; Dwir, B.; Seo, J.W.; Kapon, E.; Forro, L.; Magrez, A. controlled positioning of carbon nanotubes by dielectrophoresis: Insights into the solvent and substrate role. ACS Nano 2010, 4, 279–284. [Google Scholar] [CrossRef]
- Lima, M.D.; de Andrade, M.J.; Bergmann, C.P.; Roth, S. Thin, conductive, carbon nanotube networks over transparent substrates by electrophoretic deposition. J. Mater. Chem. 2008, 18, 776–779. [Google Scholar] [CrossRef]
- Benko, A.; Przekora, A.; Wesełucha-Birczyńska, A.; Nocuń, M.; Ginalska, G.; Błażewicz, M. Fabrication of multi-walled carbon nanotube layers with selected properties via electrophoretic deposition: Physicochemical and biological characterization. Appl. Phys. A 2016, 122, 447. [Google Scholar] [CrossRef]
- Gonzalez, V.J.; Vega-Diaz, S.M.; Morelos-Gomez, A.; Fujisawa, K.; Endo, M.; Martin Cadiz, O.; Llido, J.B.; Terrones, M. H2O2/UV layer-by-layer oxidation of multiwall carbon nanotubes: The “Onion Effect” and the control of the degree of surface crystallinity and diameter. Carbon 2018, 139, 1027–1034. [Google Scholar] [CrossRef]
- Kolanowska, A.; Wąsik, P.; Zięba, W.; Terzyk, A.P.; Boncel, S. Selective carboxylation versus layer-by-layer unsheathing of multi-walled carbon nanotubes: New insights from the reaction with boiling nitrating mixture. RSC Adv. 2019, 9, 37608–37613. [Google Scholar] [CrossRef]
- Oytun, F.; Dizman, C.; Karatepe, N.; Alpturka, O.; Basarir, F. Preparation of transparent conducting electrode on polysulfone film viamultilayer transfer of layer-by-layer assembled carbon nanotubes. Thin Solid Films 2017, 625, 168–176. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Contr. Rel. 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Jiang, J.K.; Oberdorster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Bryk, P.; Korczeniewski, E.; Kowalczyk, P.; Zawadzka, A.; Płóciennik, P.; Wiśniewski, M.; Wesołowski, R.P. Water nanodroplet on a hydrocarbon “carpet”—The mechanism of water contact angle stabilization by airborne contaminations on graphene, Au, and PTFE surfaces. Langmuir 2019, 35, 420–427. [Google Scholar] [CrossRef]
- Corbett, J.C.W.; McNeil-Watson, F.; Jack, R.O.; Howarth, M. Measuring surface zeta potential using phase analysis light scattering in a simple dip cell arrangement. Coll. Surf. A Physicochem. Eng. Asp. 2012, 396, 169–176. [Google Scholar] [CrossRef]
- Furmaniak, S.; Terzyk, A.P.; Gauden, P.A.; Harris, P.J.F.; Wiśniewski, M.; Kowalczyk, P. Simple model of adsorption on external surface of carbon nanotubes- a new analytical approach basing on molecular simulation data. Adsorption 2010, 16, 197–213. [Google Scholar] [CrossRef][Green Version]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Hamaker, H.C. Formation of deposition by electrophoresis. Trans. Farad. Soc. 1940, 36, 279–283. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Progr. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Aria, A.I.; Kidambi, P.R.; Weatherup, R.S.; Xiao, L.; Williams, J.A.; Hofmann, S. Time Evolution of the Wettability of Supported Graphene under Ambient Air Exposure. J. Phys. Chem. C 2016, 120, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans Farad. Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Badaire, S.; Poulin, P.; Maugey, M.; Zakri, C. In Situ Measurements of Nanotube Dimensions in Suspensions by Depolarized Dynamic Light Scattering. Langmuir 2004, 20, 10367–10370. [Google Scholar] [CrossRef]



| Materials before deposition | ||||||
|---|---|---|---|---|---|---|
| Oxidation time (min) | 10 | 15 | 30 | 75 | 90 | |
| No. of walls (n) | 12.5 ± 1.4 | 12.2 ± 1.8 | 11.3 ± 1.8 | 8.8 ± 1.2 | 8.1 ± 1.2 | |
| Diameter (nm) | 18.9 | 14.1 | 15.4 | 11.1 | 10.1 | |
| COOH content (mmol/g) | 2.8 | 4.0 | 2.7 | 4.2 | 2.7 | |
| BET (m2/g) | 180 | 169 | 137 | 149 | 55 | |
| ζ–potential (mV) | 35.66 ± 2.65 | −14.76 ± 5.23 | −12.46 ± 5.15 | 29.28 ± 6.76 | 36.06 ± 6.21 | |
| DLS diameter (nm) | 2170 ± 450 | 267.3 ± 8.7 | 1012 ± 58 | 263 ± 19 | 371 ± 26 | |
| Materials after deposition | ||||||
| Oxidation time (min) | 10 | 15 | 30 | 75 | 90 | Initial CF |
| Surface ζ-potential (mV) | 0.31 ± 0.42 | −0.07 ± 0.33 | −0.38 ± 1.13 | −0.64 ± 0.9 | 0.39 ± 0.97 | 0.32 ± 0.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korczeniewski, E.; Zięba, M.; Zięba, W.; Kolanowska, A.; Bolibok, P.; Kowalczyk, P.; Wiertel-Pochopień, A.; Zawała, J.; Boncel, S.; Terzyk, A.P. Electrophoretic Deposition of Layer-by-Layer Unsheathed Carbon Nanotubes—A Step Towards Steerable Surface Roughness and Wettability. Materials 2020, 13, 595. https://doi.org/10.3390/ma13030595
Korczeniewski E, Zięba M, Zięba W, Kolanowska A, Bolibok P, Kowalczyk P, Wiertel-Pochopień A, Zawała J, Boncel S, Terzyk AP. Electrophoretic Deposition of Layer-by-Layer Unsheathed Carbon Nanotubes—A Step Towards Steerable Surface Roughness and Wettability. Materials. 2020; 13(3):595. https://doi.org/10.3390/ma13030595
Chicago/Turabian StyleKorczeniewski, Emil, Monika Zięba, Wojciech Zięba, Anna Kolanowska, Paulina Bolibok, Piotr Kowalczyk, Agata Wiertel-Pochopień, Jan Zawała, Sławomir Boncel, and Artur P. Terzyk. 2020. "Electrophoretic Deposition of Layer-by-Layer Unsheathed Carbon Nanotubes—A Step Towards Steerable Surface Roughness and Wettability" Materials 13, no. 3: 595. https://doi.org/10.3390/ma13030595
APA StyleKorczeniewski, E., Zięba, M., Zięba, W., Kolanowska, A., Bolibok, P., Kowalczyk, P., Wiertel-Pochopień, A., Zawała, J., Boncel, S., & Terzyk, A. P. (2020). Electrophoretic Deposition of Layer-by-Layer Unsheathed Carbon Nanotubes—A Step Towards Steerable Surface Roughness and Wettability. Materials, 13(3), 595. https://doi.org/10.3390/ma13030595







