Formation of Cu2O Solid Solution via High-Frequency Electromagnetic Field-Assisted Ball Milling: The Reaction Mechanism
Abstract
:1. Introduction
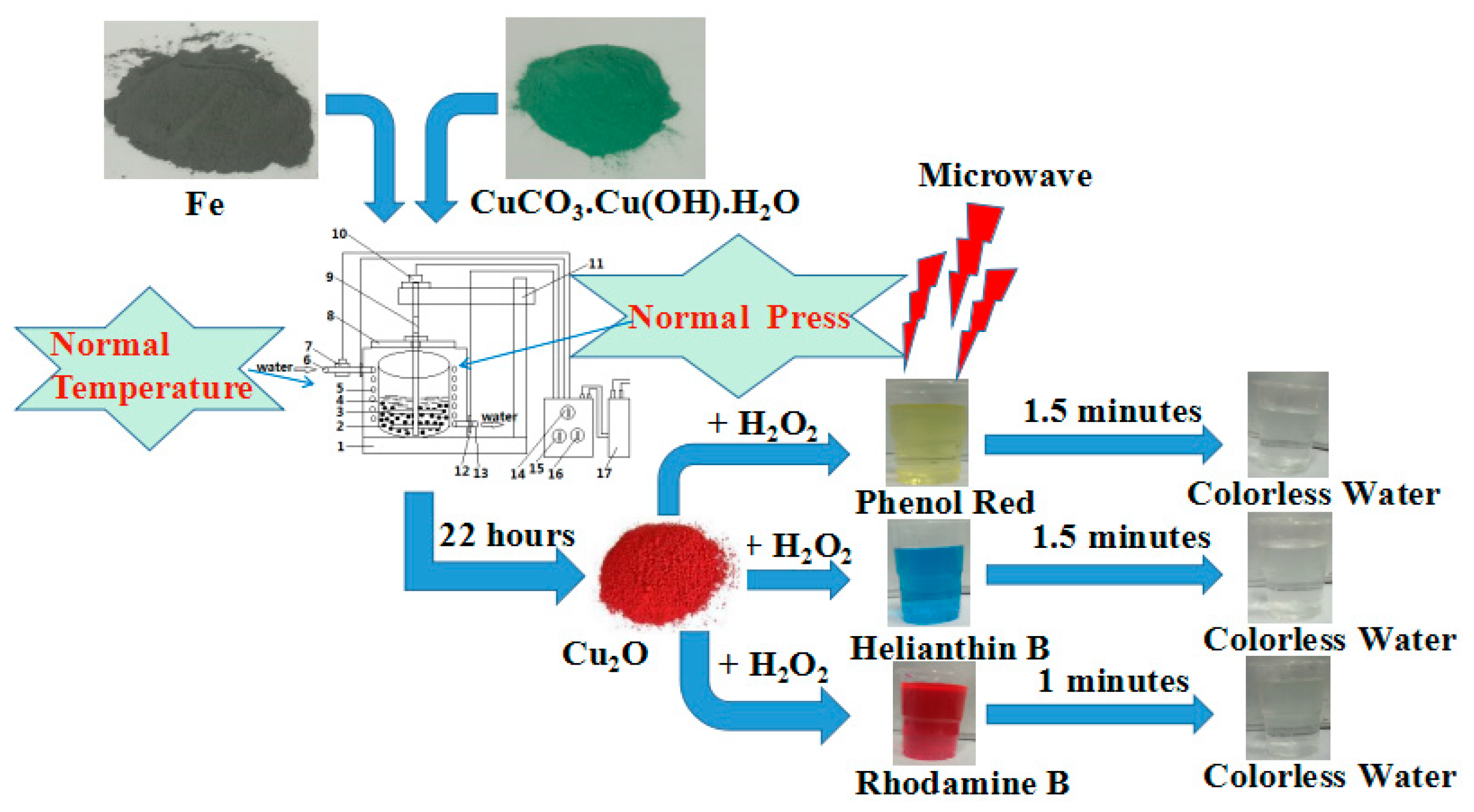
2. Experimental
3. Results and Discussion
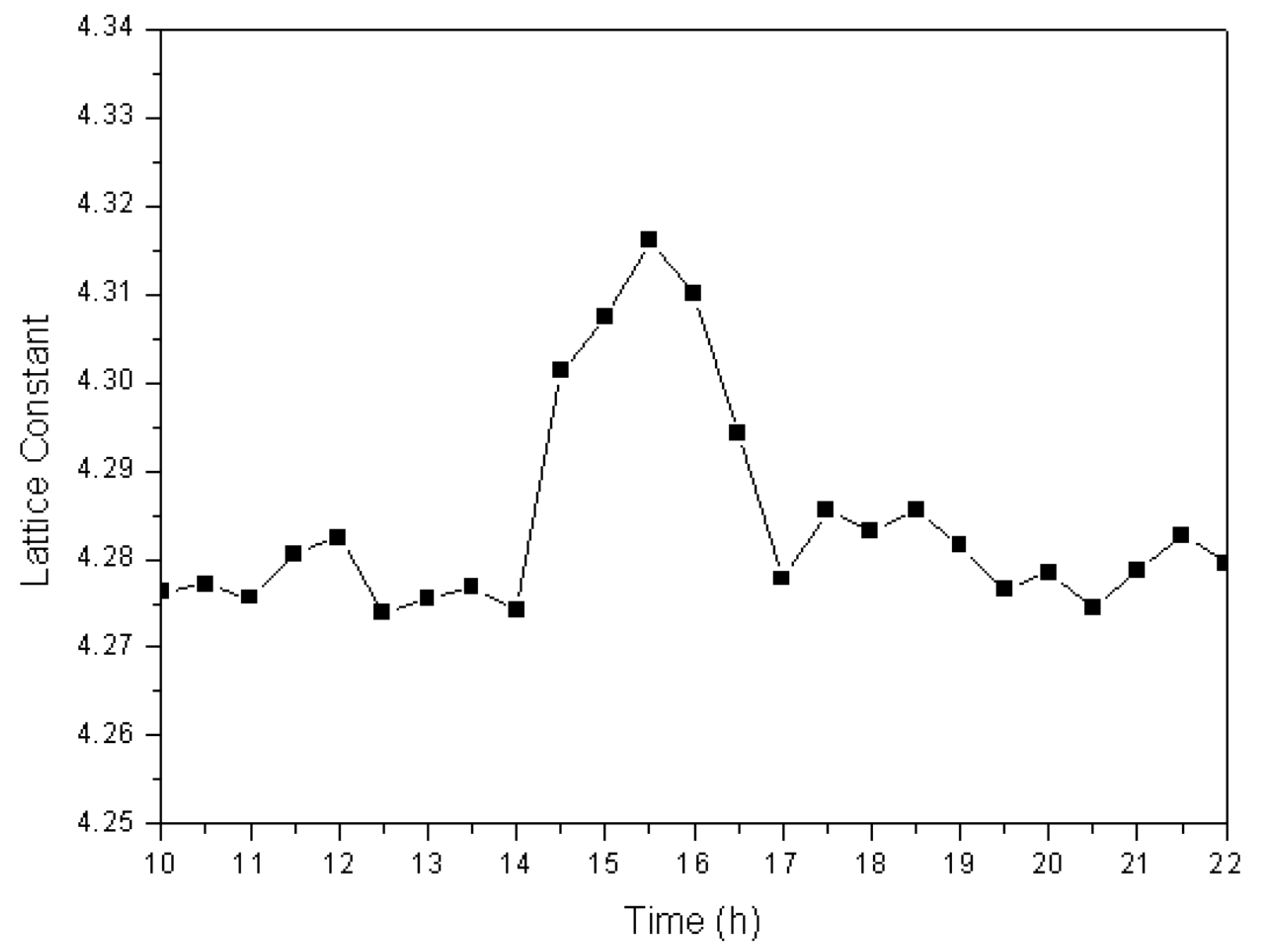
3.1. Phase Transformation
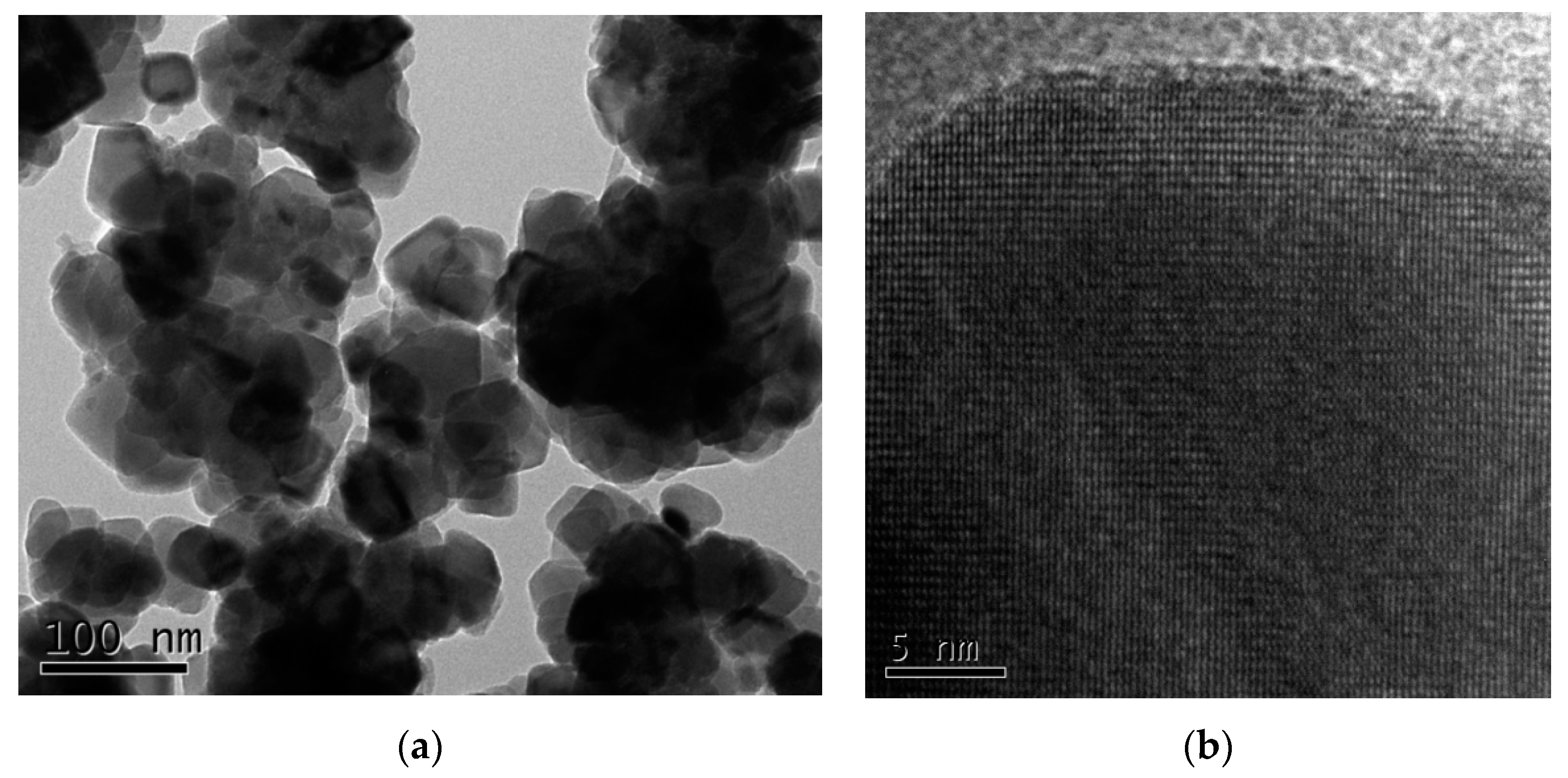
3.2. Product Microstructures and Morphologies
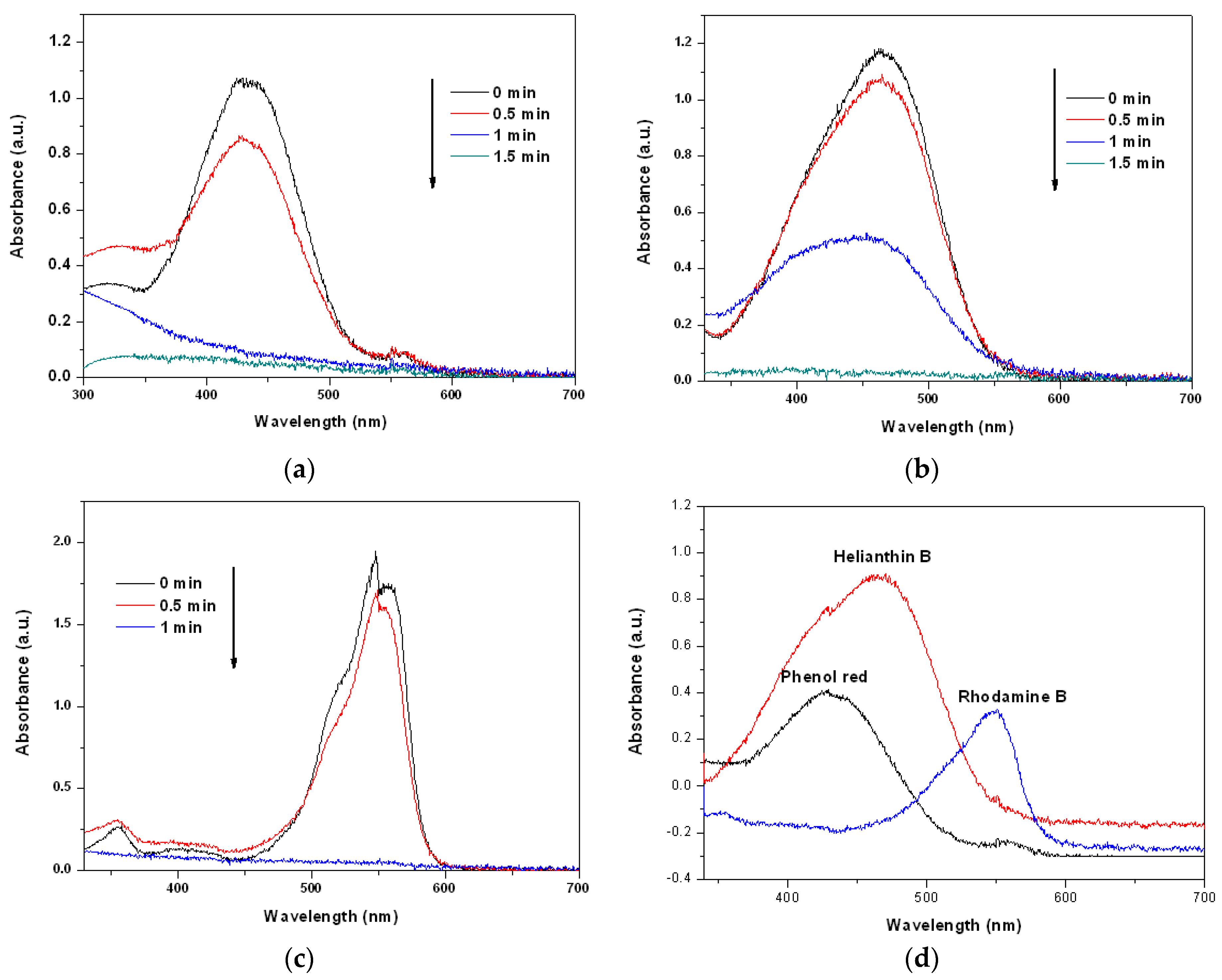
3.3. Catalytic Performance
3.4. Formation Mechanism and Reaction Kinetics
3.4.1. Solid-State Formation of Cuprous Oxide
3.4.2. Reaction Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jun, B.M.; Heo, J.; Taheri, Q.N. Adsorption of selected dyes on Ti3C2Tx MXene and Al-based metal-organic framework. Ceram. Int. 2020, 46, 2960–2968. [Google Scholar] [CrossRef]
- Jun, B.M.; Yoon, Y.; Park, C.M. Post-treatment of nanofiltration polyamide membrane through alkali-catalyzed hydrolysis to treat dyes in model wastewater. Water 2019, 11, 1645. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Ai, S.; Liang, Z. Preparation and photocatalytic properties of zinc oxide nanoparticles by microwave-assisted ball milling. Ceram. Int. 2016, 42, 3692–3696. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2018, 17, 1–19. [Google Scholar] [CrossRef]
- Jing, F.; Liang, R.; Xiong, J.; Chen, R.; Zhang, S.; Li, Y.; Wu, L. MIL-68 (Fe) as an efficient visible-light-driven photocatalyst for the treatment of a simulated waste-water contain Cr (VI) and Malachite Green. Appl. Catal. B Environ. 2017, 206, 9–15. [Google Scholar] [CrossRef]
- Xu, D.; Lai, X.; Guo, W.; Dai, P. Microwave-assisted catalytic degradation of methyl orange in aqueous solution by ferrihydrite/maghemite nanoparticles. J. Water Process Eng. 2017, 16, 270–276. [Google Scholar] [CrossRef]
- Li, H.; Jiang, P.; Zhang, W.; Chen, S.; Li, F. Hydrothermal Synthesis of BiVO4@ Cu2O Core–Shell n–p Heterojunction for Enhanced Visible-Light Photocatalytic Performance. Nanosci. Nanotechnol. Lett. 2018, 10, 451–460. [Google Scholar] [CrossRef]
- Su, Y.; Ma, H.; Nathan, A. LED-Assisted Degradation of Aromatic Organics Using Cu2O Photocatalysts. MRS Adv. 2017, 2, 3377–3381. [Google Scholar] [CrossRef] [Green Version]
- Kang, L.; Zhou, M.; Zhou, H.; Zhang, F.; Zhong, Z.; Xing, W. Controlled synthesis of Cu2O microcrystals in membrane dispersion reactor and comparative activity in heterogeneous Fenton application. Powder Technol. 2019, 343, 847–854. [Google Scholar] [CrossRef]
- Yu, X.; Kou, S.; Zhang, J.; Tang, X.; Yang, Q.; Yao, B. Preparation and characterization of Cu2O nano-particles and their photocatalytic degradation of fluroxypyr. Environ. Technol. 2018, 39, 2967–2976. [Google Scholar] [CrossRef]
- Ho, W.; Tay, Q.; Qi, H.; Huang, Z.; Li, J.; Chen, Z. Photocatalytic and adsorption performances of faceted cuprous oxide (Cu2O) particles for the removal of methyl orange (MO) from aqueous media. Molecules 2017, 22, 677. [Google Scholar] [CrossRef]
- Liu, S.H.; Wei, Y.S.; Lu, J.S. Visible-light-driven photodegradation of sulfamethoxazole and methylene blue by Cu2O/rGO photocatalysts. Chemosphere 2016, 154, 118–123. [Google Scholar] [CrossRef]
- Rostami, H.; Rostami, A.A.; Omrani, A. An electrochemical method to prepare of Pd/Cu2O/MWCNT nanostructure as an anode electrocatalyst for alkaline direct ethanol fuel cells. Electrochim. Acta 2016, 194, 431–440. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Z.; Zhou, Q.; Lu, J.; Zheng, M. A Template-Free Microwave Synthesis of One-Dimensional Cu2O Nanowires with Desired Photocatalytic Property. Materials 2018, 11, 1843. [Google Scholar] [CrossRef] [Green Version]
- Musza, K.; Szabados, M.; Ádám, A.A.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Ball Milling of Copper Powder Under Dry and Surfactant-Assisted Conditions—On the Way Towards Cu/Cu2O Nanocatalyst. J. Nanosci. Nanotechnol. 2019, 19, 389–394. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, D.; Wei, F.; Chen, N.; Liang, Z.; Luo, Y. Synthesis of graphene oxide/metal–organic frameworks hybrid materials for enhanced removal of Methylene blue in acidic and alkaline solutions. J. Chem. Technol. Biotechnol. 2018, 93, 698–709. [Google Scholar] [CrossRef]
- Ouyang, L.; Cao, Z.; Wang, H.; Hu, R.; Zhu, M. Application of dielectric barrier discharge plasma-assisted milling in energy storage materials–A review. J. Alloys Compd. 2017, 691, 422–435. [Google Scholar] [CrossRef]
- Bhosale, M.A.; Bhanage, B.M. A simple approach for sonochemical synthesis of Cu2O nanoparticles with high catalytic properties. Adv. Powder Technol. 2016, 27, 238–244. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Chen, D.; Qin, Q.; Wu, Y.; Huang, F.; Li, W. Preparation of FeOOH/Cu with High Catalytic Activity for Degradation of Organic Dyes. Materials 2019, 12, 338. [Google Scholar] [CrossRef] [Green Version]
- Shkodich, N.F.; Vadchenko, S.G.; Nepapushev, A.A.; Kovalev, D.Y.; Kovalev, I.D.; Ruvimov, S.; Mukasyan, A.S. Crystallization of amorphous Cu50Ti50 alloy prepared by high-energy ball milling. J. Alloys Compd. 2018, 741, 575–579. [Google Scholar] [CrossRef]
- Yang, P.; Liu, Y.; Zhao, X.; Cheng, J.; Li, H. Electromagnetic wave absorption properties of FeCoNiCrAl0.8 high entropy alloy powders and its amorphous structure prepared by high-energy ball milling. J. Mater. Res. 2016, 31, 2398–2406. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Qin, Q.; Wang, F.; Wang, W.; Luo, Y. The synthesis of Cu/Fe/Fe3O4 catalyst through the aqueous solution ball milling method assisted by high-frequency electromagnetic field. Superlattices Microstruct. 2018, 118, 123–129. [Google Scholar]
- Chen, D.; Ni, S.; Fang, J.J. Preparation of Cu2O nanoparticles in cupric chloride solutions with a simple mechanochemical approach. J. Alloys Compd. 2010, 504, S345–S348. [Google Scholar] [CrossRef]
- De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Kuhnert, N. Microwave-assisted reactions in organic synthesis—Are there any nonthermal microwave effects? Angew. Chem. Int. Ed. 2002, 41, 1863–1866. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Qin, Q.; Wang, F.; Chen, D. A study of the mechanism of microwave-assisted ball milling preparing ZnFe2O4. J. Magn. Magn. Mater. 2016, 409, 6–9. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y. Synthesis of NiFe2O4 nanoparticles by a low temperature microwave-assisted ball milling technique. Sci. China Technol. Sci. 2012, 55, 1535–1538. [Google Scholar] [CrossRef]
- Xie, W.; Cheng, H.; Chu, Z.; Chen, Z.; Long, C. Effect of carbonization temperature on the structure and microwave absorbing properties of hollow carbon fibres. Ceram. Int. 2011, 37, 1947–1951. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, J.; Deng, L.; Ma, C.; Li, J.; Li, Y.; Yang, R. Targeted intracellular controlled drug delivery and tumor therapy through in situ forming Ag nanogates on mesoporous silica nanocontainers. ACS Appl. Mater. Interfaces 2015, 7, 11930–11938. [Google Scholar] [CrossRef]
- Sheikhzadeh, M.; Sanjabi, S. Structural characterization of stainless steel/TiC nanocomposites produced by high-energy ball-milling method at different milling times. Mater. Des. 2012, 39, 366–372. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Z.; Yang, X.; Wu, Y.; Jia, C.; Bo, T.; Wang, L. Surface Modification Mechanism of Stearic Acid to Zirconia Powders Induced by Ball Milling for Water-Based Injection Molding. J. Am. Ceram. Soc. 2011, 94, 1327–1330. [Google Scholar] [CrossRef]
- Lei, Y.; Lin, X.; Liao, H. New insights on microwave induced rapid degradation of methyl orange based on the joint reaction with acceleration effect between electron hopping and Fe2+-H2O2 reaction of NiFeMnO4 nanocomposites. Sep. Purif. Technol. 2018, 192, 220–229. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Chen, Y.D.; Qin, Q.D. Synthesis of FeCoNiCuZn single-phase high-entropy alloy by high-frequency electromagnetic-field assisted ball milling. J. Magn. Magn. Mater. 2020, 498, 166151. [Google Scholar]
- Zhang, Y.Z.; Kang, Z.T.; Chen, D. Synthesis and microwave absorbing properties of Mn–Zn nanoferrite produced by microwave assisted ball milling. J. Mater. Sci. Mater. Electron. 2014, 25, 4246–4251. [Google Scholar] [CrossRef]
- Ward, C.R.; French, D. Determination of glass content and estimation of glass composition in fly ash using quantitative X-ray diffraction. Fuel 2006, 85, 2268–2277. [Google Scholar] [CrossRef]
- Uvarov, V. The influence of X-ray diffraction pattern angular range on Rietveld refinement results used for quantitative analysis, crystallite size calculation and unit-cell parameter refinement. J. Appl. Crystallogr. 2019, 52, 252–261. [Google Scholar] [CrossRef]
- Ward, C.R. Quantitaive X-ray powder diffraction analysis of clay minerals in Australian coals using Rietveld methods. Appl. Clay Sci. 2002, 21, 227–240. [Google Scholar]
- Ward, C.R.; Taylor, J.C.; Matulis, C.E. Quantification of mineral matter in the Argonne Premium Coals using interactive Rietveld-based X-ray diffraction. Int. J. Coal Geol. 2001, 46, 67–82. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-state kinetic models: basics and mathematical fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef]
- Tanaka, H. Thermal analysis and kinetics of solid state reactions. Thermochim. Acta 1995, 267, 29–44. [Google Scholar] [CrossRef]



| Model | F(a) = kt | K | R2 | Reaction Time |
|---|---|---|---|---|
| One-dimensional diffusion | a2 = kt | 0.04336 | 0.94 | 23.06 h |
| Two-dimensional diffusion | (1 − a) × ln(1 − a) + a = kt | 0.03329 | 0.91 | 30.04 h |
| Three-dimensional diffusion (Jander) | (1 − (1 − a)1/3))2 = kt | 0.01217 | 0.85 | 80.53 h |
| Three-dimensional diffusion (Ginstling–Brounshtein) | 1 − 2/3 × a − (1 − a)2/3 = kt | 0.00874 | 0.89 | 38.13 h |
| Phase boundary (planar) | a = kt | 0.03928 | 0.95 | 25.46 h |
| Phase boundary (cylindrical) | 1 − (1 − a)1/2 = kt | 0.03037 | 0.94 | 32.89 h |
| Phase boundary (spherical) | 1 − (1 − a)1/3 = kt | 0.02355 | 0.93 | 42.04 h |
| Nucleation and growth (Avrami) | ((−ln(1 − a))1/2 = kt | 0.05202 | 0.94 | 71.45 h |
| Nucleation and growth (Erofeev) | ((−ln(1 − a))1/3 = kt | 0.03588 | 0.94 | 66.88 h |
| Nucleation and growth (Avrami–Erofeev) | [−ln(1 − a)]1/m = kt, 0.5 ≤ m ≤ 4 | 0.02842 | 0.93 | 67.84 h |
| 1-D nucleation and constant growth rate | ln a = kt | 0.07575 | 0.88 | −1.32 × 10−5 h |
| Random nucleation and rapid growth | −ln(1 − a) = kt | 0.10478 | 0.89 | 131.85 h |
| Chemical reaction (C1.5) | (1 − a)−1/2 − 1 = kt | 0.08924 | 0.81 | −11.21 h |
| Chemical reaction (C2) | 1/(1 − a) − 1 = kt | 0.316 | 0.71 | 3,164,553.80 h |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, Y.; Li, J.; Li, W.; Chen, D.; Qin, Q. Formation of Cu2O Solid Solution via High-Frequency Electromagnetic Field-Assisted Ball Milling: The Reaction Mechanism. Materials 2020, 13, 618. https://doi.org/10.3390/ma13030618
Zhang Y, Chen Y, Li J, Li W, Chen D, Qin Q. Formation of Cu2O Solid Solution via High-Frequency Electromagnetic Field-Assisted Ball Milling: The Reaction Mechanism. Materials. 2020; 13(3):618. https://doi.org/10.3390/ma13030618
Chicago/Turabian StyleZhang, Yingzhe, Yudao Chen, Juan Li, Wei Li, Ding Chen, and Qingdong Qin. 2020. "Formation of Cu2O Solid Solution via High-Frequency Electromagnetic Field-Assisted Ball Milling: The Reaction Mechanism" Materials 13, no. 3: 618. https://doi.org/10.3390/ma13030618
APA StyleZhang, Y., Chen, Y., Li, J., Li, W., Chen, D., & Qin, Q. (2020). Formation of Cu2O Solid Solution via High-Frequency Electromagnetic Field-Assisted Ball Milling: The Reaction Mechanism. Materials, 13(3), 618. https://doi.org/10.3390/ma13030618






