Seleninic Acid Potassium Salts as Water-Soluble Biocatalysts with Enhanced Bioavailability
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Procedures and Analysis Data
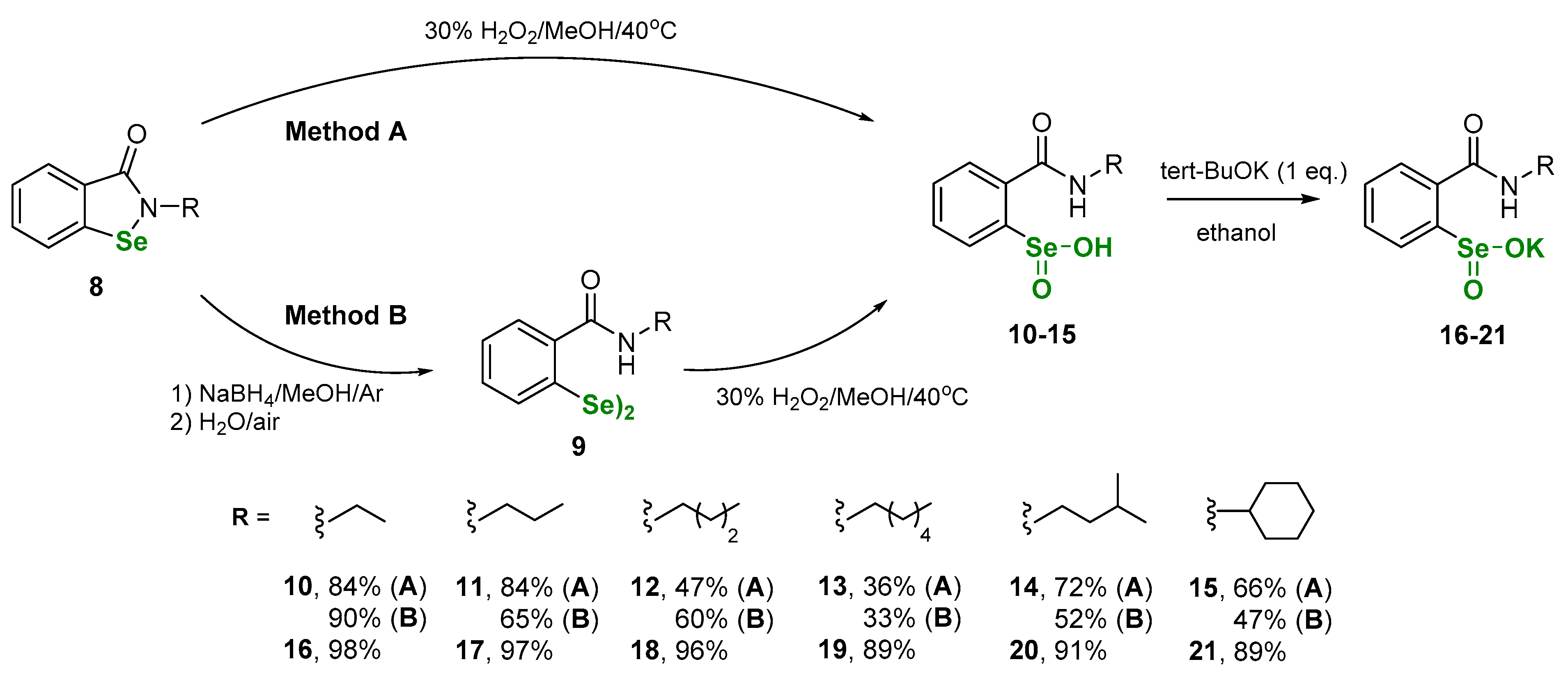
2.2.1. Synthesis of 2-(N-alkylcarboxyamido)benzeneselenenic acids 10–15
2.2.2. Synthesis of 2-(N-alkylcarboxyamido)benzeneselenenic acid potassium salts 16–21
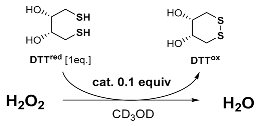
2.3. Antioxidant Activity Assay
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barbosa, N.V.; Nogueira, C.W.; Nogara, P.A.; de Bem, A.F.; Aschnerc, M.; Rocha, J.B.T. Organoselenium compounds as mimics of selenoproteins and thiol modifier agents. Metallomics 2017, 9, 1703–1734. [Google Scholar] [CrossRef] [PubMed]
- Orian, L.; Toppo, S. Organochalcogen peroxidase mimetics as potential drugs: a long story of a promise still unfulfilled. Free Rad. Biol. Med. 2014, 66, 65–74. [Google Scholar]
- Weekley, C.M.; Harris, H.H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef] [PubMed]
- Ungati, H.; Govindaraj, V.; Narayanan, M.; Mugesh, G. Probing the formation of a Seleninic Acid in Living Cells by a Fluorescence Switching of a Glutathione Peroxidase Mimetic. Angew. Chem. Int. Ed. 2019, 58, 8156–8160. [Google Scholar] [CrossRef] [Green Version]
- Pacuła, A.J.; Mangiavacchi, F.; Sancineto, L.; Lenardao, E.J.; Ścianowski, J.; Santi, C. An Update on “Selenium Containing Compounds from Poison to Drug Candidates: A Review on the GPx-like Activity”. Curr. Chem. Biol. 2015, 9, 97–112. [Google Scholar] [CrossRef]
- Candrini, G.; Malavasi, W.; Preti, C.; Tosi, G.; Zannini, P. Substituted benzeneseleninic acids as bidentate ligands. Synthesis and spectroscopic studies of manganese(H) and iron complexes. Spetrochim. Acta. 1983, 39, 635–639. [Google Scholar] [CrossRef]
- Graziosi, G.; Preti, C.; Tosi, G.; Zannini, P. Osmium (111) Halide Complexes with para- and meta Substituted Benzeneseleninato Ligands. Aust. J. Chem. 1985, 38, 1675–1684. [Google Scholar] [CrossRef]
- Yao, N.-T.; Zhang, R.-F.; Zhang, S.-L.; Li, Q.-L.; Ma, C.-L. A novel octa-nuclear 32-membered zirconocene macrocycle based on the aromatic selenite. Dalton Trans. 2017, 46, 524–528. [Google Scholar] [CrossRef]
- Ugandhar, U.; Baskar, V. Monoorganoantimony(V) Phosphonates and PhosphoSelininates. Dalton Trans. 2016, 45, 6269–6274. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, R.-F.; Zhang, S.-L.; Ru, J.; Du, J.-Y.; Ma, C.-L. Novel cobalt (II) coordination complexes based on 3,4,5-trifluorobenzeneseleninic acid and different N-donor ligands. Polyhedron 2018, 146, 172–179. [Google Scholar] [CrossRef]
- Syper, L.; Młochowski, J. Benzeneperoxyseleninic Acids - Synthesis and Properties. Tetrahedron 1987, 43, 207–213. [Google Scholar] [CrossRef]
- Hori, T.; Sharpless, K.B. Synthetic Applications of Arylselenenic and Arylseleninic Acids. Conversion of Olefins to Allylic Alcohols and Epoxides. J. Org. Chem. 1978, 43, 1689–1694. [Google Scholar] [CrossRef]
- Taylor, T.R.; Flood, L.A. Polystyrene-Bound Phenylseleninic Acid: Catalytic Oxidations of Olefins, Ketones, and Aromatic Systems. J. Org. Chem. 1983, 48, 5160–5164. [Google Scholar] [CrossRef]
- Back, G.T.; Collins, S.; Kerr, R.G. Oxidation of Hydrazines with Benzeneseleninic Acid and Anhydride. J. Org. Chem. 1981, 46, 1564–1570. [Google Scholar] [CrossRef]
- Teskey, Ch.J.; Adler, P.; Gonzalves, C.; Maulide, N. Chemoselective α,β-Dehydrogenation of Saturated Amides. Angew.Chem. Int. Ed. 2019, 58, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Yuan, D.; Yua, L. Green and Practical Oxidative Deoximation of Oximes to Ketones or Aldehydes with Hydrogen Peroxide/Air by Organoselenium Catalysis. Adv. Synth. Catal. 2017, 359, 1194–1201. [Google Scholar] [CrossRef]
- Sarma, B.K.; Mugesh, G. Antioxidant Activity of the Anti-Inflmmatory Compound Ebselen: A Reversible Cyclization Pathway via Selenenic and Seleninic Acid Intermediates. Chem. Eur. J. 2008, 14, 10603–10614. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Mugesh, G. Amide-Based Glutathione Peroxidase Mimics: Effect of Secondary and Tertiary Amide Substituents on Antioxidant Activity. Chem. Asian J. 2009, 4, 974–983. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Vernekar, A.A.; Jakka, S.R.; Roy, G.; Mugesh, G. Mechanistic investigations on the efficient catalytic decomposition of peroxynitrite by ebselen analogues. Org. Biomol. Chem. 2011, 9, 5193–5200. [Google Scholar] [CrossRef]
- Du, P.; Viswanathan, U.M.; Xu, Z.; Ebrahimnejad, H.; Hanf, B.; Burkholz, T.; Schneider, M.; Bernhardt, I.; Kirsch, G.; Jacob, C. Synthesis of amphiphilic seleninic acid derivatives with considerable activity against cellular membranes and certain pathogenic microbes. J. Haz. Mat. 2014, 269, 74–82. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Ścianowski, J.; Aleksandrzak, K.B. Highly efficient synthesis and antioxidant capacity of N-substituted benzisoselenazol-3(2H)-ones. RSC Advances 2014, 4, 48959–48962. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Kaczor, K.B.; Wojtowicz, A.; Antosiewicz, J.; Janecka, A.; Długosz, A.; Janecki, T.; ´Scianowski, J. New glutathione peroxidase mimetics—Insights into antioxidant and cytotoxic activity. Bioorg. Med. Chem. 2017, 25, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Pacuła, A.J.; Kaczor, K.B.; Antosiewicz, J.; Janecka, A.; Długosz, A.; Janecki, T.; Wojtczak, A.; Ścianowski, J. New Chiral Ebselen Analogues with Antioxidant and Cytotoxic Potential. Molecules 2017, 22, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Pacuła, A.J.; Obieziurska, M.; Ścianowski, J.; Kaczor, K.B.; Antosiewicz, J. Synthesis of biologically active diaryl diselenides under water control. Arkivoc 2018, iii, 144–155. [Google Scholar]
- Kumakura, F.; Mishra, B.; Priyadarsini, K.I.; Iwaoka, M. A Water-Soluble Cyclic Selenide with Enhanced Glutathione Peroxidase-Like Catalytic Activities. Eur. J. Org. Chem. 2010, 3, 440–444. [Google Scholar] [CrossRef]
- Tidei, C.; Piroddi, M.; Galli, F.; Santi, C. Oxidation of thiols promoted by PhSeZnCl. Tetrahedron Lett. 2012, 53, 232–234. [Google Scholar] [CrossRef]



| Catalyst (0.1 equiv.) | Remaining DTTred (%) | ||||
|---|---|---|---|---|---|
| 3 min | 5 min | 15 min | 30 min | 60 min | |
| Ebselen, 7 | 84 | 75 | 64 | 58 | 52 |
| Benzseleninic acids | |||||
| 10 | 89 | 86 | 78 | 67 | 51 |
| 11 | 81 | 75 | 70 | 63 | 51 |
| 12 | 85 | 82 | 75 | 65 | 47 |
| 13 | 89 | 85 | 69 | 46 | 19 |
| 14 | 76 | 56 | 38 | 24 | 12 |
| 15 | 85 | 64 | 37 | 18 | 2 |
| Benzseleninic acid salts 16–21 | 0 | 0 | 0 | 0 | 0 |
| Catalyst (0.01 equiv.) | Remaining DTT (%) | t-Test | Mann-Whitney Test | ||||
|---|---|---|---|---|---|---|---|
| 3 min | 5 min | 15 min | 30 min | 60 min | p-value | p-value | |
| Ebselen, 7 | 97 | 96 | 95 | 94 | 92 | <0.001 | <0.015 |
| 16 | 24 | 11 | 0 | 0 | 0 | <0.005 | <0.015 |
| 17 | 76 | 45 | 21 | 14 | 11 | <0.005 | <0.015 |
| 18 | 71 | 63 | 26 | 17 | 14 | <0.01 | <0.015 |
| 19 | 81 | 63 | 17 | 0 | 0 | <0.001 | <0.015 |
| 20 | 59 | 16 | 0 | 0 | 0 | <0.005 | <0.015 |
| 21 | 78 | 18 | 3 | 0 | 0 | <0.001 | <0.015 |
| Catalyst. (0.0075 equiv.) | 16 | 17 | 18 | 19 | 20 | 21 | PhSeOOK | No Catalyst |
|---|---|---|---|---|---|---|---|---|
| DTTred (%) | 19 | 47 | 15 | 46 | 79 | 26 | 49 | 93 |
| Structure | MCF-7 IC50 (µM) | HL-60 IC50 (µM) | Structure | MCF-7 IC50 (µM) | HL-60 IC50 (µM) |
|---|---|---|---|---|---|
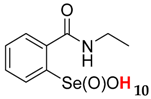 | 40.1 ± 1.2 | 11.7 ± 1.0 |  | 31.5 ± 1.2 | 56.8 ± 4.4 |
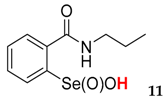 | 71.9 ± 4.1 | 23.5 ± 1.1 | 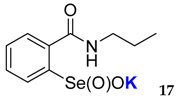 | 20.6 ± 2.5 | 63.1 ± 0.5 |
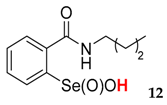 | 42.5 ± 0.8 | 62.1 ± 8.0 | 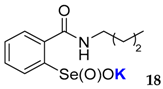 | 101.4 ± 2.3 | 92.6 ± 4.7 |
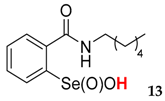 | 65.4 ± 0.9 | 113 ± 9.1 | 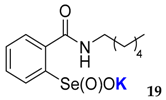 | 45 ± 0.2 | 200 ± 14.3 |
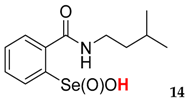 | 40.8 ± 4.5 | 51.5 ± 4.5 | 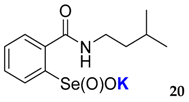 | 26.5 ± 3.5 | 72.5 ± 4.3 |
 | 45.9 ± 5.3 | 70.8 ± 1.3 | 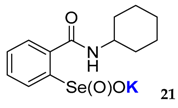 | 16.6 ± 1.1 | 42.1 ± 3.1 |
| Carboplatin | 3.80 ± 0.45 | 2.9 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obieziurska, M.; Pacuła, A.J.; Laskowska, A.; Długosz-Pokorska, A.; Janecka, A.; Ścianowski, J. Seleninic Acid Potassium Salts as Water-Soluble Biocatalysts with Enhanced Bioavailability. Materials 2020, 13, 661. https://doi.org/10.3390/ma13030661
Obieziurska M, Pacuła AJ, Laskowska A, Długosz-Pokorska A, Janecka A, Ścianowski J. Seleninic Acid Potassium Salts as Water-Soluble Biocatalysts with Enhanced Bioavailability. Materials. 2020; 13(3):661. https://doi.org/10.3390/ma13030661
Chicago/Turabian StyleObieziurska, Magdalena, Agata J. Pacuła, Anna Laskowska, Angelika Długosz-Pokorska, Anna Janecka, and Jacek Ścianowski. 2020. "Seleninic Acid Potassium Salts as Water-Soluble Biocatalysts with Enhanced Bioavailability" Materials 13, no. 3: 661. https://doi.org/10.3390/ma13030661







