A BODIPY-Based Fluorogenic Probe for Specific Imaging of Lipid Droplets
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Spectroscopy
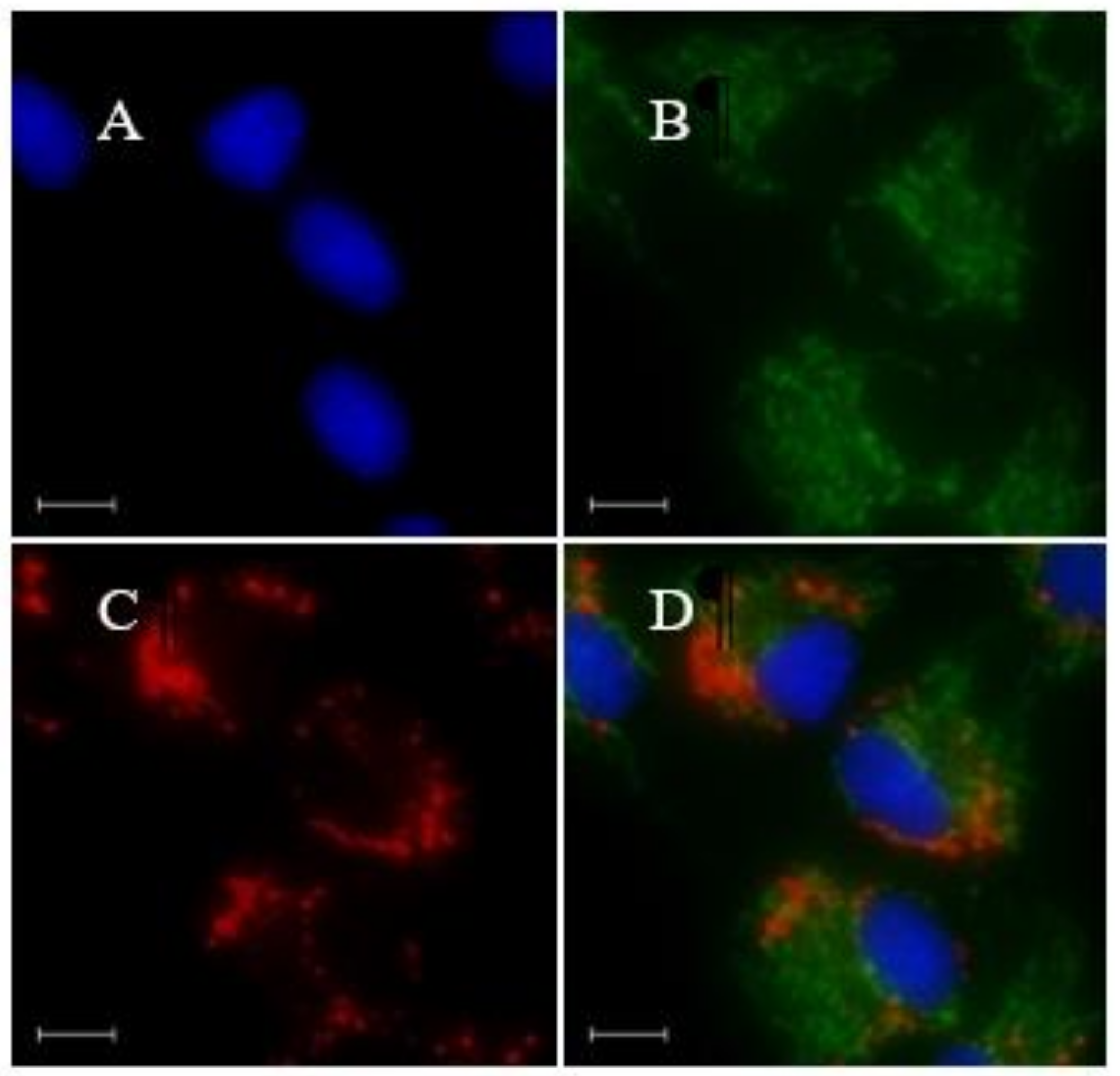
2.3. Cell Imaging
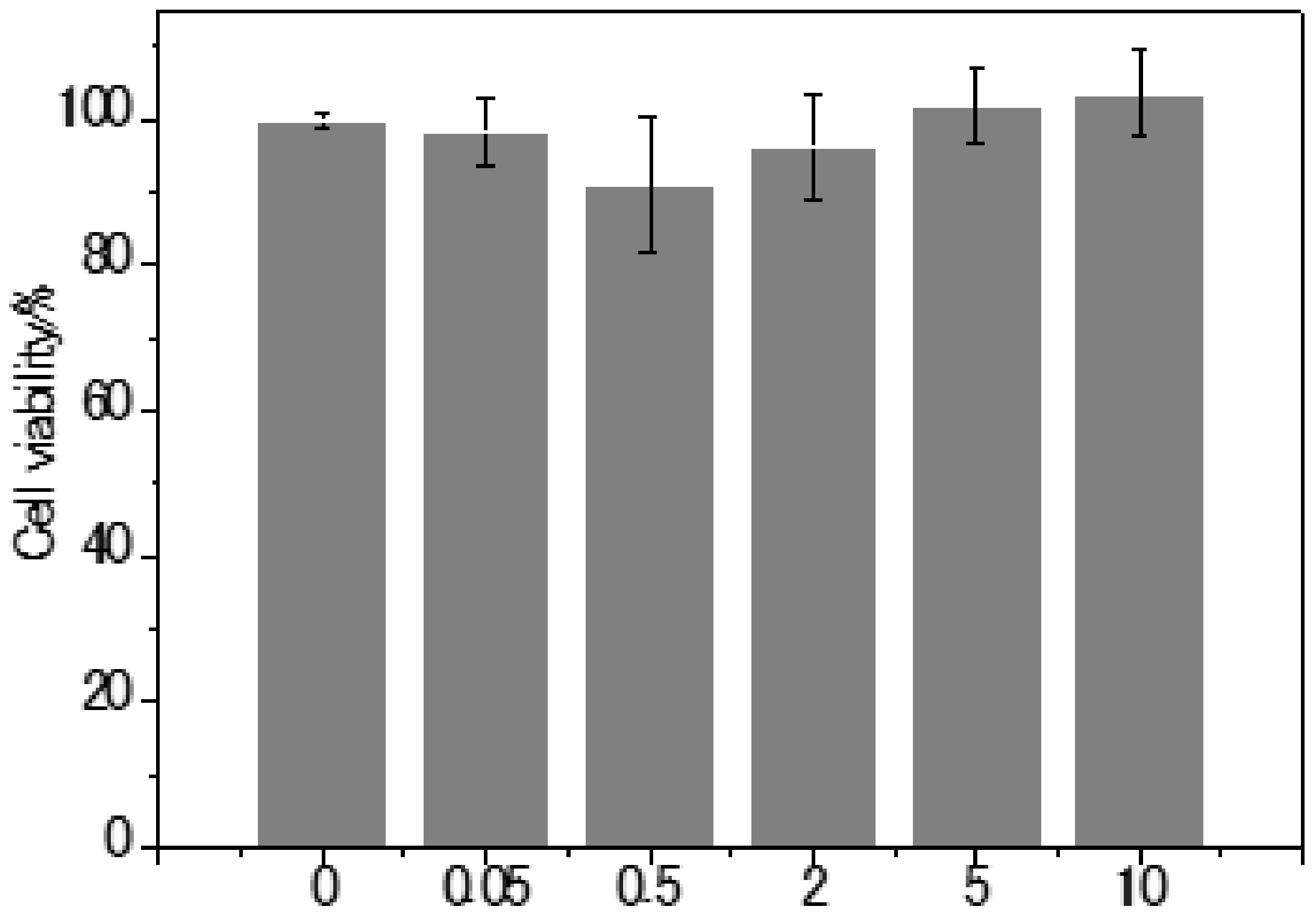
2.4. Cell Viability
2.5. Photostability
3. Results
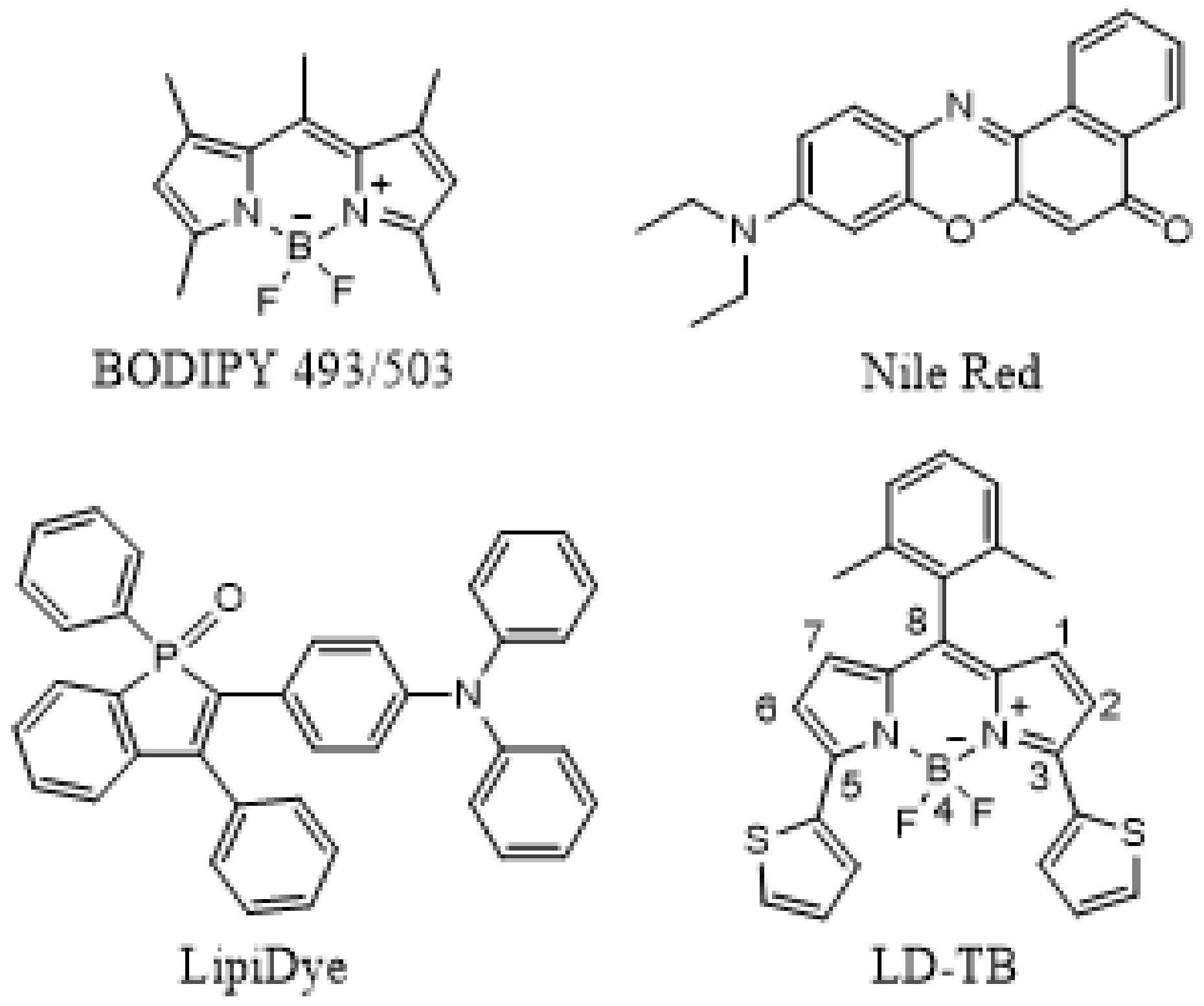
3.1. Synthesis and Photophysical Studies of LD-TB
3.2. LD-TB Stains Specifically Lipid Droplets in Cells
3.3. LD-TB is Not Cytotoxic to Live Cells
3.4. LD-TB Displays High Photostability
3.5. Multicolor Imaging of LD-TB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thiam, A.R.; Jr, R.V.F.; Walther, T.C.; Farese, R.V. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 2013, 14, 775–786. [Google Scholar]
- Murphy, D.J. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog. Lipid Res. 2001, 40, 325–438. [Google Scholar]
- Farese, R.V., Jr.; Walther, T.C. Lipid droplets finally get a little respect. Cell 2009, 139, 855–860. [Google Scholar]
- Martin, S.; Parton, R.G. Opinion: Lipid droplets: A unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 2006, 7, 373–378. [Google Scholar]
- Murphy, S.; Martin, S.; Parton, R.G. Lipid droplet-organelle interactions; sharing the fats. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2009, 1791, 441–447. [Google Scholar]
- Olzmann, J.A.; Richter, C.M.; Kopito, R.R. Spatial regulation of UBXD8 and p97/VCP controls ATGL-mediated lipid droplet turnover. Proc. Natl. Acad. Sci. USA 2013, 110, 1345–1350. [Google Scholar]
- Rambold, A.S.; Cohen, S.; Lippincott-Schwartz, J. Fatty acid trafficking in starved cells: Regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 2015, 32, 678–692. [Google Scholar]
- Fujimoto, T.; Ohsaki, Y.; Cheng, J.; Suzuki, M.; Shinohara, Y. Lipid droplets: A classic organelle with new outfits. Histochem. Cell Biol. 2008, 130, 263–279. [Google Scholar]
- Boren, J.; Brindle, K. Apoptosis-induced mitochondrial dysfunction causes cytoplasmic lipid droplet formation. Cell Death Differ. 2012, 19, 1561–1571. [Google Scholar]
- Greenberg, A.S.; Coleman, R.A.; Kraemer, F.B.; McManaman, J.L.; Obin, M.S.; Puri, V.; Yan, Q.-W.; Miyoshi, H.; Mashek, U.G. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Investig. 2011, 121, 2102–2110. [Google Scholar]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar]
- Nadler, A.; Schultz, C. The Power of Fluorogenic Probes. Angew. Chem. Int. Ed. 2013, 52, 2408–2410. [Google Scholar]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design Strategies for Water-Soluble Small Molecular Chromogenic and Fluorogenic Probes. Chem. Rev. 2013, 114, 590–659. [Google Scholar]
- Su, D.; Teoh, C.L.; Wang, L.; Liu, X.; Chang, Y.-T. Motion-induced change in emission (MICE) for developing fluorescent probes. Chem. Soc. Rev. 2017, 46, 4833–4844. [Google Scholar]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Accounts Chem. Res. 2017, 50, 366–375. [Google Scholar]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar]
- Listenberger, L.L.; Brown, D.A. Fluorescent Detection of Lipid Droplets and Associated Proteins. Curr. Protoc. Cell Biol. 2007, 35, 24.2.1–24.2.11. [Google Scholar]
- Gao, M.; Su, H.; Li, S.; Lin, Y.; Ling, X.; Qin, A.; Tang, B.Z. An easily accessible aggregation-induced emission probe for lipid droplet-specific imaging and movement tracking. Chem. Commun. 2017, 53, 921–924. [Google Scholar]
- Jiang, M.; Gu, X.; Lam, J.W.Y.; Zhang, Y.; Kwok, R.T.K.; Wong, K.S.; Tang, B.Z. Two-photon AIE bio-probe with large Stokes shift for specific imaging of lipid droplets† †Electronic supplementary information (ESI) available: Experimental section, NMR, mass and absorption, HOMO, LUMO, photophysical properties, cell viability, cell imaging, photostability, and two-photon excited fluorescence spectra of TPA-PI. Chem. Sci. 2017, 8, 5440–5446. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, T.; Liu, H.; Chen, Y.; Kwok, R.T.K.; Ma, C.; Zhang, P.; Sung, H.H.-Y.; Williams, I.D.; Lam, J.W.Y.; et al. Bright Near-Infrared Aggregation-Induced Emission Luminogens with Strong Two-Photon Absorption, Excellent Organelle Specificity, and Efficient Photodynamic Therapy Potential. ACS Nano 2018, 12, 8145–8159. [Google Scholar]
- Hu, R.; Chen, B.; Wang, Z.; Qin, A.; Zhao, Z.; Lou, X.; Tang, B.Z. Intriguing “chameleon” fluorescent bioprobes for the visualization of lipid droplet-lysosome interplay. Biomater. 2019, 203, 43–51. [Google Scholar]
- Collot, M.; Fam, T.-K.; AshokKumar, P.; Faklaris, O.; Galli, T.; Danglot, L.; Klymchenko, A.S. Ultrabright and Fluorogenic Probes for Multicolor Imaging and Tracking of Lipid Droplets in Cells and Tissues. J. Am. Chem. Soc. 2018, 140, 5401–5411. [Google Scholar]
- Sharma, A.; Jha, A.K.; Mishra, S.; Jain, A.; Chauhan, B.S.; Kathuria, M.; Rawat, K.S.; Gupta, N.M.; Tripathi, R.; Mitra, K.; et al. Imaging and Quantitative Detection of Lipid Droplets by Yellow Fluorescent Probes in Liver Sections of Plasmodium Infected Mice and Third Stage Human Cervical Cancer Tissues. Bioconj. Chem. 2018, 29, 3606–3613. [Google Scholar]
- Sharma, A.; Umar, S.; Kar, P.; Singh, K.; Sachdev, M.; Goel, A. A new type of biocompatible fluorescent probe AFN for fixed and live cell imaging of intracellular lipid droplets. Analyst 2016, 141, 137–143. [Google Scholar]
- Collot, M.; Bou, S.; Fam, T.K.; Richert, L.; Mély, Y.; Danglot, L.; Klymchenko, A.S. Probing Polarity and Heterogeneity of Lipid Droplets in Live Cells Using a Push–Pull Fluorophore. Anal. Chem. 2018, 91, 1928–1935. [Google Scholar]
- Ashoka, A.H.; AshokKumar, P.; Kovtun, Y.P.; Klymchenko, A.S. Solvatochromic Near-Infrared Probe for Polarity Mapping of Biomembranes and Lipid Droplets in Cells under Stress. J. Phys. Chem. Lett. 2019, 10, 2414–2421. [Google Scholar]
- Zhai, J.; Zhang, Y.; Yang, C.; Xu, Y.; Qin, Y. A long wavelength hydrophobic probe for intracellular lipid droplets. Analyst 2014, 139, 52–54. [Google Scholar]
- Tang, J.; Zhang, Y.; Yin, H.-Y.; Xu, G.; Zhang, J.-L. Precise Labeling and Tracking of Lipid Droplets in Adipocytes Using a Luminescent ZnSalen Complex. Chem. Asian J. 2017, 12, 2533–2538. [Google Scholar]
- O’Connor, D.; Byrne, A.; Dolan, C.; Keyes, T.E. Phase partitioning, solvent-switchable BODIPY probes for high contrast cellular imaging and FCS. N. J. Chem. 2018, 42, 3671–3682. [Google Scholar]
- Li, G.; Otsuka, Y.; Matsumiya, T.; Suzuki, T.; Li, J.; Takahashi, M.; Yamada, K. A Straightforward Substitution Strategy to Tune BODIPY Dyes Spanning the Near-Infrared Region via Suzuki–Miyaura Cross-Coupling. Materials 2018, 11, 1297. [Google Scholar]
- Yamada, K.; Toyota, T.; Takakura, K.; Ishimaru, M.; Sugawara, T. Preparation of BODIPY probes for multicolor fluorescence imaging studies of membrane dynamics. N. J. Chem. 2001, 25, 667–669. [Google Scholar]
- Ni, Y.; Wu, J. Far-red and near infrared bodipy dyes: Synthesis and applications for fluorescent ph probes and bio-imaging. Org. Biomol. Chem. 2014, 12, 3774–3791. [Google Scholar]
- Landrum, M.; Smertenko, A.; Edwards, R.; Hussey, P.J.; Steel, P.G. BODIPY probes to study peroxisome dynamics in vivo. Plant J. 2010, 62, 529–538. [Google Scholar]
- Zhu, S.; Zhang, J.; Vegesna, G.; Luo, F.-T.; Green, S.A.; Liu, H. Highly water-soluble neutral bodipy dyes with controllable fluorescence quantum yields. Org. Lett. 2010, 13, 438–441. [Google Scholar]
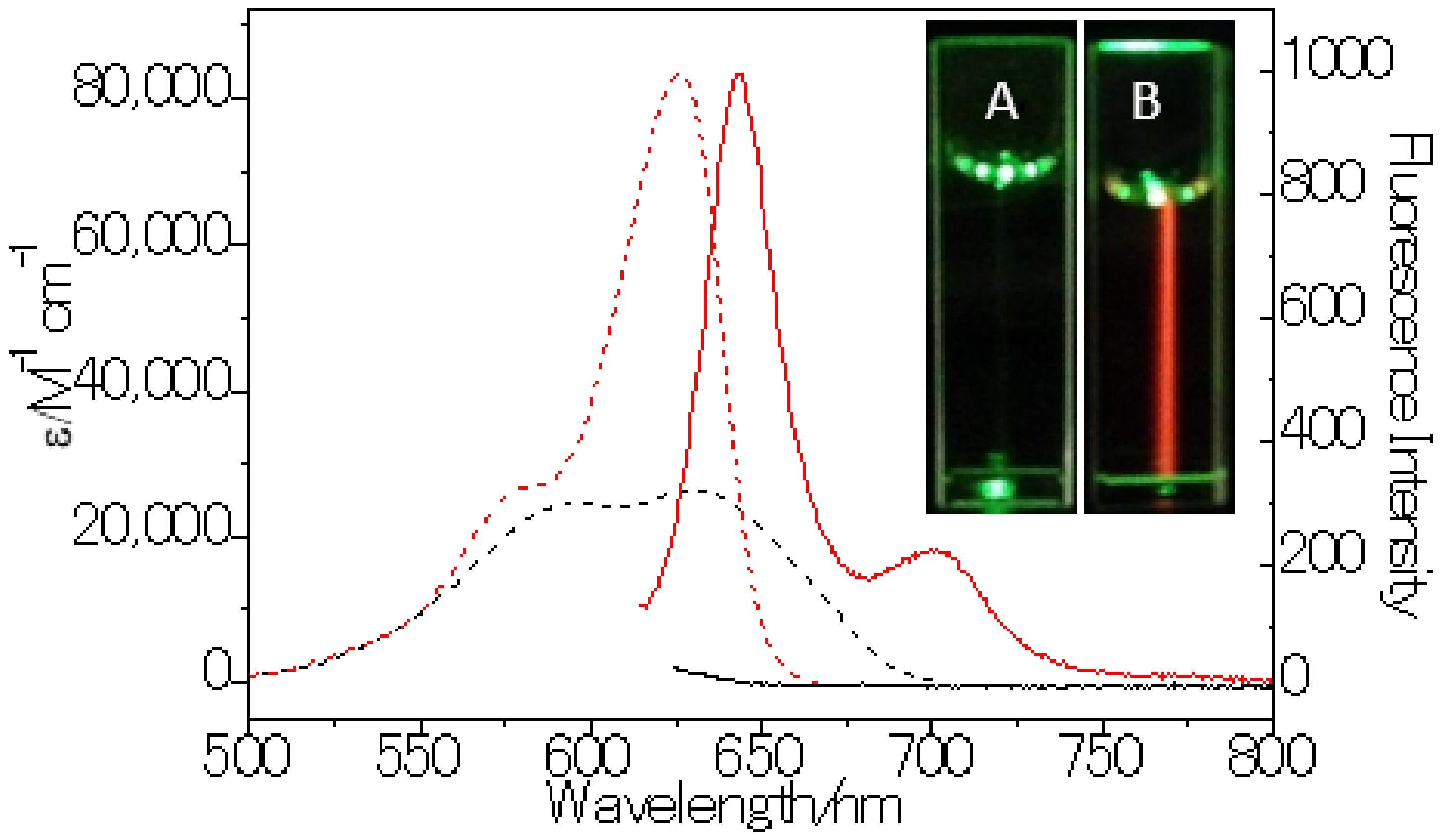


| Solvent | λabs /nm | ε /M−1 cm−1 | FWHMabs /nm | λem /nm | FWHMem /nm | Φ | Stokes shift/nm | Brightness (ε × Φ) |
|---|---|---|---|---|---|---|---|---|
| Cyclohexane | 621 | 83,000 | 50 | 638 | 23 | 0.84 | 17 | 69,720 |
| Toluene | 625 | 74,000 | 42 | 644 | 27 | 0.90 | 19 | 66,600 |
| Ethyl acetate | 617 | 73,000 | 59 | 638 | 27 | 0.85 | 21 | 62,050 |
| Acetone | 617 | 72,000 | 56 | 639 | 27 | 0.85 | 22 | 61,200 |
| DMSO | 628 | 69,000 | 44 | 650 | 29 | 0.84 | 22 | 57,960 |
| 0.5% DMSO/H2O | 630 | 26,000 | 105 | - | - | <0.01 | - | <260 |
| Sunflower oil | 626 | 84,000 | 38 | 643 | 26 | 0.85 | 17 | 71,400 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Li, J.; Otsuka, Y.; Zhang, S.; Takahashi, M.; Yamada, K. A BODIPY-Based Fluorogenic Probe for Specific Imaging of Lipid Droplets. Materials 2020, 13, 677. https://doi.org/10.3390/ma13030677
Li G, Li J, Otsuka Y, Zhang S, Takahashi M, Yamada K. A BODIPY-Based Fluorogenic Probe for Specific Imaging of Lipid Droplets. Materials. 2020; 13(3):677. https://doi.org/10.3390/ma13030677
Chicago/Turabian StyleLi, Guanglei, Jianye Li, Yu Otsuka, Shuai Zhang, Masashi Takahashi, and Koji Yamada. 2020. "A BODIPY-Based Fluorogenic Probe for Specific Imaging of Lipid Droplets" Materials 13, no. 3: 677. https://doi.org/10.3390/ma13030677
APA StyleLi, G., Li, J., Otsuka, Y., Zhang, S., Takahashi, M., & Yamada, K. (2020). A BODIPY-Based Fluorogenic Probe for Specific Imaging of Lipid Droplets. Materials, 13(3), 677. https://doi.org/10.3390/ma13030677




