On the Microstructure and Isothermal Oxidation at 800 and 1200 °C of the Nb-24Ti-18Si-5Al-5Cr-5Ge-5Sn (at.%) Silicide-Based Alloy
Abstract
1. Introduction
2. Experimental
3. Results
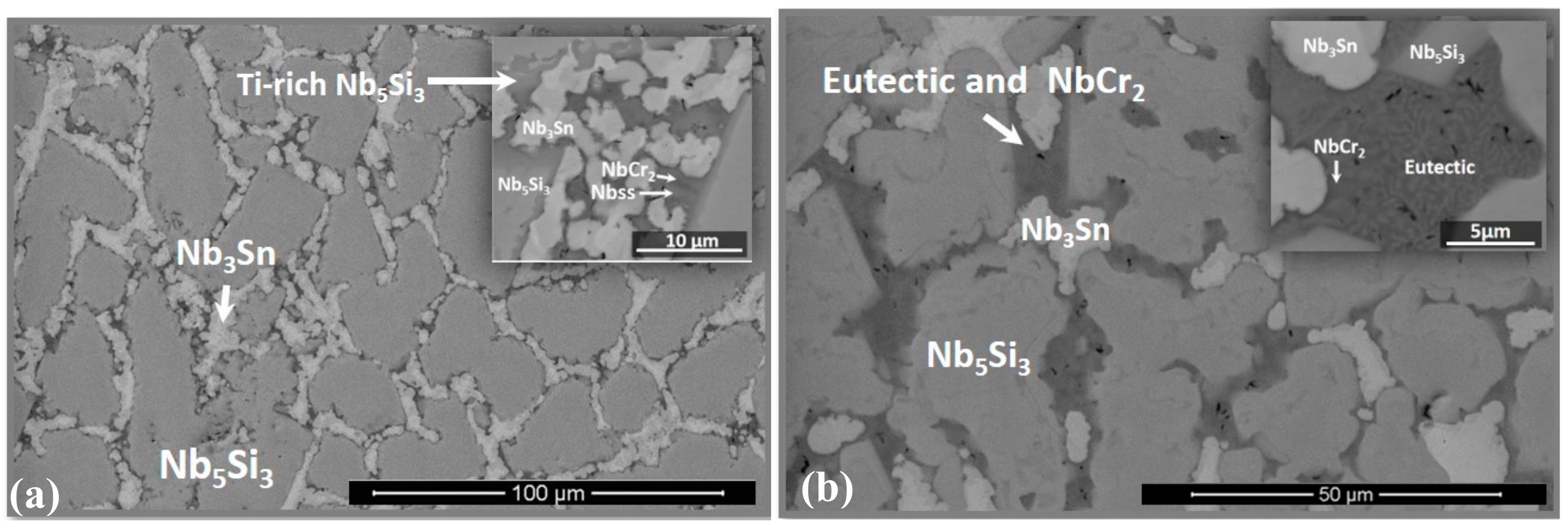
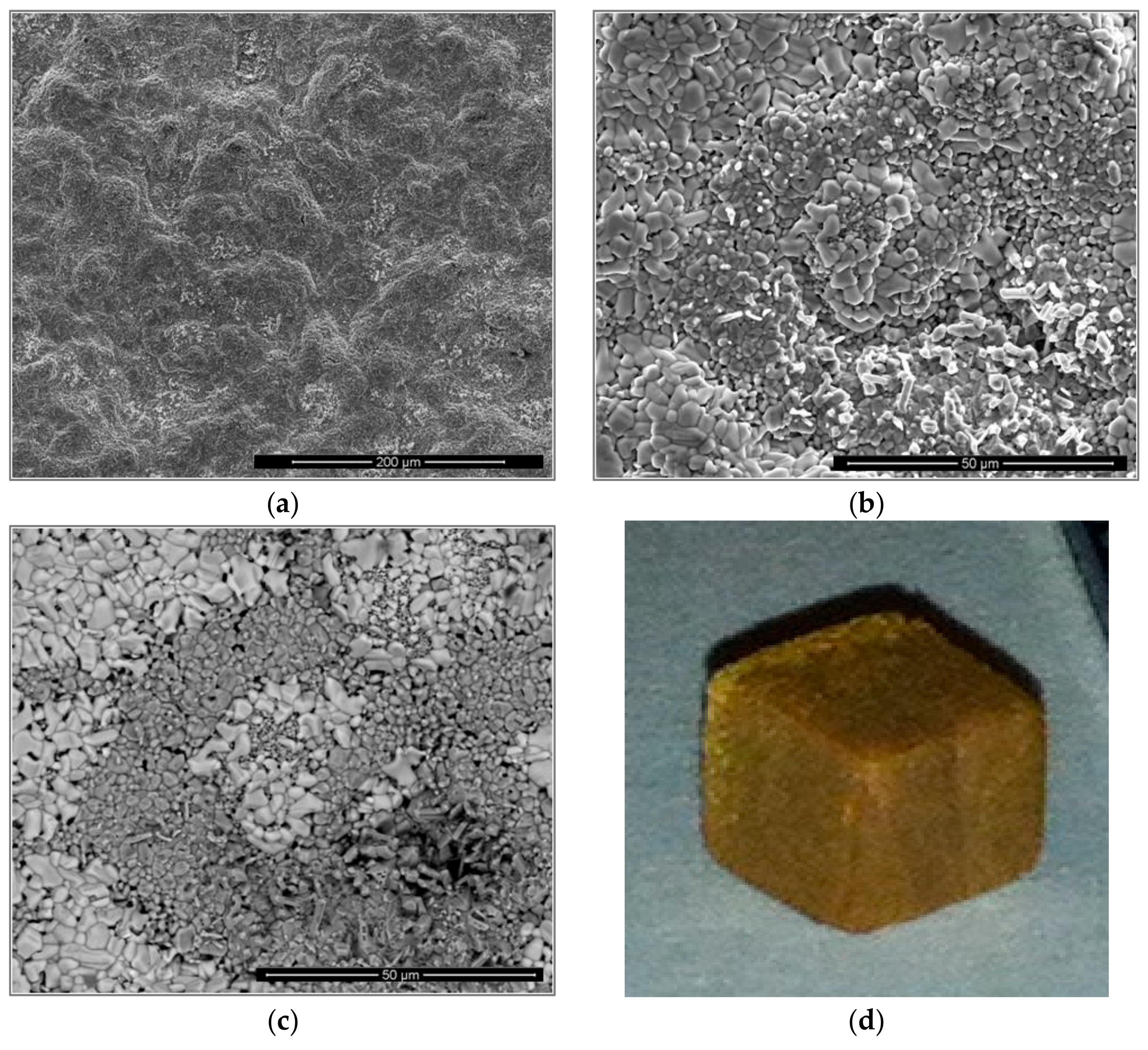
3.1. As-Cast Alloy
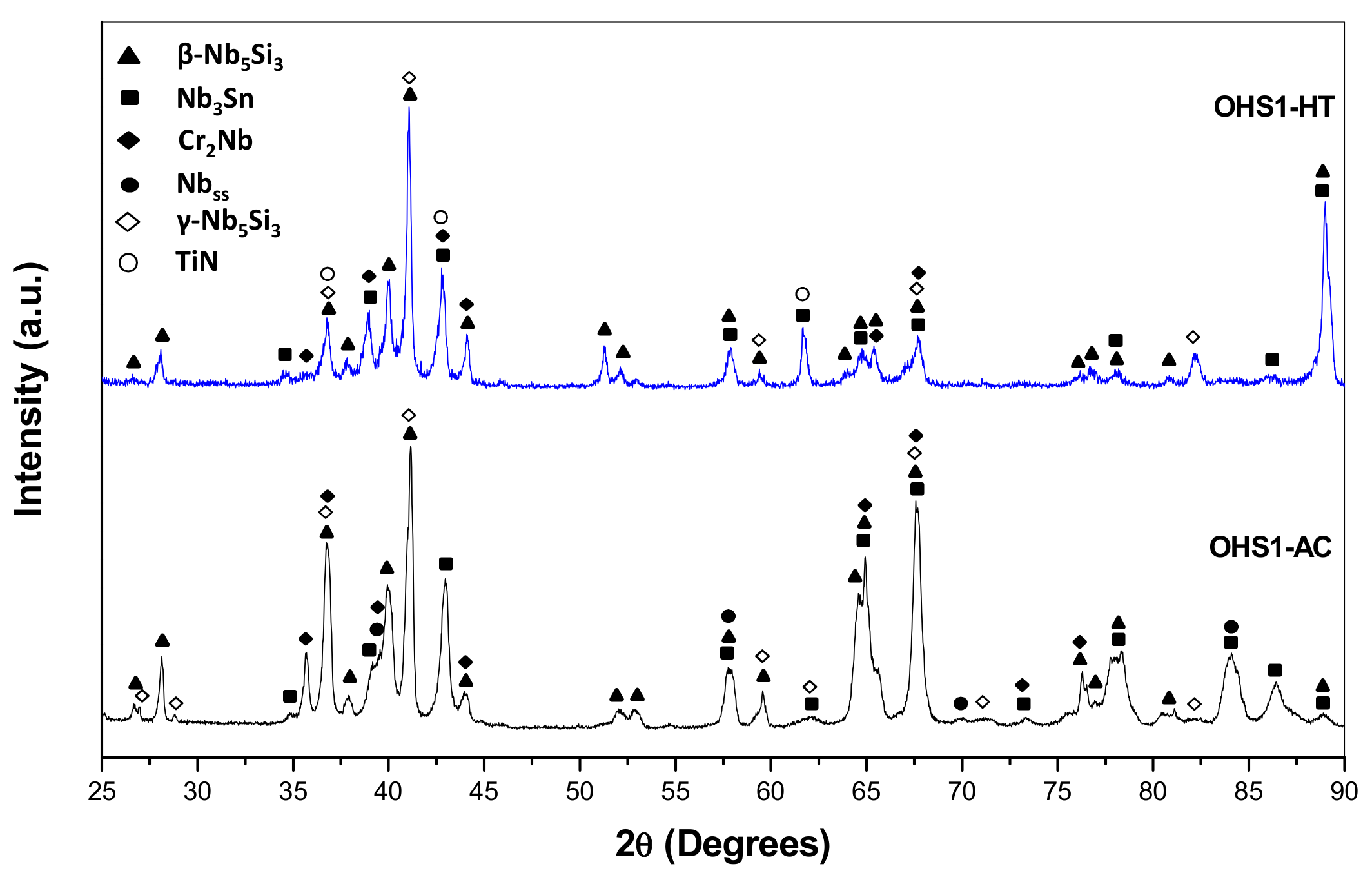
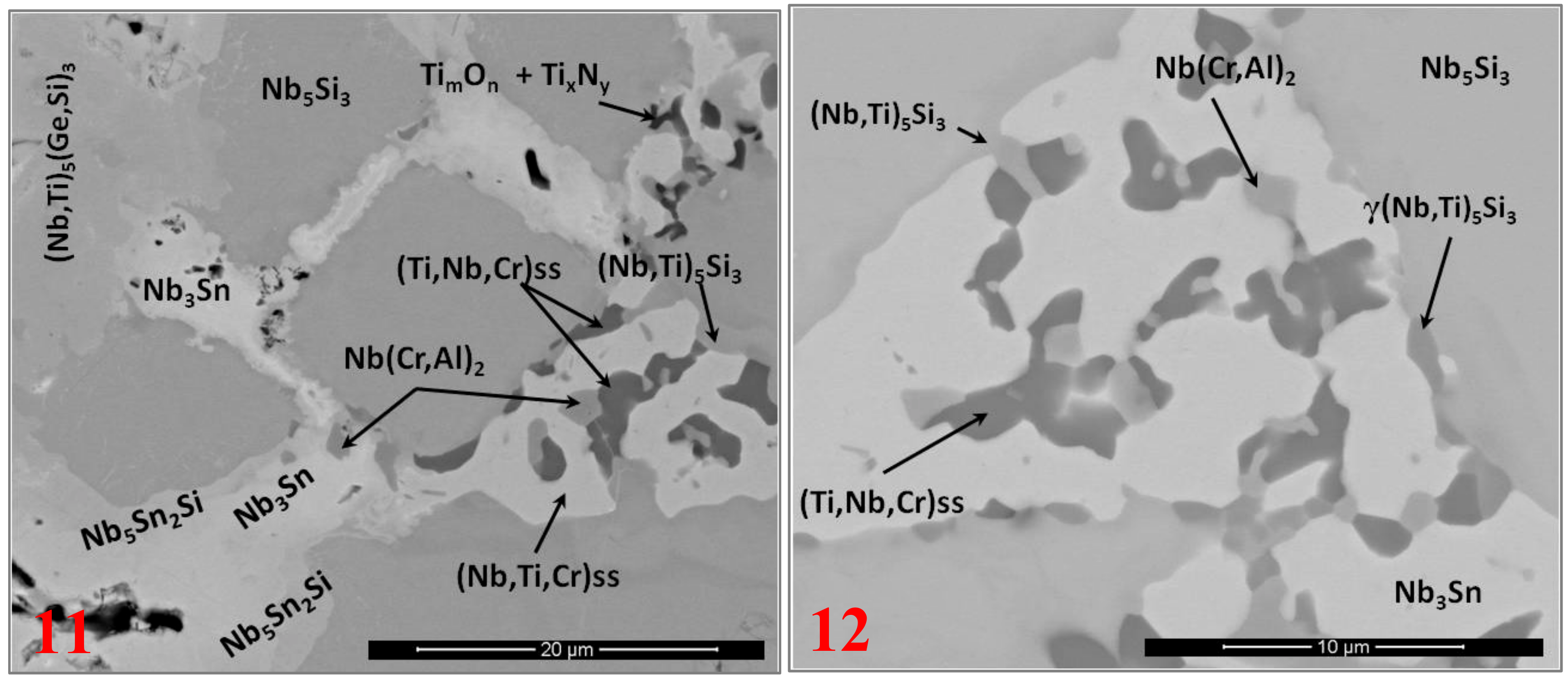
3.2. Heat-Treated Microstructure
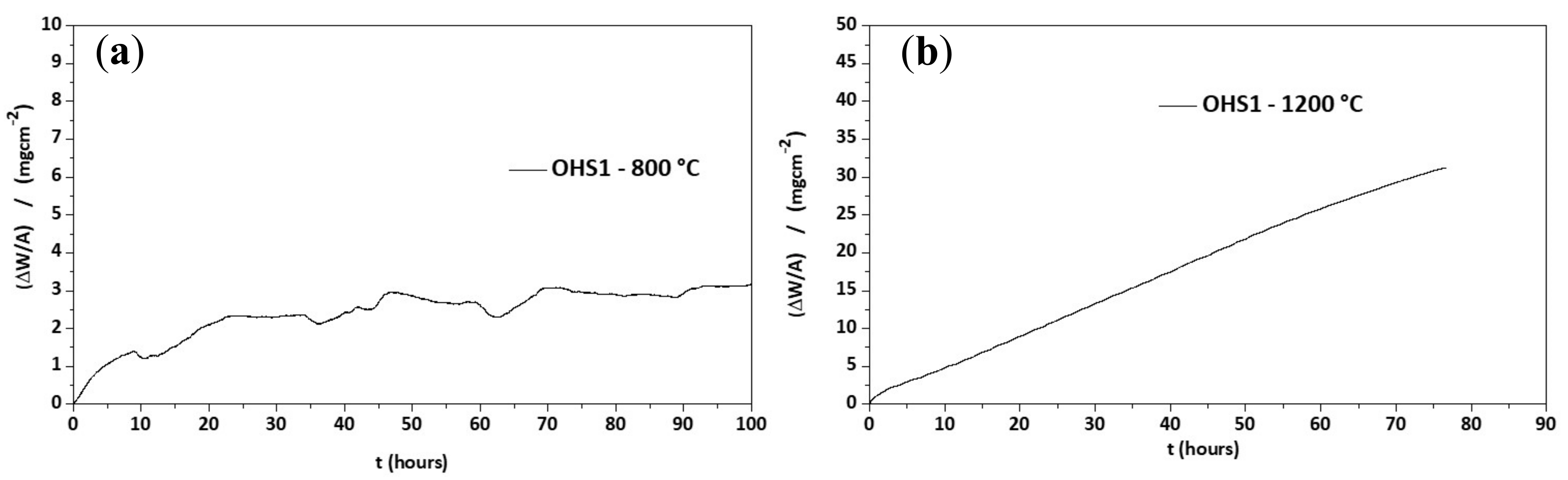
3.3. Oxidation
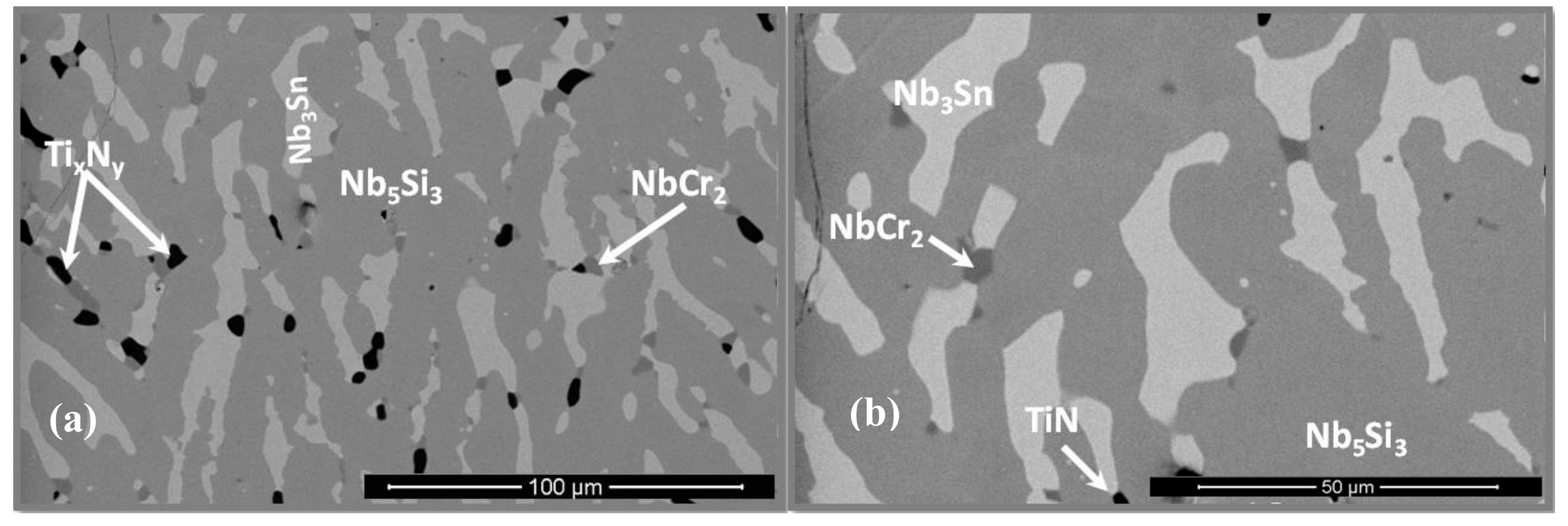
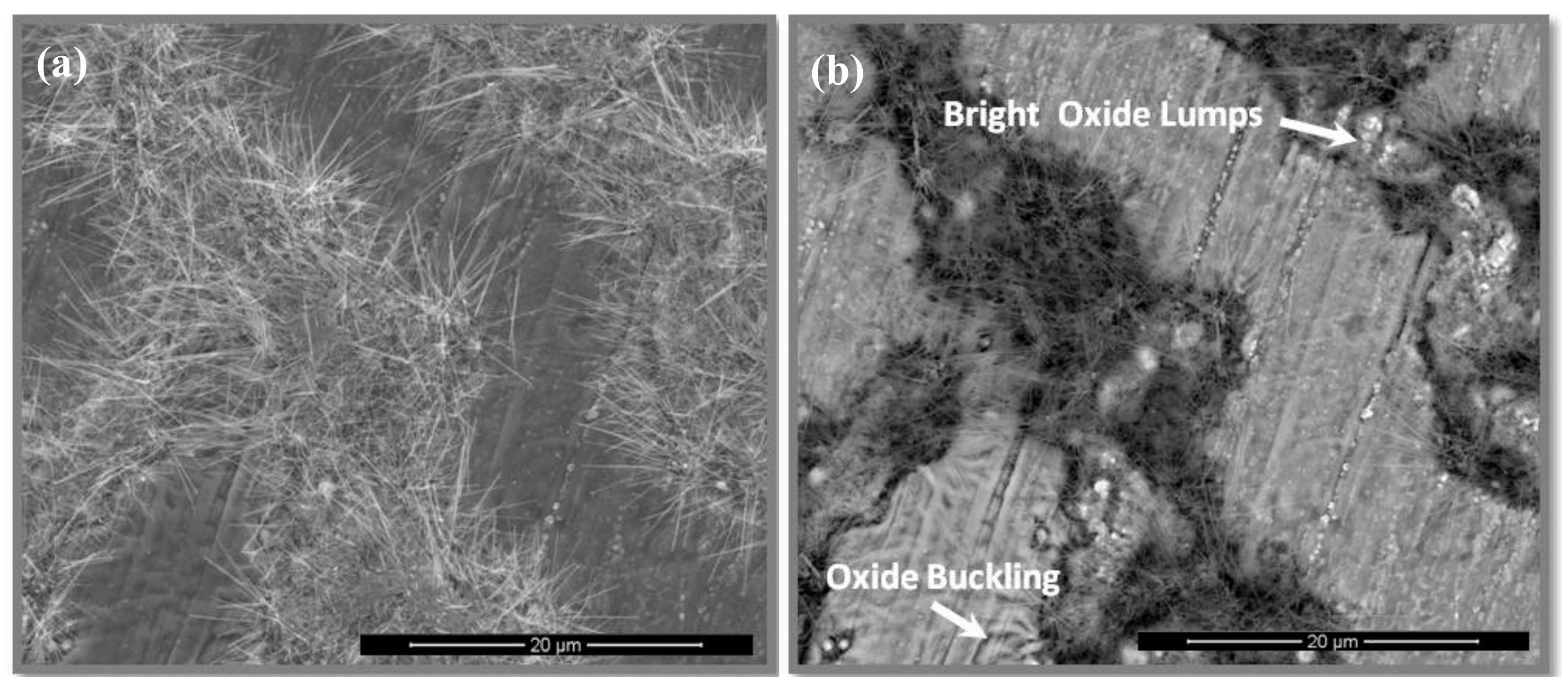
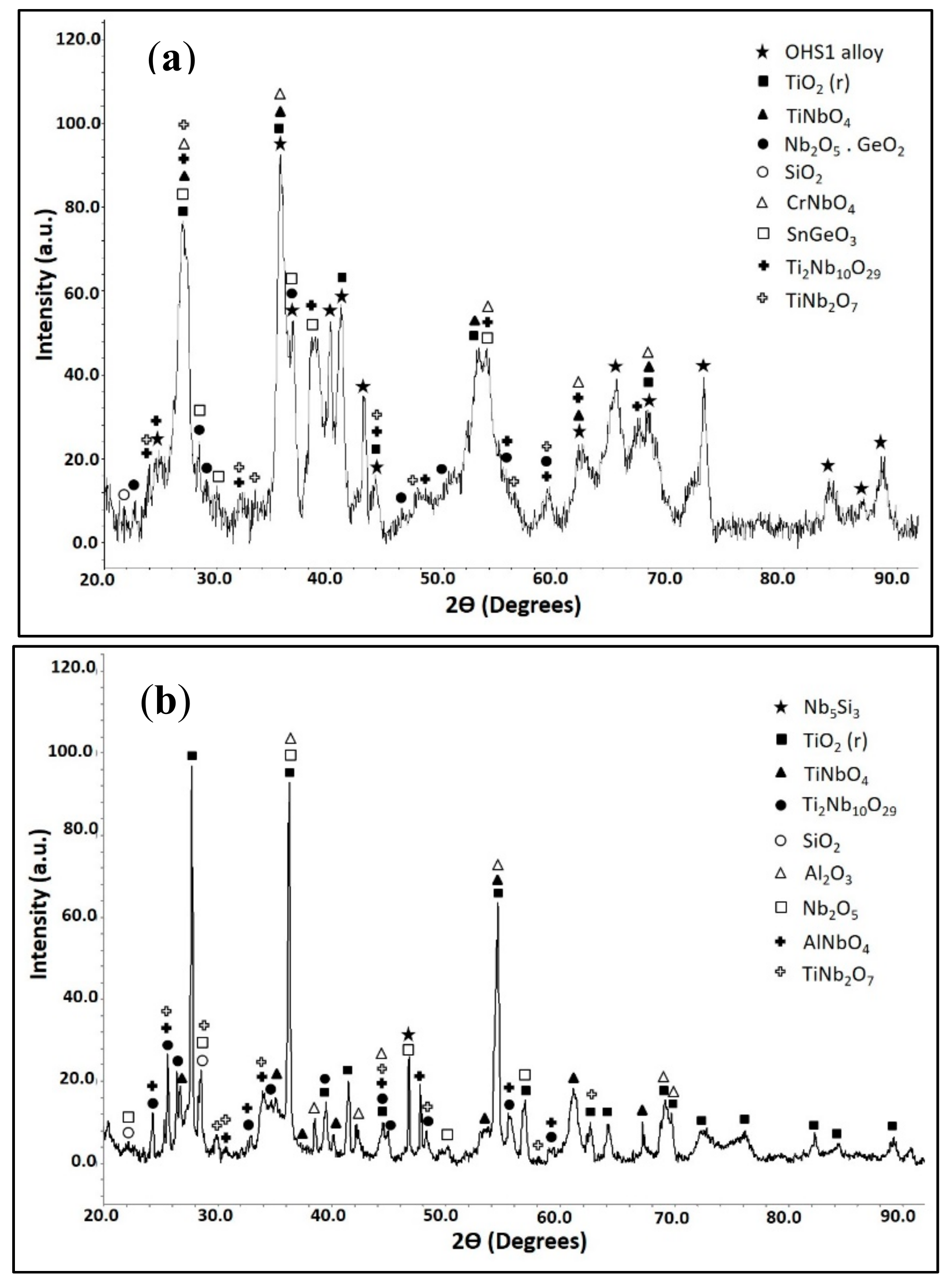
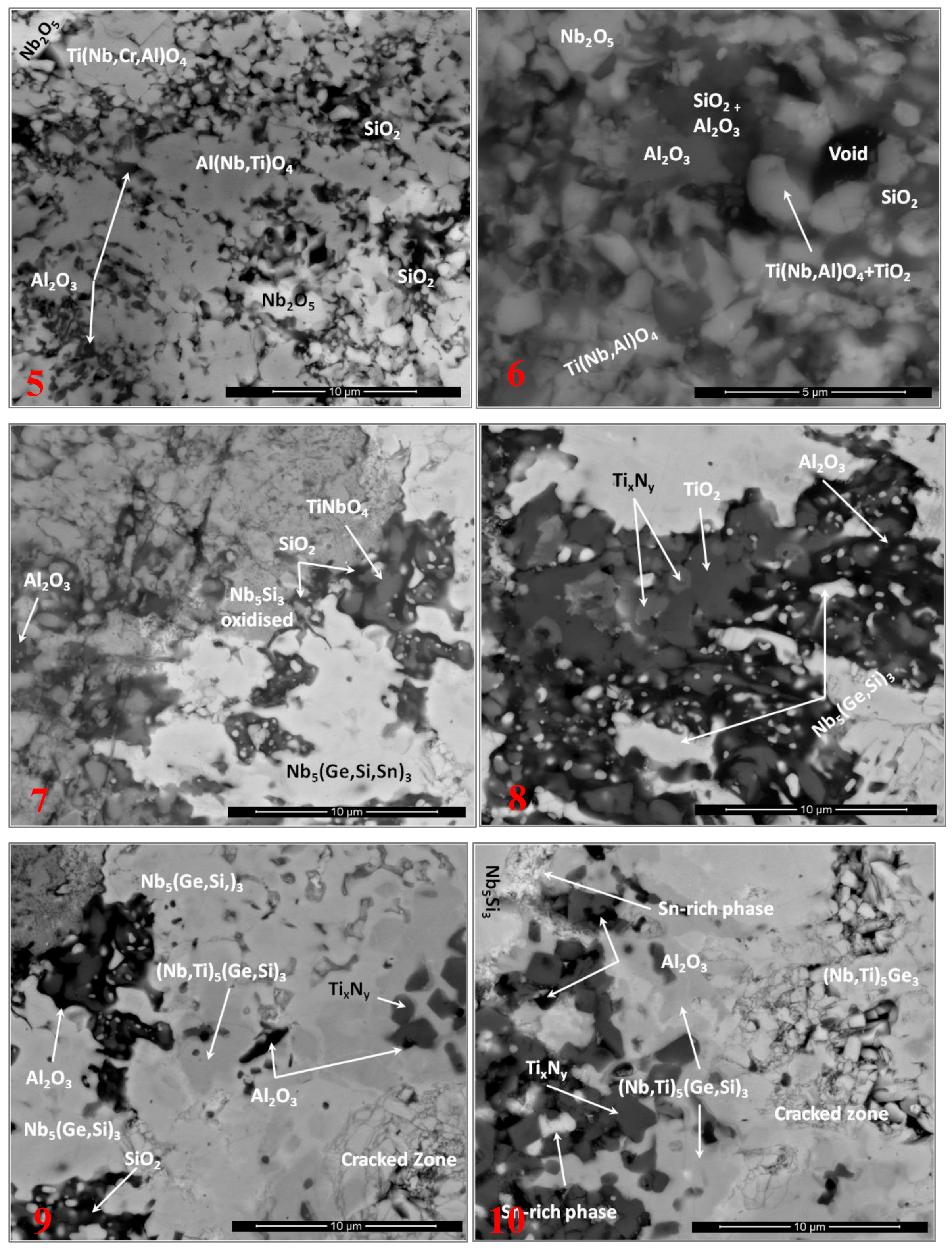
3.4. The Scale at 800 °C
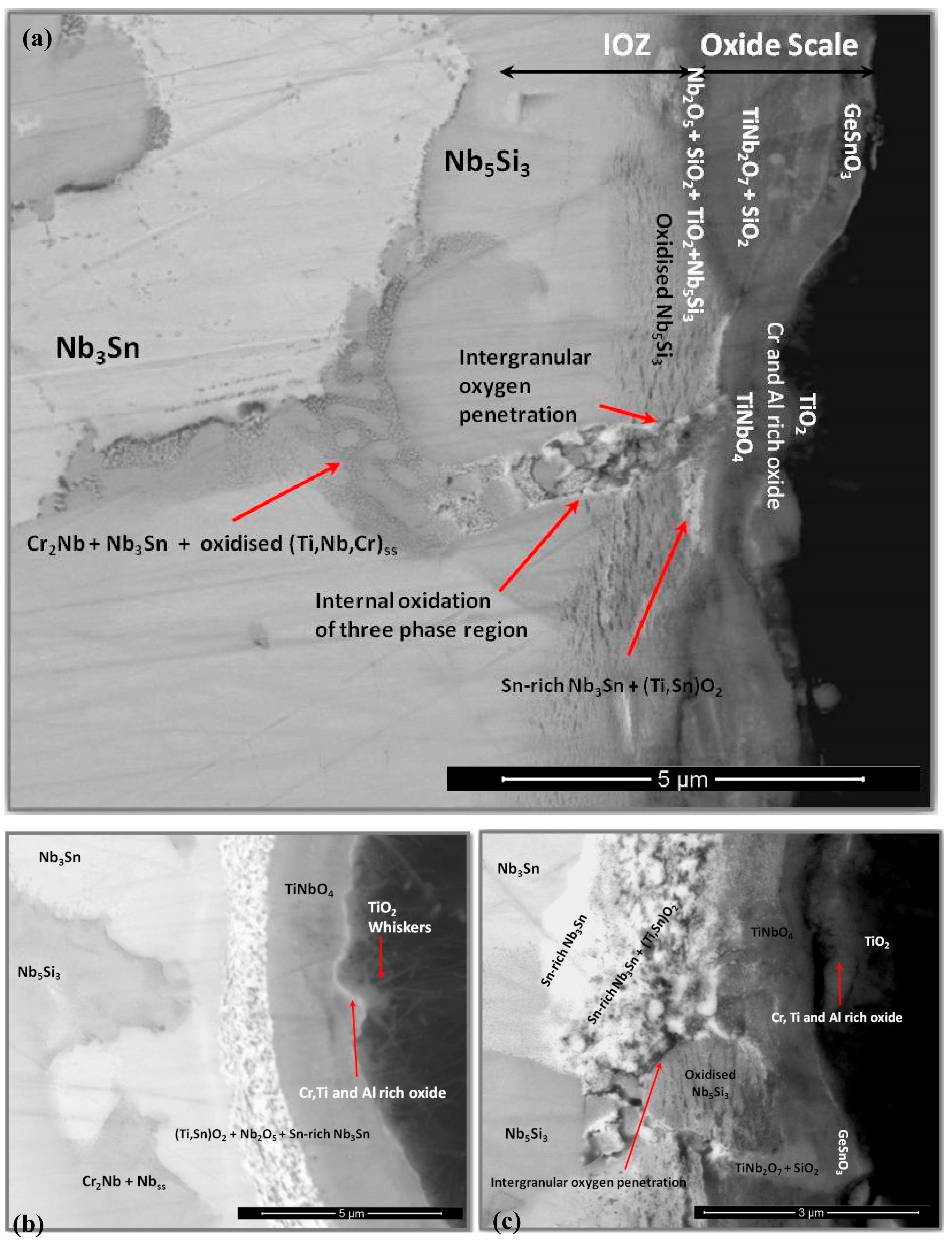
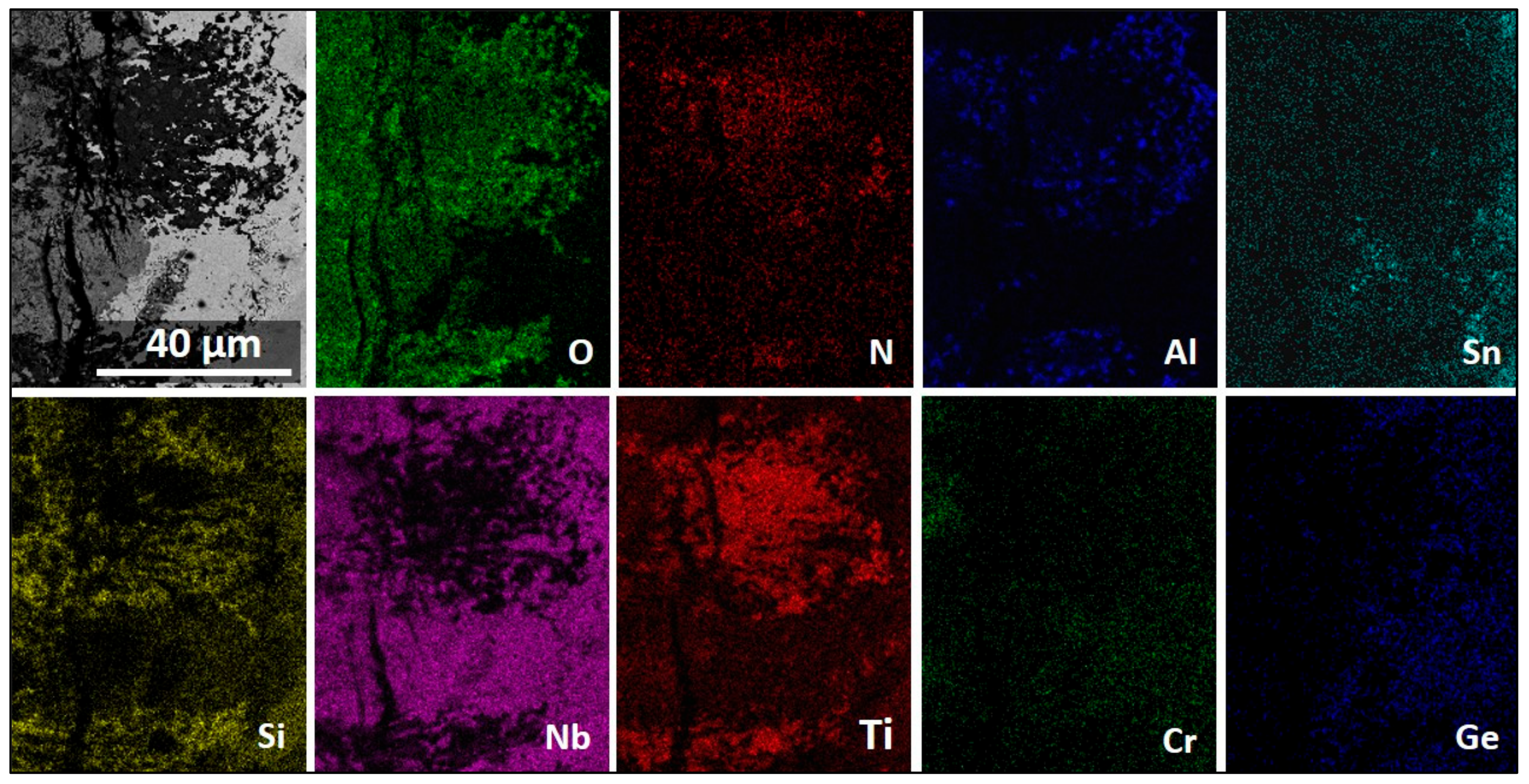
3.5. The Scale at 1200 °C
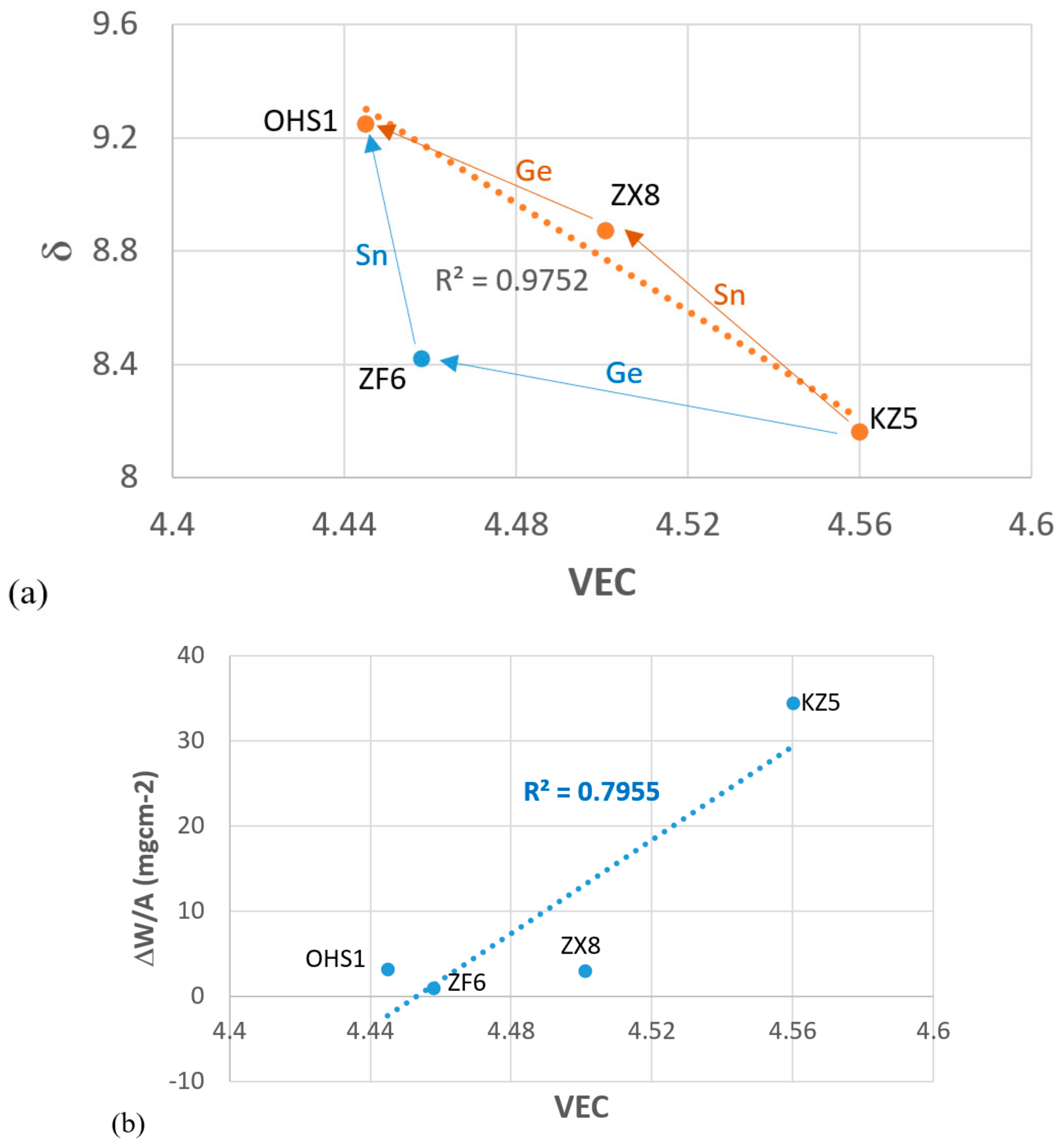
4. Discussion
4.1. Macrosegregation
4.2. Microstructure
4.3. Oxidation
4.3.1. Oxidation at 800 °C
4.3.2. Oxidation at 1200 °C
4.3.3. Oxide Scales
4.4. Alloy OHS1: High-Entropy Alloy (HEA) or Complex Concentrated Alloy (CCA)?
5. Summary and Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dimiduk, D.M.; Subramanian, P.R.; Mendiratta, M.G. Exploration of Nb-based advanced intermetallic materials. Acta Metall. Sin. Engl. Lett. 1995, 8, 519–530. [Google Scholar]
- Bewlay, B.P.; Jackson, M.R.; Zhao, J.; Subramanian, P.R. A Review of Very-High-Temperature Nb-silicide–Based Composites. Metall. Mater. Trans. A 2003, 34, 2043–2052. [Google Scholar] [CrossRef]
- Tsakiropoulos, P.; Superalloys, B.N.B. Encyclopedia of Aerospace Engineering; Ckley, R.B., Shyy, W., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Bewlay, B.P.; Lipsitt, H.A.; Jackson, M.R.; Reeder, W.J.; Sutcliff, J.A. Solidification pr Cessing of high temperature intermetallic eutectic-based alloys. Mater. Sci. Eng. A 1995, 192, 534–543. [Google Scholar] [CrossRef]
- Jackson, M.R.; Bewlay, B.P.; Zhao, J. Niobium-Silicide Based Composites Resistant to High Temperature Oxidation. U.S. Patent US 6913655, 29 December 2004. [Google Scholar]
- Fujikara, M.; Kasama, A.; Tanaka, R.; Hanada, S. Effect of alloy chemistry on the high temperature strengths and room temperature fracture toughness of advanced Nb-based alloys. Mater. Trans. 2004, 45, 493–501. [Google Scholar] [CrossRef]
- Kim, Y.; Menon, S.; Woodward, C. NbTiSiMo-X Alloys: Composition, Microstructure, Refinement and Properties; Report AFRL-RX-WP-TP-2009–4122; PN Publications: New Delhi, India, 2009. [Google Scholar]
- Drawin, S.; Justin, J.F. Advanced lightweight silicide and nitride based materials for turbo-engine applications. J. Aerosp. Lab. 2011, AL03-06, 1–13. [Google Scholar]
- Subramanian, P.R.; Mendiratta, M.G.; Dimiduk, D.M. High temperature silicides and refractory alloys. Mater. Res. S Ciety Symp. Pr Ceed. 1994, 322, 491–502. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On Nb silicide based alloys: Alloy design and selection. Materials 2018, 11, 844. [Google Scholar] [CrossRef]
- Prokoshkin, D.A.; Vasileva, E.V. Alloys of Niobium; Samarin, A.M., Ed.; translated from Russian by Kaner, N.; translation edited by Molly Gleiser; Israel Program for Scientific Translations: Jerusalem, Israel, 1965. [Google Scholar]
- Subramanian, P.R.; Mendiratta, M.G.; Dimiduk, D.M. Development approaches for advanced intermetallic materials—Historical perspective and selected successes. In Structural Intermetallics; Darolia, R., Lewandowski, J.J., Liu, C.T., Martin, P.L., Miracle, D.B., Nathal, M.V., Eds.; Springer: Singapore, 1993; pp. 619–630. [Google Scholar]
- Subramanian, P.R.; Mendiratta, M.G.; Dimiduk, D.M.; Stucke, M.A. Advanced intermetallic alloys—beyond gamma titanium aluminides. Mater. Sci. Eng. 1997, 240, 1–13. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On the alloying and properties of tetragonal Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 69. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. Alloying and properties of C14-NbCr2 and A15-Nb3X X=Al,Ge,Si,Sn in Nb-silicide based alloys. Materials 2018, 11, 395. [Google Scholar] [CrossRef]
- Perkins, R.A.; Chiang, K.T.; Meier, G.H. Formation of alumina on Nb-Al alloys. Scripta Metall. 1988, 22, 419–424. [Google Scholar] [CrossRef]
- Svedberg, R.C.; Ammon, R.L. Oxidation Resistant Niobium Alloy—Mechanically Alloying Niobium Intermetallic and Niobium Alloy. U.S. Patent #4,836,849, 6 June 1989. [Google Scholar]
- Menon, E.S.K.; Mendiratta, M.G.; Dimiduk, D.M. Oxidation behavior of complex niobium based alloys. In Proceedings of the International Symposium Niobium 2001, Orlando, FL, USA, 2–5 December 2001; pp. 121–145, ISBN 0971206805. [Google Scholar]
- Chan, K.S. Cyclic oxidation response of multiphase niobium-based alloys. Metal. Mater. Trans. 2004, 35, 589–597. [Google Scholar] [CrossRef]
- Geng, J.; Tsakiropoulos, P.; Shao, G. A Thermo-gravimetric and microstructural study of the oxidation of Nbss/Nb5Si3 based in situ composites with Sn addition. Intermetallics 2007, 15, 270–281. [Google Scholar] [CrossRef]
- Vellios, N. Design of Niobium Silicide Based Alloys. Ph.D. Thesis, University of Surrey, Guildford, UK, 2008. [Google Scholar]
- Knittel, S.; Mathieu, S.; Portebois, L.; Vilasi, M. Effect of tin addition on Nb-Si based in situ composites. Part II: Oxidation behaviour. Intermetallics 2014, 47, 43–52. [Google Scholar] [CrossRef]
- Zelenitsas, K.; Tsakiropoulos, P. Effect of Al, Cr and Ta additions on the oxidation behaviour of Nb–Ti–Si in situ composites at 800 C. Mater. Sci. Eng. 2006, 416, 269–280. [Google Scholar] [CrossRef]
- Geng, J.; Tsakiropoulos, P.; Shao, G. Oxidation of Nb–Si–Cr–Al in situ composites with Mo,Ti and Hf additions. Mater. Sci. Eng. 2006, 441, 26–38. [Google Scholar] [CrossRef]
- Portillo, B.I.; Varma, S.K. Oxidation behaviour of Nb-20Mo-15Si-5B-20Ti alloy in air from 700 to 1300. Alloys Compd. 2010, 497, 68–73. [Google Scholar] [CrossRef]
- Jun, W.; Xiping, G.; Jinming, G. Effects of B on the microstructure and oxidation resistance of Nb-Ti-Si based ultrahigh-temperature alloy. Chin. J. Aeronaut. 2009, 22, 544–550. [Google Scholar] [CrossRef]
- Perkins, R.A.; Chiang, K.T.; Meier, G.H. Effect of Alloying, Rapid Solidification, and Surface Kinetics on the High-Temperature Environmental Resistance of Niobium; AFOSR report, LMSC-F195926; PN Publications: New Delhi, India, 1987. [Google Scholar]
- Mitra, R. Mechanical behaviour and oxidation resistance of structural silicides. Int. Mater. Rev. 2006, 51, 13–64. [Google Scholar] [CrossRef]
- Yao, D.; Zhou, C.; Yang, J.; Chen, H. Experimental studies and modelling of the oxidation of multiphase Nb-base alloys. Corros. Sci. 2009, 51, 2619–2627. [Google Scholar] [CrossRef]
- Levin, E.M.; Robbins, C.R.; McMurdie, H.F. Phase Diagrams for Ceramists; American Ceramic Society: Columbus, OH, USA, 1964; pp. 142–363. [Google Scholar]
- Tsakiropoulos, P. On the macrosegregation of silicon in niobium silicide based alloys. Intermetallics 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Xu, Z.; Utton, C.; Tsakiropoulos, P. A study of the effect of 2 at.% Sn on the microstructure and isothermal oxidation at 800 and 1200 C of Nb-24Ti-18Si based alloys with Al and/or Cr additions. Materials 2018, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Utton, C.; Tsakiropoulos, P. A study of the Phase diagram for ceramics. effect of 5 at.% Sn on the microstructure and isothermal oxidation at 800 and 1200 C of Nb-24Ti-18Si based alloys with Al and/or Cr additions. Materials 2020, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tsakiropoulos, P. On the microstructure and hardness of the Nb-24Ti-18Si-5Al-5Cr-5Ge and Nb-24Ti-18Si-5Al-5Cr-5Ge-5Hf at.% silicide based alloys. Materials 2019, 12, 2655. [Google Scholar] [CrossRef] [PubMed]
- Zacharis, E.; Utton, C.; Tsakiropoulos, P. A study of the effects of Hf and Sn on the microstructure, hardness and oxidation of Nb-18Si silicide based alloys without Ti addition. Materials 2018, 11, 2247. [Google Scholar] [CrossRef] [PubMed]
- Thandorn, T.; Tsakiropoulos, P. Study of the role of B addition on the microstructure of the Nb-24Ti-18Si-8B alloy. Intermetallics 2010, 18, 1033–1038. [Google Scholar] [CrossRef]
- Jackson, M.R.; Bewlay, B.P.; Zhao, J. Niobium Silicide Based Composites Resistant to Low Temperature Pesting. U.S. Patent 6,419,765, 16 July 2002. [Google Scholar]
- Li, Z.; Tsakiropoulos, P. The effect of Ge addition on the oxidation of Nb-24Ti-18Si silicide based alloys. Materials 2019, 12, 3120. [Google Scholar] [CrossRef]
- Papadimitriou, I.; Utton, C.; Tsakiropoulos, P. The impact of Ti and temperature on the stability of Nb5Si3 phases: A first principles study. Sci. Technol. Adv. Mater. 2017, 18, 467–469. [Google Scholar] [CrossRef]
- Li, Z.; Tsakiropoulos, P. Study of the effect of Cr and Ti additions in the microstructure of Nb-18Si-5Ge based in situ composites. Intermetallics 2012, 26, 18–25. [Google Scholar] [CrossRef]
- Li, Z.; Tsakiropoulos, P. The microstructures of Nb-18Si-5Al-5Ge and Nb-24Ti-18Si-5Al-5Ge in situ composites. J. Alloys Compd. 2013, 550, 553–560. [Google Scholar] [CrossRef]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Cr and Al additions in the microstructure of Nb-Ti-Si in situ composites. Intermetallics 2005, 13, 1079–1095. [Google Scholar] [CrossRef]
- Kofstad, P. Chapter: Formation of Compact Scales at High Temperatures. In High Temperature Oxidation of Metals; Corrosion Monographs Series; IntechOpen: Oslo, Norway, 1966. [Google Scholar]
- Geng, J.; Tsakiropoulos, P. A study of the microstructure and oxidation of Nb-Si-Cr-Al-Mo in situ composites alloyed with Ti, Hf and Sn. Intermetallics 2007, 15, 382–395. [Google Scholar] [CrossRef]
- Tsakiropoulos, P.; Zelenitsas, K.; Vellios, N. Study of the effect of Al, Cr and Sn additions on the microstructure and properties of Nb silicide based alloys. Mater. Res. S C. Symp. Proc. C 2011, 1295. [Google Scholar] [CrossRef]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Ta and Cr additions in the microstructure of Nb-Ti-Si-Al in situ composites. Intermetallics 2006, 14, 639–659. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; Okamoto, H.; Gokhale, A.B.; Abbaschian, R. The Nb-Si Niobium-silicon system. J. Phase Equilibria 1993, 14, 502–509. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. Alloying and hardness of eutectics with Nbss and Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 592. [Google Scholar] [CrossRef]
- Vellios, N.; Tsakiropoulos, P. A study of the role of Fe and Sn additions in the microstructure of Nb-24Ti-18Si-5Cr silicide based alloys. Intermetallics 2010, 18, 1729–1736. [Google Scholar] [CrossRef]
- Okamoto, H. Phase Diagrams for Binary Alloys: Desk Handbook; ASM International: Metals Park, OH, USA, 2000. [Google Scholar]
- Vellios, N.; Tsakiropoulos, P. The role of Sn and Ti additions in the microstructure of Nb-18Si base alloys. Intermetallics 2007, 15, 1518–1528. [Google Scholar] [CrossRef]
- Li, Z.; Tsakiropoulos, P. Study of the effects of Ge addition on the microstructure of Nb–18Si in situ composites. Intermetallics 2010, 18, 1072–1078. [Google Scholar]
- Li, Z.; Tsakiropoulos, P. Study of the effect of Ti and Ge in the microstructure of Nb-24Ti-18Si-5Ge in situ composite. Intermetallics 2011, 19, 1291–1297. [Google Scholar] [CrossRef]
- Papadimitiou, C.; Utton, P. Tsakiropoulos. On the Nb-Ge binary system. Metall. Mater. Trans. 2015, 46, 5526–5536. [Google Scholar] [CrossRef]
- Bendersky, L.; Biancaniello, F.S.; Boettinger, W.J.; Perepezko, J.H. Microstructural characterization of rapidly solidified Nb-Si alloys. Mater. Sci. Eng. 1987, 89, 151–159. [Google Scholar] [CrossRef]
- Kofstad, P.; Kjollesdal, H. Oxidation of Niobium columbium in the temperature range 500 to 1200 C. Trans. Metall. S Ciety AIME 1961, 221, 285–294. [Google Scholar]
- Nelson, J.; Ghadyani, M.; Utton, C.; Tsakiropoulos, P. A study of the effects of Al, Cr, Hf and Ti additions on the microstructure and oxidation of Nb-24Ti-18Si silicide based alloys. Materials 2018, 11, 1579. [Google Scholar] [CrossRef]
- Manning, W.R.; Hunter, O.; Calderwood, F.W.; Stacy, D.W. Thermal expansion of Nb2O5. J. Am. Ceram. S Ciety 1972, 55, 342–347. [Google Scholar] [CrossRef]
- Papadimitriou, C.; Utton, C.; Scott, A.; Tsakiropoulos, P. Ab initio study of the intermetallics in Nb-Si binary system. Intermetallics 2014, 54, 125–132. [Google Scholar] [CrossRef]
- Lefez, B.; Jouen, S.; Hannoyer, B.; Bacos, M.; Beucher, E. Oxidation behaviour of the 47Nb-16Si-25Ti-8Hf-2Al-2Cr alloy sheet and vibrational spectroscopy. Mater. High Temp. 2009, 26, 15–20. [Google Scholar] [CrossRef]
- Nico, C.; Monteiro, T.; Graca, M.P.F. Niobium oxides and niobates physical properties: Review and prospects. Prog. Mater. Sci. 2016, 80, 1–37. [Google Scholar] [CrossRef]
- Moore, D.K.; Cherniak, D.J.; Watson, E.B. Oxygen diffusion in rutile from 750 to 1000 C and 0.1 to 1000 Mpa. Am. Mineral. 1998, 83, 700–711. [Google Scholar] [CrossRef]
- McCarthy, K.A.; Ballard, S.S.; Doerner, E.C. Thermal conductivity of sapphire and rutile as a function of temperature. Phys. Rev. 1952, 88, 153. [Google Scholar]
- Grant, F.A. Properties of rutile titanium dioxide. Rev. Mod. Phys. 1959, 31, 646–674. [Google Scholar] [CrossRef]
- Kofstad, P. Note on the defect structure of rutile TiO2. J. Less Common Met. 1967, 13, 635–638. [Google Scholar] [CrossRef]
- Hurlen, T. Oxidation of Niobium; Technical Scientific Note no.1 Norwegian Report; Central Institute for Industrial Research: Oslo, Norway, 1959. [Google Scholar]
- Argent, B.B.; Phelps, B. The oxidation of Niobium-Titanium and Niobium-Molybdenum alloys. J. Less Common Met. 1960, 2, 181–190. [Google Scholar] [CrossRef]
- Ardit, M.; Dondi, M.; Cruciani, G. Structural stability, cation ordering, and l Cal relaxation along the AlNbO4-Al0.5Cr0.5NbO4 join. Am. Mineral. 2012, 97, 910–917. [Google Scholar] [CrossRef]
- Music, D.; Stelzer, B. Intrinsic thermal shock behaviour of common rutile oxides. Physics 2019, 1, 22. [Google Scholar] [CrossRef]
- Felten, E.J. The interaction of the alloy Nb-25Ti with air, oxygen and nitrogen. II The reaction of Nb-25Ti in air and oxygen between 650 and 1000 C. J. Less Common Metals 1969, 17, 199–206. [Google Scholar] [CrossRef]
- Ghadyani, M.; Utton, C.; Tsakiropoulos, P. Microstructures and isothermal oxidation of the alumina scale forming Nb1.7Si2.4Ti2.4Al3Hf0.5 and Nb1.3Si2.4Ti2.4Al3.5Hf0.4 alloys. Materials 2019, 12, 222. [Google Scholar] [CrossRef]
- Dejneka, M.J.; Chapman, C.L.; Misture, S.T. Strong, low thermal expansion niobate ceramics. J. Am. Ceram. 2011, 48, 2249–2261. [Google Scholar] [CrossRef]
- Choosuwan, H.; Guo, R.; Bhalla, A.S.; Balachandran, U. Negative thermal expansion behaviour in single crystal and ceramic of Nb2O5 based compositions. J. Appl. Phys. 2002, 91, 5051. [Google Scholar] [CrossRef]
- Levin, E.M. Phase equilibria in the system niobium pentoxide-germanium dioxide, Journal of Research of the National Bureau of Standards-A. Phys. Chem. 1966, 70, 5–10. [Google Scholar]
- Pradep, K.G.; Tasan, C.C.; Yao, M.J.; Deng, Y.; Springer, H.; Raabe, D. Non-equiatomic high entropy alloys: Approach towards rapid alloy screening and property-oriented design. Mater. Sci. Eng. A 2015, 648, 183–192. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On Nb silicide based alloys. Part II. J. Alloys Compd. 2018, 748, 569–576. [Google Scholar] [CrossRef]



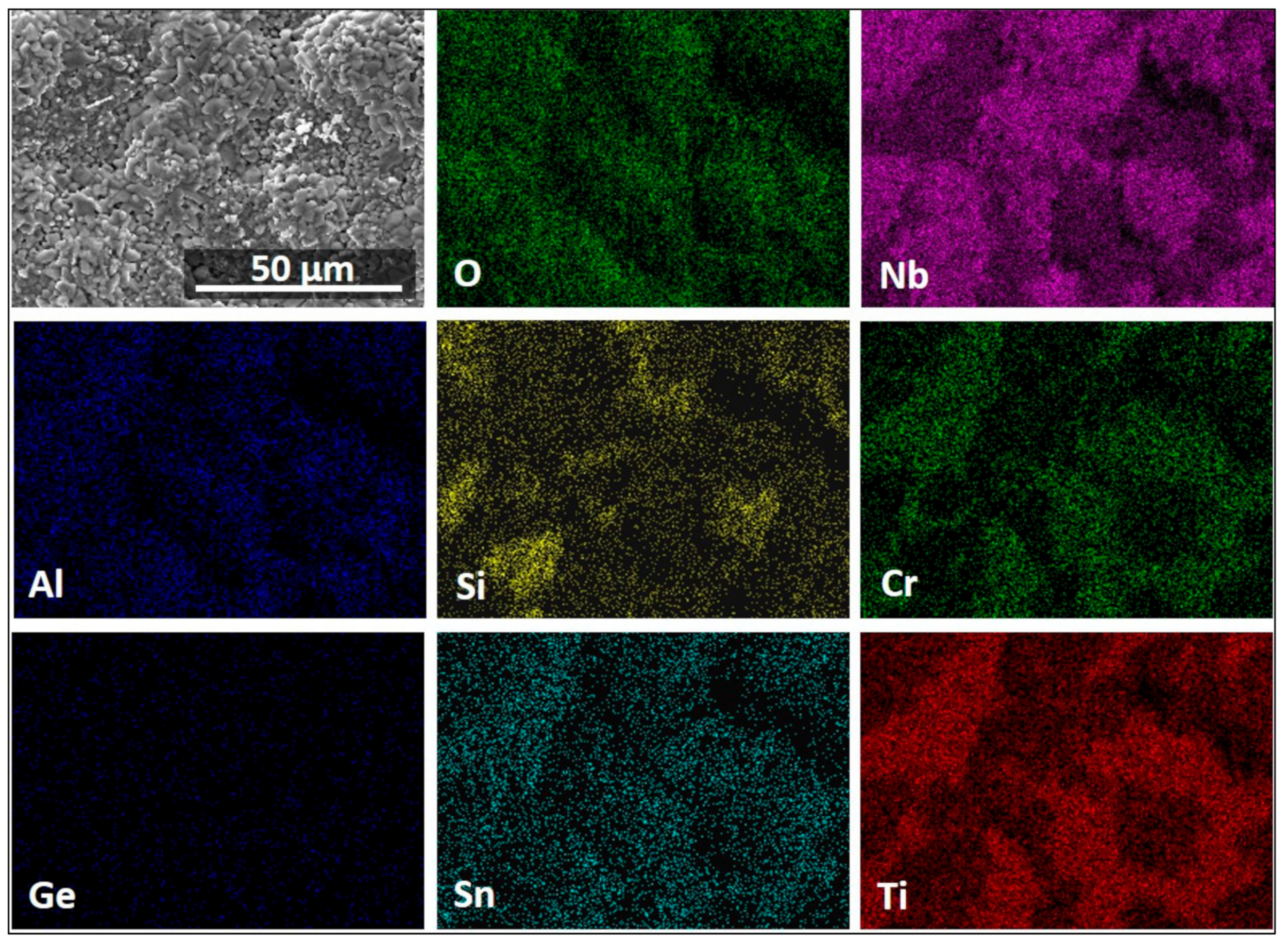


| Area/Phase | Nb (at.%) | Ti (at.%) | Si (at.%) | Cr (at.%) | Al (at.%) | Ge (at.%) | Sn (at.%) |
|---|---|---|---|---|---|---|---|
| Top | 40.0 ± 1.1 | 23.2 ± 1.4 | 18.8 ± 1.6 | 4.3 ± 0.6 | 4.6 ± 0.4 | 5.2 ± 0.4 | 3.9 ± 0.5 |
| 42.5–38.5 | 25.0–20.4 | 22.3–15.9 | 5.1–3.3 | 5.3–3.9 | 5.6–4.5 | 4.8–3.1 | |
| Center | 41.6 ± 1.7 | 22.1 ± 1.7 | 18.7 ± 1.6 | 4.1 ± 0.5 | 4.7 ± 0.5 | 4.7 ± 0.5 | 4.1 ± 0.4 |
| 43.6–38.0 | 25.4–20.1 | 21.2–15.5 | 5.3–3.2 | 5.6–3.4 | 5.7–3.7 | 5.1–3.2 | |
| Bottom | 39.2 ± 0.6 | 24.2 ± 0.8 | 17.9 ± 0.8 | 4.8 ± 0.4 | 4.8 ± 0.4 | 5.2 ± 0.3 | 3.9 ± 0.4 |
| 40.7–38.0 | 25.8–22.8 | 19.1–16.7 | 5.4–4.2 | 5.6–4.0 | 5.6–4.7 | 4.5–3.4 | |
| Nbss | 23.3 ± 0.7 | 30.6 ± 1.0 | 5.3 ± 1.0 | 29.4 ± 1.6 | 7.2 ± 0.7 | 1.5 ± 0.5 | 2.7 ± 0.2 |
| 24.3–22.2 | 32.1–29.5 | 6.8–3.9 | 31.9–27.4 | 8.4–6.3 | 2.2–0.7 | 3.1–2.4 | |
| NbCr2 | 23.1 ± 1.1 | 17.6 ± 1.2 | 7.3 ± 0.4 | 45.3 ± 2.6 | 5.1 ± 0.4 | 0.9 ± 0.1 | 0.7 ± 0.4 |
| 24.9–21.7 | 19.6–16.2 | 8.0–7.0 | 48.2–42.0 | 5.8–4.5 | 1.1–0.9 | 1.4–0.3 | |
| Nb3Sn | 49.0 ± 0.9 | 24.9 ± 0.5 | 2.9 ± 0.1 | 4.4 ± 0.3 | 5.8 ± 0.3 | 1.6 ± 0.2 | 11.4 ± 0.4 |
| 50.3–47.8 | 25.7–24.3 | 3.1–2.7 | 4.6–3.8 | 6.2–5.3 | 1.9–1.4 | 12.1–11.0 | |
| Ti-rich Nb3Sn | 41.9 ± 0.9 | 29.4 ± 1.0 | 2.8 ± 0.2 | 7.4 ± 0.7 | 7.3 ± 0.3 | 1.5 ± 0.1 | 9.7 ± 0.5 |
| 43.2–40.4 | 31.2–27.7 | 3.2–2.4 | 8.2–6.4 | 7.8–6.8 | 1.7–1.3 | 10.5–8.8 | |
| Nb5Si3 | 43.6 ± 0.3 | 18.6 ± 0.3 | 27.3 ± 0.5 | 1.2 ± 0.1 | 2.0 ± 0.2 | 6.2 ± 0.1 | 1.1 ± 0.2 |
| 43.8–43.1 | 19.1–18.5 | 27.7–26.7 | 1.3–1.1 | 2.2–1.8 | 6.3–6.1 | 1.4–1.0 | |
| Ti-rich Nb5Si3 | 38.8 ± 2.0 | 23.0 ± 1.9 | 20.6 ± 0.6 | 1.9 ± 0.4 | 5.2 ± 0.4 | 6.7 ± 0.2 | 3.8 ± 0.2 |
| 40.5–36.1 | 25.3–21.1 | 21.3–19.6 | 2.5–1.6 | 5.8–4.8 | 7.1–6.4 | 4.1–3.5 |
| Area/Phase | Nb (at.%) | Ti (at.%) | Si (at.%) | Cr (at.%) | Al (at.%) | Ge (at.%) | Sn (at.%) |
|---|---|---|---|---|---|---|---|
| Top | 39.6 ± 0.4 | 24.1 ± 0.5 | 18.2 ± 0.8 | 4.2 ± 0.3 | 4.6 ± 0.3 | 5.1 ± 0.2 | 4.2 ± 0.3 |
| 39.9–39.1 | 24.6–23.6 | 19.2–17.4 | 4.5–3.9 | 5.0–4.2 | 5.3–4.8 | 4.7–4.0 | |
| Bulk | 40 ± 1.0 | 23.7 ± 0.6 | 17.8 ± 1.0 | 4.6 ± 0.4 | 4.7 ± 0.2 | 5.0 ± 0.2 | 4.2 ± 0.5 |
| 41.1–38.4 | 24.6–23.2 | 19.1–16.3 | 5.0–4.0 | 5.0–4.5 | 5.3–4.6 | 5.2–3.8 | |
| Bottom | 39.4 ± 0.6 | 24.4 ± 0.4 | 18.0 ± 0.7 | 4.4 ± 0.5 | 4.5 ± 0.2 | 5.2 ± 0.2 | 4.1 ± 0.2 |
| 40.3–38.8 | 24.9–24.0 | 18.6–17.1 | 4.9–3.8 | 4.7–4.3 | 5.4–5.0 | 4.3–3.8 | |
| NbCr2 | 26.0 ± 0.6 | 10.1 ± 0.7 | 8.8 ± 0.2 | 50.5 ± 1.0 | 3.5 ± 0.4 | 0.7 ± 0.0 | 0.4 ± 0.3 |
| 26.6–25.3 | 10.5–9.1 | 9.1–8.6 | 51.7–49.3 | 3.9–3.1 | 0.8–0.7 | 0.7–0.1 | |
| Nb3Sn | 47 ± 0.4 | 23.8 ± 0.4 | 2.6 ± 0.2 | 5.8 ± 0.2 | 5.0 ± 0.6 | 1.3 ± 0.1 | 14.5 ± 0.8 |
| 47.5–46.7 | 24.1–23.1 | 2.8–2.4 | 6.1–5.5 | 5.5–4.0 | 1.3–1.2 | 15.8–14.0 | |
| Nb5Si3 | 37.1 ± 0.4 | 24.2 ± 0.8 | 22.0 ± 1.3 | 3.2 ± 0.3 | 4.8 ± 0.3 | 5.8 ± 0.7 | 2.9 ± 0.4 |
| 37.6–6.5 | 25.0–23.5 | 23.5–20.9 | 3.5–3.0 | 5.0–4.5 | 6.4–5.0 | 3.2–2.5 | |
| Ti-rich Nb5Si3 | 33.2 ± 0.2 | 27.2 ± 1.3 | 24.9 ± 0.7 | 2.1 ± 0.4 | 4 ± 0.5 | 4.9 ± 0.7 | 3.7 ± 0.2 |
| 34.8–32.6 | 29.5–26.6 | 25.6–24.1 | 2.7–1.7 | 4.9–3.7 | 6.7–5.1 | 4.1–3.5 |
| Temperature | n | Kl (g·cm−2·s−1) | Kp (g2·cm−4·s−1) | Weight Gain (mg/cm2) |
|---|---|---|---|---|
| 800 °C | 0.46 | - | 2.4 × 10−11 (0–100) h | 3.19 |
| 1200 °C | 0.83 | 1.1 × 10−7 (> 3.1) h | 4.9 × 10−10 (0–3.1) h | 31.28 |
| Alloy | ΔHm (kJ/mol) | Tm (K) | ΔHm/T (J/mol·K) | ΔHmsd/ΔHmsp | Tmsd (K) | Tmsp (K) | Tmsd/Tmsp | [ΔHm/Tm] × [ΔHmsd/ΔHmsp]−1 | MACSi (at.%) |
|---|---|---|---|---|---|---|---|---|---|
| ZX8 OHS1 ZF6 KZ5 | 26.9 27.7 27.7 27.5 | 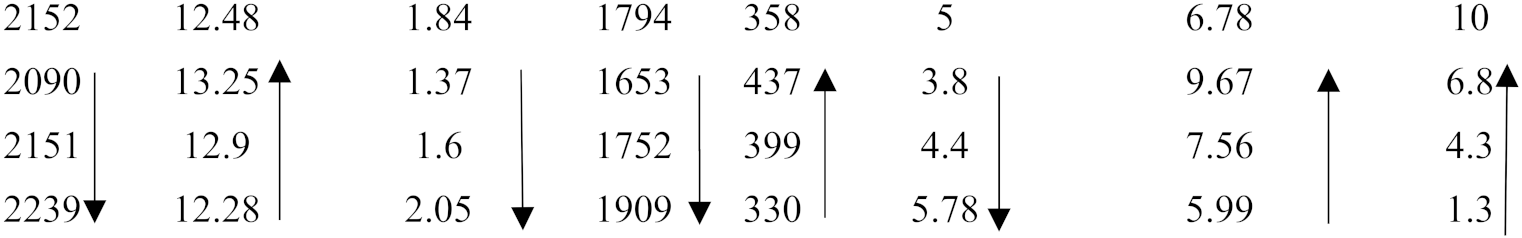 | |||||||
| Alloy | ΔHm (kJ/mol) | Tm (K) | ΔHm/Tm (J/mol·K) | ΔHmsd/ΔHmsp | Tmsd (K) | Tmsp (K) | Tmsd/Tmsp | [ΔHm/Tm] × [ΔHmsd/ΔHmsp]−1 | MACSi (at%) |
|---|---|---|---|---|---|---|---|---|---|
| OHS1 ZX6 (Al) ZF5 (Al) KZ7 (Al) | 27.7 27.3 28 27.7 | 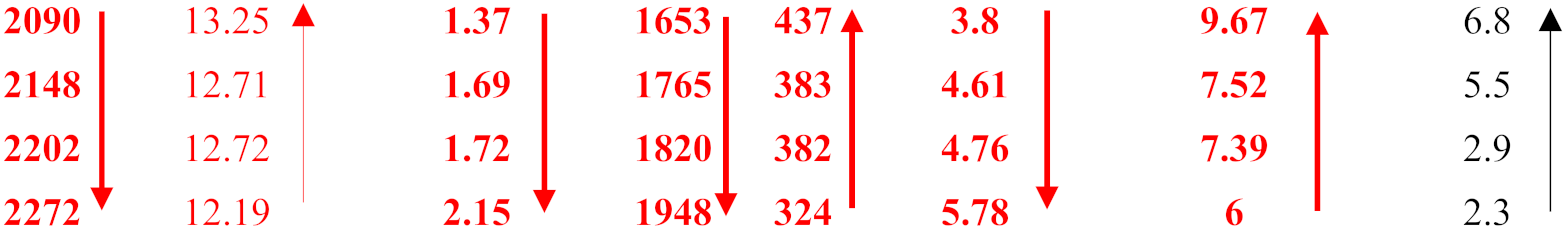 | |||||||
| Alloy | ΔHm (kJ/mol) | Tm (K) | ΔHm/Tm (J/mol·K) | ΔHmsd/ΔHmsp | Tmsd (K) | Tmsp (K) | Tmsd/Tmsp | [ΔHm/Tm] × [ΔHmsd/ΔHmsp]−1 | MACSi (at%) |
|---|---|---|---|---|---|---|---|---|---|
| ZX4 (Cr) OHS1 ZF4 (Cr) KZ4 (Cr) | 27.8 27.7 28.4 28.2 |  | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Negrete, O.; Tsakiropoulos, P. On the Microstructure and Isothermal Oxidation at 800 and 1200 °C of the Nb-24Ti-18Si-5Al-5Cr-5Ge-5Sn (at.%) Silicide-Based Alloy. Materials 2020, 13, 722. https://doi.org/10.3390/ma13030722
Hernández-Negrete O, Tsakiropoulos P. On the Microstructure and Isothermal Oxidation at 800 and 1200 °C of the Nb-24Ti-18Si-5Al-5Cr-5Ge-5Sn (at.%) Silicide-Based Alloy. Materials. 2020; 13(3):722. https://doi.org/10.3390/ma13030722
Chicago/Turabian StyleHernández-Negrete, Ofelia, and Panos Tsakiropoulos. 2020. "On the Microstructure and Isothermal Oxidation at 800 and 1200 °C of the Nb-24Ti-18Si-5Al-5Cr-5Ge-5Sn (at.%) Silicide-Based Alloy" Materials 13, no. 3: 722. https://doi.org/10.3390/ma13030722
APA StyleHernández-Negrete, O., & Tsakiropoulos, P. (2020). On the Microstructure and Isothermal Oxidation at 800 and 1200 °C of the Nb-24Ti-18Si-5Al-5Cr-5Ge-5Sn (at.%) Silicide-Based Alloy. Materials, 13(3), 722. https://doi.org/10.3390/ma13030722






