Ni2P/rGO/NF Nanosheets As a Bifunctional High-Performance Electrocatalyst for Water Splitting
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Ni2P/rGO/NF Synthesis
2.3. Characterization and Electrochemical Measurements
3. Results and Discussion
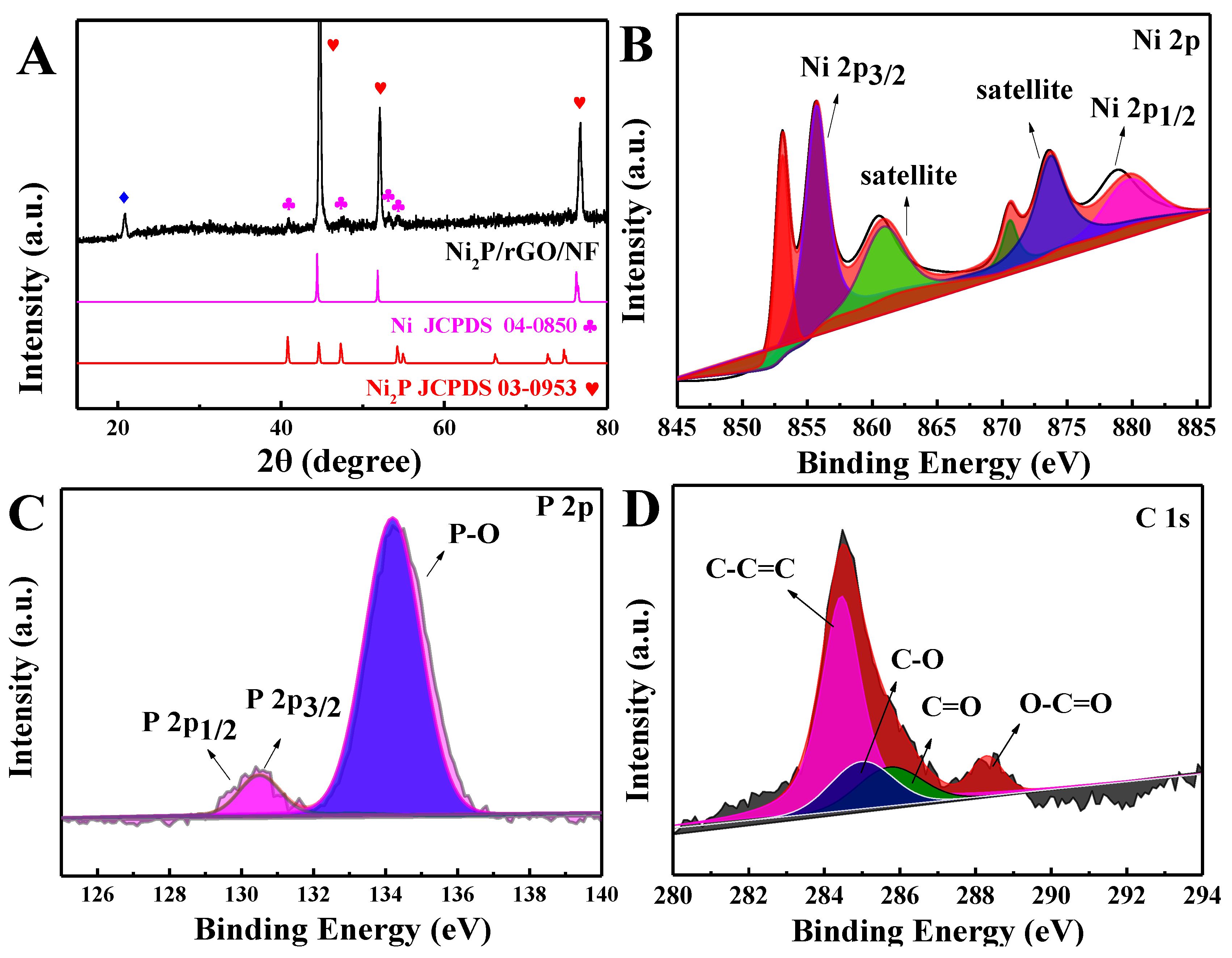
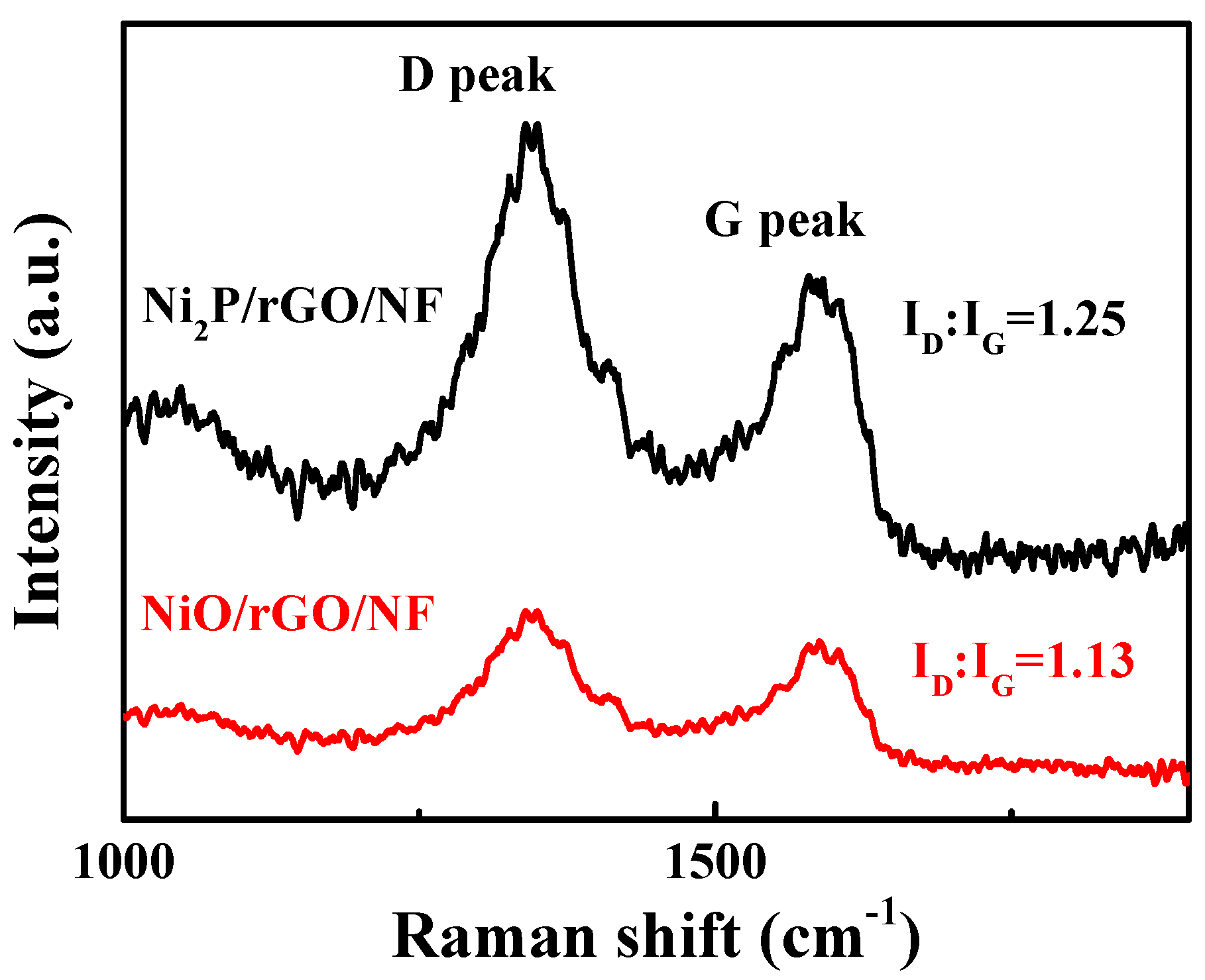
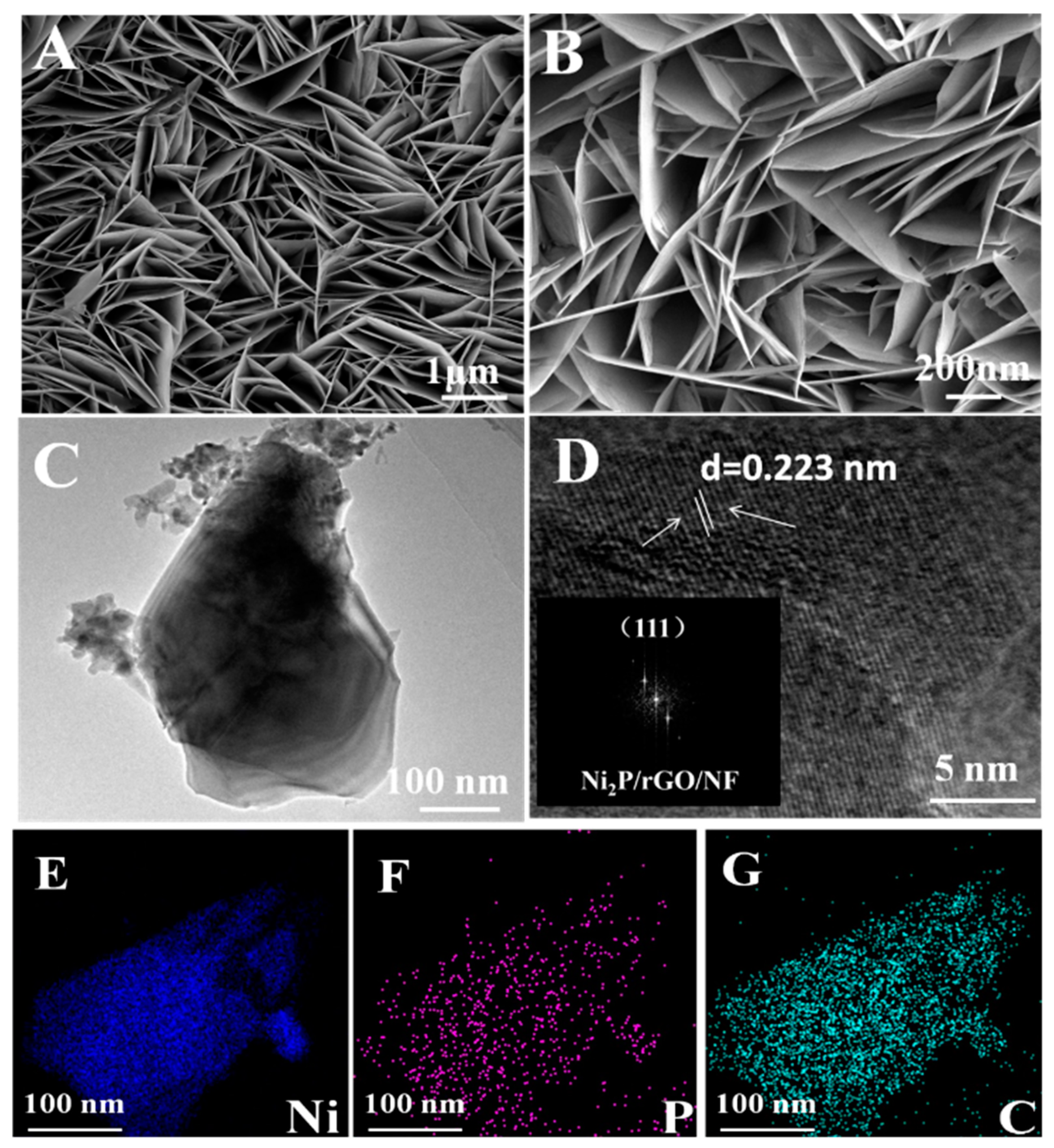
3.1. Physical Characterization
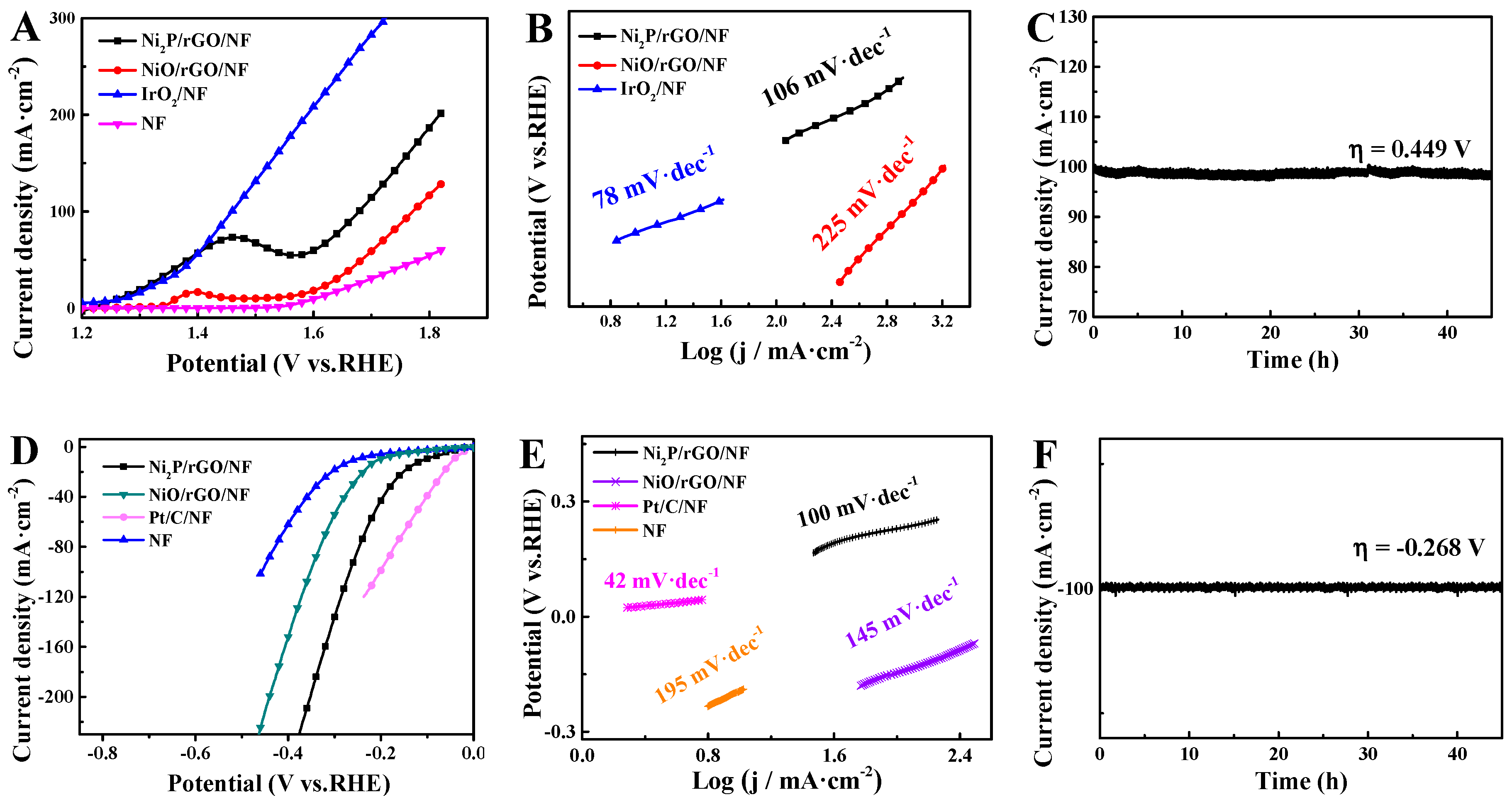
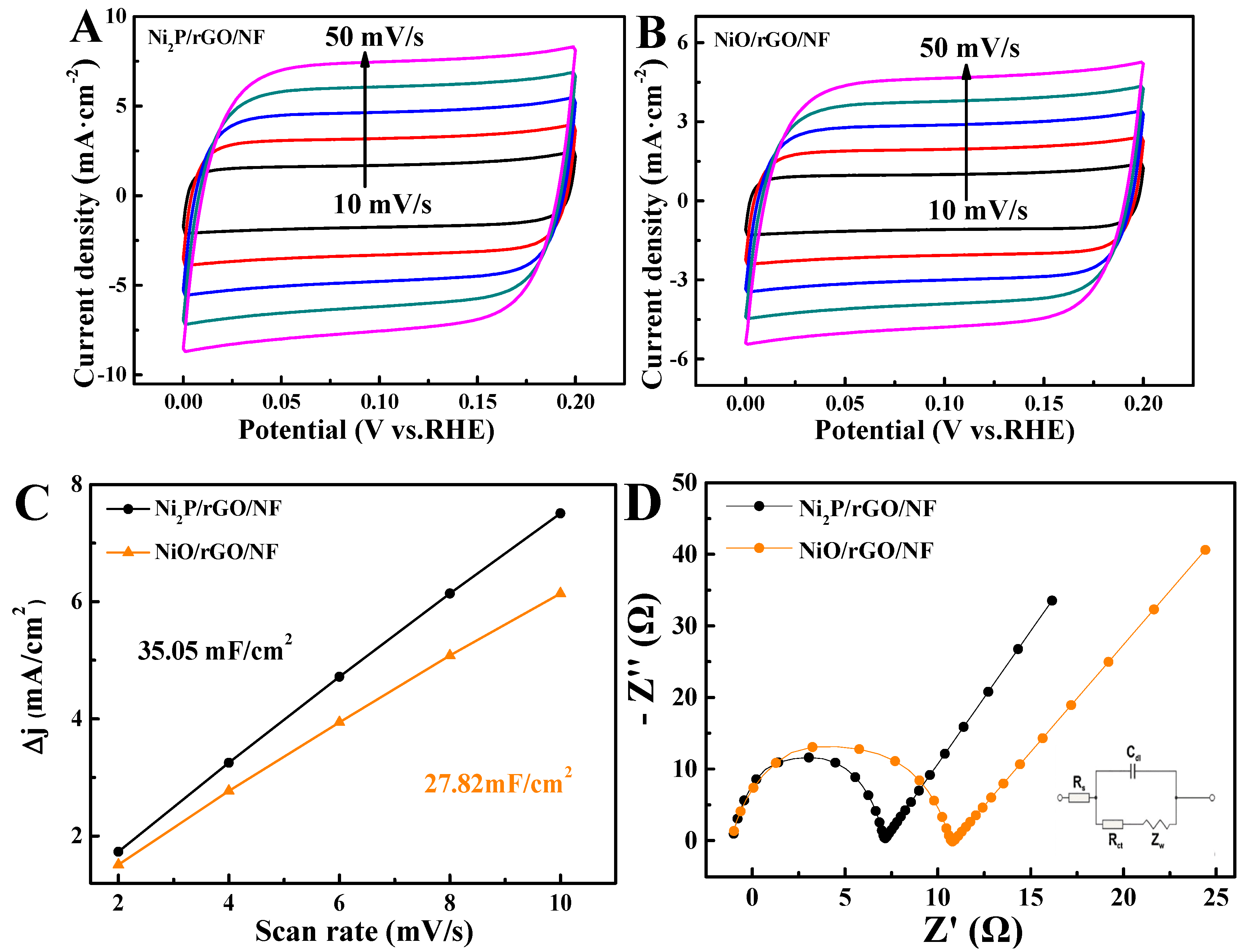
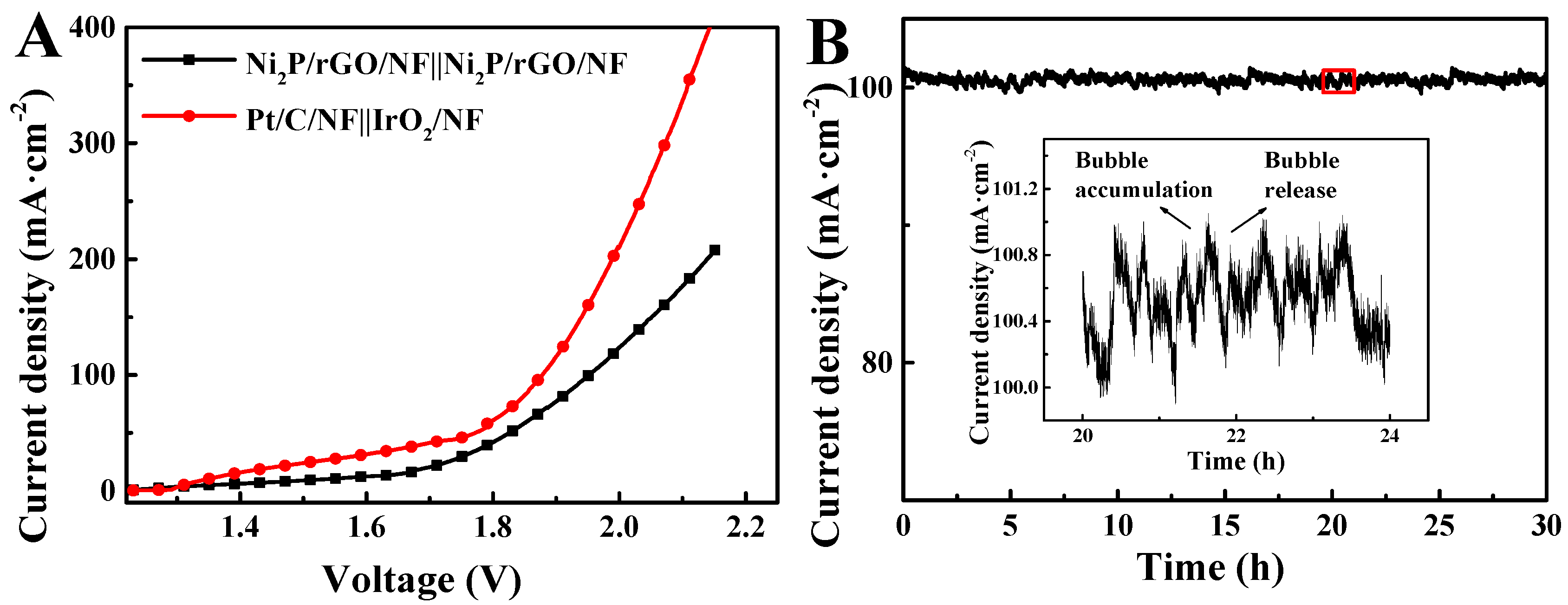
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khajehsaeidi, Z.; Sangpour, P.; Ghaffarinejad, A. A novel co-electrodeposited Co/MoSe2/reduced graphene oxide nanocomposite as electrocatalyst for hydrogen evolution. Int. J. Hydrog. Energy 2019, 44, 19816–19826. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sankar, S.S.; Sangeetha, K.; Karthik, P.E.; Kundu, S. Precision and correctness in the evaluation of electrocatalytic water splitting: Revisiting activity parameters with a critical assessment. Energy Environ. Sci. 2018, 11, 744–771. [Google Scholar] [CrossRef]
- You, H.; Wu, Z.; Jia, Y.; Xu, X.; Xia, Y.; Han, Z.; Wang, Y. Highefficiency and mechano-/photo-bi-catalysis of piezoelectricZnO@photoelectric-TiO2 core-shell nanofibers for dye decomposition. Chemosphere 2017, 183, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Q.; Yu, F.; Zhu, Q.; Sun, J.Y.; Qin, F.; Yu, L.; Bao, J.M.; Yu, Y.; Chen, S.; Ren, Z.F. Water splitting by electrolysis at high current densities under 1.6volts. Energy Environ. Sci. 2018, 11, 2858–2864. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative strategies for electrocatalytic water splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Li, Y.C.; He, B.; Liu, X.Q.; Hu, X.Q.; Huang, J.; Ye, S.Q.; Zhu, S.; Yang, W.; Zhen, L. Graphene confined MoS2 particles for accelerated electrocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2019, 44, 8070–8078. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Z.; Fujitsuka, M.; Majima, T. Z-Scheme Photocatalytic Water Splitting on a 2D Heterostructure of Black Phosphorus/Bismuth Vanadate Using Visible Light. Angew. Chem. Int. Edit. 2018, 57, 2160–2164. [Google Scholar] [CrossRef]
- Nam, S.; Mai, C.T.K.; Oh, I. Ultrastable Photoelectrodes for Solar Water Splitting Based on Organic Metal Halide Perovskite Fabricated by Lift-Off Process. ACS Appl. Mater. Inter. 2018, 10, 14659–14664. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, D.; Tong, X.; Liu, M. Ultrathin Lutetium Oxide Film as an Epitaxial Hole-Blocking Layer for Crystalline Bismuth Vanadate Water Splitting Photoanodes. Adv. Funct. Mater. 2018, 28, 1705512. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent Progress in Cobalt-based Heterogeneous Catalysts for Electrochemical Water Splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Yang, J.; Chhowalla, M. Recent Strategies for Improving the Catalytic Activity of 2D TMD Nanosheets toward the Hydrogen Evolution Reaction. Adv. Mater. 2016, 28, 6197–6206. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, Q.; Asiri, A.M.; Sun, X.; Luo, Y. Self-supported FeP Nanorod Arrays: A Cost-effective 3D Hydrogen Evolution Cathode with High Catalytic Activity. ACS Catal. 2014, 4, 4065–4069. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Zhu, J.; Zhang, C.; Liu, T. Self-templated Growth of Vertically Aligned 2H-1T MoS2 for Efficient Electrocatalytic Hydrogen Evolution. ACS Appl. Mater. Inter. 2016, 8, 31702–31708. [Google Scholar] [CrossRef] [PubMed]
- Nitin, K.C.; Haneul, J.; Byeongyoon, K.; Lee, K. Nanostructured Materials on 3D Nickel Foam as Electrocatalysts for Water Splitting. Nanoscale 2017, 9, 12231–12247. [Google Scholar]
- Song, L.; Kang, X.; Zhang, S. CNT/g-C3N4 photocatalysts with enhanced hydrogen evolution ability for water splitting based on a noncovalent interaction. Int. J. Energy Res. 2018, 42, 1649–1656. [Google Scholar] [CrossRef]
- Hu, Y.P.; Li, F.; Long, Y.; Yang, H.D.; Gao, L.L.; Long, X.F.; Hu, H.G.; Xu, N.; Jin, J.; Ma, J.T. Ultrafine CoPS nanoparticles encapsulated in N, P, and S tri-doped porous carbon as an efficient bifunctional water splitting electrocatalyst in both acid and alkaline solutions. J. Mater. Chem. A 2018, 6, 10433–10440. [Google Scholar] [CrossRef]
- Weng, B.C.; Grice, C.R.; Meng, W.W.; Guan, L.; Xu, F.H.; Yu, Y.; Wang, C.L.; Zhao, D.W.; Yan, Y.F. Metal–Organic Framework-Derived CoWP@C Composite Nanowire Electrocatalyst for Efficient Water Splitting. ACS Energy Lett. 2018, 3, 1434–1442. [Google Scholar] [CrossRef]
- Kong, D.S.; Cha, J.J.; Wang, H.T.; Lee, H.R.; Cui, Y. First-Row Transition Metal Dichalcogenide Catalysts for Hydrogen Evolution Reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Wang, X.; Zheng, B.; Zhang, W.; Chen, Y. Scalable Synthesis of Porous Hollow CoSe2–MoSe2/Carbon Microspheres for Highly Efficient Hydrogen Evolution Reaction in Acidic and Alkaline Media. J. Mater. Chem. A 2018, 6, 12701–12707. [Google Scholar] [CrossRef]
- Zheng, B.J.; Chen, Y.F.; Qi, F.; Wang, X.Q.; Zhang, W.L.; Li, Y.R.; Li, X.S. 3D-Hierarchical MoSe2 Nanoarchitecture as a Highly Efficient Electrocatalyst for Hydrogen Evolution. 2D Mater. 2017, 4, 025092. [Google Scholar] [CrossRef]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured Nickel Phosphide as an Electrocatalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Kim, A.R.; Nahm, K.S.; Lee, H.K.; Jin Yoo, D. Synchronized synthesis of Pd@C-RGO carbocatalyst for improved anode and cathode performance for direct ethylene glycol fuel cell. Chem. Commun. 2014, 50, 14623–14626. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Zheng, B.J.; Yu, B.; Wang, B.; Hou, W.Q.; Zhang, W.L.; Chen, Y.F. In-Situ Synthesis of Hierarchical MoSe2-CoSe2 Nanotubes as Efficient Electrocatalyst for Hydrogen Evolution Reaction in both Acidic and Alkaline Medium. J. Mater. Chem. A 2018, 6, 7842–7850. [Google Scholar] [CrossRef]
- Yu, B.; Qi, F.; Chen, Y.F.; Wang, X.Q.; Zheng, B.J.; Zhang, W.L.; Li, Y.R.; Zhang, L.C. Nanocrystalline Co0.85Se Anchored on Graphene Nanosheets as a Highly Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. ACS Appl. Mater. Inter. 2017, 9, 30703–30710. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zhang, K.; Lin, H.L.; Li, X.; Chan, H.C.; Yang, L.C.; Gao, Q.S. MoS2–Ni3S2 Heteronanorods as Efficient and Stable Bifunctional Electrocatalysts for Overall Water Splitting. ACS Catal. 2017, 7, 2357–2366. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.Y.; Ge, X.M.; Liu, Z.L.; Wang, J.Y.; Wang, X. Ultrathin MoS2 Nanoplates with Rich Active Sites as Highly Efficient Catalyst for Hydrogen Evolution. ACS Appl. Mater. Inter. 2013, 5, 12794–12798. [Google Scholar] [CrossRef]
- Ravikumar, C.H.; Nair, G.V.; Muralikrishna, S.; Nagaraju, D.H.; Balakrishna, R.G. Nanoflower like Structures of MoSe2 and MoS2 as Efficient Catalysts for Hydrogen Evolution. Mater. Lett. 2018, 220, 133–135. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, R.; Lu, W.B.; Wang, Z.; Liu, D.N.; Hao, S.; Du, G.; Asiri, A.M.; Sun, X.P. Energy-Saving Electrolytic Hydrogen Generation: Ni2P Nanoarray as a High-Performance Non-Noble-Metal Electrocatalyst. Angew. Chem. Int. Edit. 2016, 129, 860–864. [Google Scholar] [CrossRef]
- Hu, S.N.; Feng, C.Q.; Wang, S.Q.; Liu, J.W.; Wu, H.M.; Zhang, L.; Zhang, J.J. Ni3N/NF as Bifunctional Catalysts for Both Hydrogen Generation and Urea Decomposition. ACS Appl. Mater. Inter. 2019, 11, 13168–13175. [Google Scholar] [CrossRef]
- Yang, L.Q.; Liu, Y.L.; Wang, L.; Zhao, Z.J.; Xing, C.J.; Shi, S.H.; Yuan, M.L.; Ge, Z.M.; Cai, Z.Y. Co5.47N/rGO@NF as a High-Performance Bifunctional Catalyst for Urea-Assisted Hydrogen Evolution. Catal. Lett. 2019, 49, 31111–31118. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Qi, F.; Zheng, B.; He, J.; Lin, J.; Zhang, W.; Li, Y.; Chen, Y. Self-Assembled Coral-like Hierarchical Architecture Constructed by NiSe2 Nanocrystals with Comparable Hydrogen-Evolution Performance of Precious Platinum Catalyst. ACS Appl. Mater. Interf. 2017, 9, 7154–7159. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Da, P.F.; Zhang, T.; Mao, J.; Liu, H.; Ling, T. Designing Hybrid NiP2/NiO Nanorod Arrays for Efficient Alkaline Hydrogen Evolution. ACS Appl. Mater. Interf. 2018, 10, 17896–17902. [Google Scholar] [CrossRef] [PubMed]
- Min, S.D.; Zhao, C.G.; Chen, G.R.; Qian, X.Z. One-Pot Hydrothermal Synthesis of Reduced Graphene Oxide/Ni(OH)2 Films on Nickel Foam for High Performance Supercapacitors. Electrochim. Acta 2014, 115, 155–164. [Google Scholar] [CrossRef]
- Song, R.; Luo, B.; Geng, J.; Song, D.; Jing, D. Photothermocatalytic Hydrogen Evolution over Ni2P/TiO2 for Full-Spectrum Solar Energy Conversion. Ind. Eng. Chem. Res. 2018, 57, 7846–7854. [Google Scholar] [CrossRef]
- Kuai, C.G.; Zhang, Y.; Wu, D.Y.; Sokaras, D.; Mu, L.Q.; Spence, S.; Nordlund, D.; Lin, F.; Du, X.W. Fully Oxidized Ni-Fe Layered Double Hydroxide with 100% Exposed Active Sites for Catalyzing Oxygen Evolution Reaction. ACS Catal. 2019, 9, 6027–6032. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Xu, Z.W.; Yan, K.; Zhao, H.B.; Zhang, J.D. One-Step Synthesis of CuO–Cu2O Heterojunction by Flame Spray Pyrolysis for Cathodic Photoelectrochemical Sensing of l-Cysteine. ACS Appl. Mater. Inter. 2017, 9, 40452–40460. [Google Scholar] [CrossRef]
- Qiao, L.Q.; Zhou, M.; Li, Y.H.; Zhang, A.J.; Deng, J.; Liao, M.J.; Xiao, P.; Zhang, Y.H.; Zhang, S.T. Enhancing Electrochemical Hydrogen Generation by Platinum-Modification of p-Type Silicon Wires Array under Visible Light. J. Electrochem. Soc. 2014, 161, H458–H463. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Bifunctional Electrocatalysts: Hierarchical NiCo2S4 Nanowire Arrays Supported on Ni Foam: An Efficient and Durable Bifunctional Electrocatalyst for Oxygen and Hydrogen Evolution Reactions. Adv. Funct. Mater. 2016, 26, 4661–4672. [Google Scholar] [CrossRef]
- Wang, J.M.; Ma, X.; Qu, F.L.; Asiri, A.M.; Sun, X.P. Fe-doped Ni2P nanosheet array for high-efficiency electrochemical water oxidation. Inorg. Chem. 2017, 56, 1041–1044. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, M.R.; Sheng, W.C.; Yan, Y.S. Hollow chevrel-phase NiMo3S4 for hydrogen evolution in alkaline electrolytes. Angew. Chem. Int. Ed. 2016, 55, 15240–15245. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.D.; Gao, H.Y.; Xing, L.W.; Chen, X.; Dong, W.J.; Huang, X.B.; Wang, G. 3D Self-Supported Porous NiO@NiMoO4 Core–Shell Nanosheets for Highly Efficient Oxygen Evolution Reaction. Inorg. Chem. 2019, 58, 6758–6764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.H.; Jia, S.P.; Xu, H.Q.; Zang, J.B.; Liu, J.; Xu, X.P. A hybrid of NiMo-Mo2C/C as non-noble metal electrocatalyst for hydrogen evolution reaction in an acidic solution. Electrochim. Acta 2016, 222, 747–754. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Li, F.; Liu, B.; Zhang, P. Ni2P/rGO/NF Nanosheets As a Bifunctional High-Performance Electrocatalyst for Water Splitting. Materials 2020, 13, 744. https://doi.org/10.3390/ma13030744
Huang J, Li F, Liu B, Zhang P. Ni2P/rGO/NF Nanosheets As a Bifunctional High-Performance Electrocatalyst for Water Splitting. Materials. 2020; 13(3):744. https://doi.org/10.3390/ma13030744
Chicago/Turabian StyleHuang, Jinyu, Feifei Li, Baozhong Liu, and Peng Zhang. 2020. "Ni2P/rGO/NF Nanosheets As a Bifunctional High-Performance Electrocatalyst for Water Splitting" Materials 13, no. 3: 744. https://doi.org/10.3390/ma13030744
APA StyleHuang, J., Li, F., Liu, B., & Zhang, P. (2020). Ni2P/rGO/NF Nanosheets As a Bifunctional High-Performance Electrocatalyst for Water Splitting. Materials, 13(3), 744. https://doi.org/10.3390/ma13030744




