Willow Bark for Sustainable Energy Storage Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.3. Electrode Preparation
2.4. Material Characterization
2.4.1. Evaluation of Accessible Surface Area
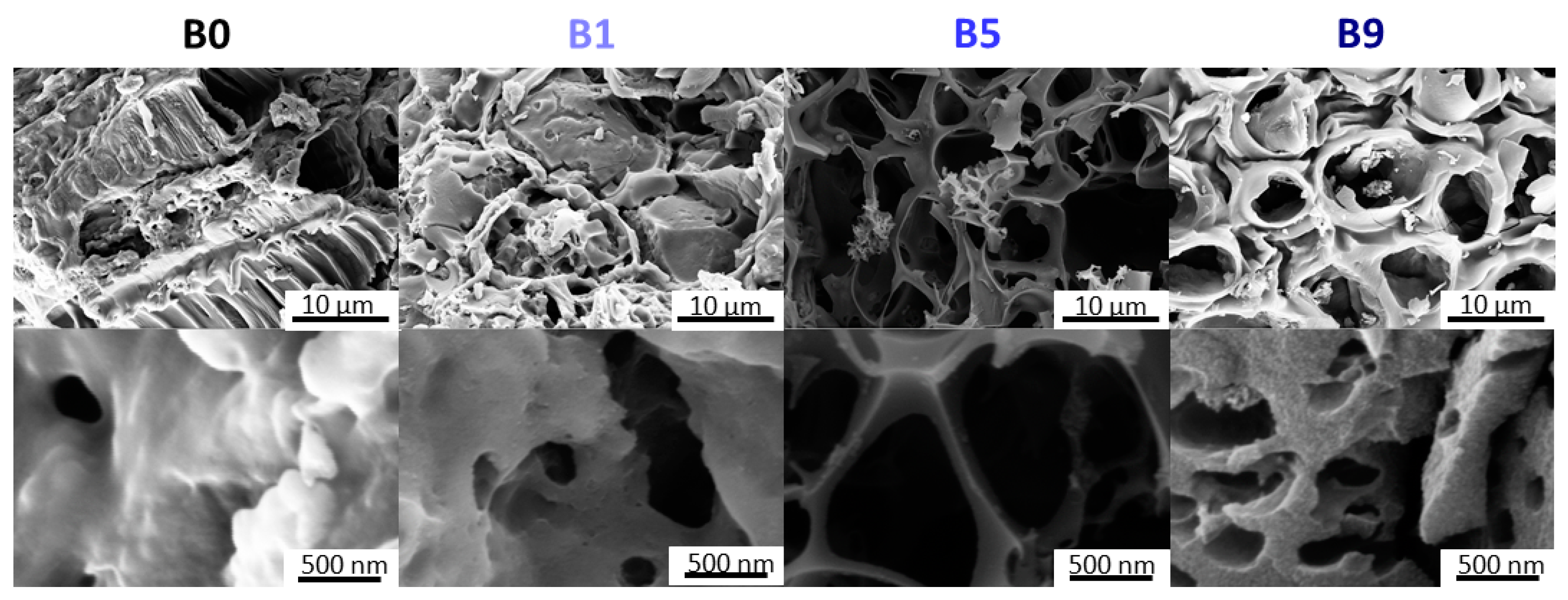
2.4.2. Scanning Electron Microscopy
2.4.3. Cell Preparation
3. Results and Discussion
3.1. Activation and Material Characterization
3.2. Supercapacitor
3.3. Comparison with Other Biobased Supercapacitor Electrodes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oramahi, H.A.; Diba, F. Maximizing the Production of Liquid Smoke from Bark of Durio by Studying its Potential Compounds. Procedia Environ. Sci. 2013, 17, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Şensöz, S. Slow pyrolysis of wood barks from Pinus brutia Ten. and product compositions. Bioresour. Technol. 2003, 89, 307–311. [Google Scholar] [CrossRef]
- Hengst, G.E.; Dawson, J.O. Bark properties and fire resistance of selected tree species from the central hardwood region of North America. Can. J. For. Res. 1994, 24, 688–696. [Google Scholar] [CrossRef]
- Bauer, G.; Speck, T.; Blömer, J.; Bertling, J.; Speck, O. Insulation capability of the bark of trees with different fire adaptation. J. Mat. Sci. 2010, 45, 5950–5959. [Google Scholar] [CrossRef]
- Sudakova, I.G.; Ivanov, I.P.; Ivanchenko, N.M.; Kuznetsov, B.N. Protective compositions for wood on the basis of the suberin of birch bark. Chem. Plant Raw Mater. 2005, 1, 59–63. [Google Scholar]
- Pásztory, Z.; Mohácsiné, I.R.; Gorbacheva, G.; Börcsök, Z. The utilization of tree bark. BioResources 2016, 11, 7859–7888. [Google Scholar] [CrossRef]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient storage mechanisms for building better supercapacitors. Nat. Energy 2016, 1, 16070. [Google Scholar] [CrossRef]
- Beguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251, 2283. [Google Scholar] [CrossRef]
- Lin, Z.; Goikolea, E.; Balducci, A.; Naoi, K.; Taberna, P.L.; Salanne, M.; Yushin, G.; Simon, P. Materials for supercapacitors: When Li-ion battery power is not enough. Mater. Today 2018, 21, 419–436. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Lee, J.H.; Roh, K.C. Herbaceous Biomass Waste-Derived Activated Carbons for Supercapacitors. J. Electrochem. Sci. Technol. 2019, 9, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Sevilla, M.; Diez, N.; Ferrero, G.A.; Fuertes, A.B. Sustainable supercapacitor electrodes produced by the activation of biomass with sodium thiosulfate. Energy Storage Mater. 2019, 18, 356–365. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, Y.; Zhang, L.; Xie, Y.; Ye, X. Facile synthesis of three-dimensional porous carbon for high-performance supercapacitors. J. Alloys Compd. 2019, 787, 1–8. [Google Scholar] [CrossRef]
- Liew, S.Y.; Thielemans, W.; Freunberger, S.; Spirk, S. Polysaccharide Based Supercapacitors; Springer International Publishing: Basel, Switzerland, 2017. [Google Scholar]
- Manchala, S.; Tandava, V.S.R.K.; Jampaiah, D.; Bhargava, S.K.; Shanker, V. Novel and Highly Efficient Strategy for the Green Synthesis of Soluble Graphene by Aqueous Polyphenol Extracts of Eucalyptus Bark and Its Applications in High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 11612–11620. [Google Scholar] [CrossRef]
- Momodu, D.; Madito, M.; Barzegar, F.; Bello, A.; Khaleed, A.; Olaniyan, O.; Dangbegnon, J.; Manyala, N. Activated carbon derived from tree bark biomass with promising material properties for supercapacitors. J. Solid State Electrochem. 2016, 21, 859–872. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Qiu, Z.; Zhou, J.; Si, W.; Cui, H.; Zhuo, S. Hierarchical porous carbons from alkaline poplar bark extractive-based phenolic resins for supercapacitors. Electrochim. Acta 2015, 180, 1007–1013. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, Q.; Wei, X.; Gao, Y.; Li, H. A Facile and Low-Cost Route to Heteroatom Doped Porous Carbon Derived from Broussonetia Papyrifera Bark with Excellent Supercapacitance and CO2 Capture Performance. Sci. Rep. 2016, 6, 22646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spirk, S. Polysaccharides as Battery Components; Springer International Publishing: Basel, Switzerland, 2018; p. 59. [Google Scholar]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Understanding chemical reactions between carbons and NaOH and KOH. Carbon 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Phiri, J.; Dou, J.; Vuorinen, T.; Gane, P.A.C.; Maloney, T.C. Highly Porous Willow Wood-Derived Activated Carbon for High-Performance Supercapacitor Electrodes. ACS Omega 2019, 4, 18108–18117. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Laheäär, A.; Przygocki, P.; Abbas, Q.; Béguin, F. Appropriate methods for evaluating the efficiency and capacitive behavior of different types of supercapacitors. Electrochem. Commun. 2015, 60, 21–25. [Google Scholar] [CrossRef]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Hydrothermal Carbonization of Abundant Renewable Natural Organic Chemicals for High-Performance Supercapacitor Electrodes. Adv. Energy Mater. 2011, 1, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Gogotsi, Y.; Simon, P. True performance metrics in electrochemical energy storage. Science 2011, 334, 917–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobele, G.; Volperts, A.; Telysheva, G.; Zhurinsh, A.; Vervikishko, D.; Sametov, A.; Shkolnikov, E.; Ozolinsh, J. Wood-based activated carbons for supercapacitors with organic electrolyte. Holzforschung 2015, 69, 777–784. [Google Scholar] [CrossRef]
- Ma, Y.-Z.; Guo, Y.; Zhou, C.; Wang, C.-Y. Biomass-derived dendritic-like porous carbon aerogels for supercapacitors. Electrochim. Acta 2016, 210, 897–904. [Google Scholar] [CrossRef]
- Kurniawan, A.; Ong, L.K.; Kurniawan, F.; Lin, C.X.; Soetaredjo, F.E.; Zhao, X.S.; Ismadji, S. Easy approach to synthesize N/P/K co-doped porous carbon microfibers from cane molasses as a high performance supercapacitor electrode material. RSC Adv. 2014, 4, 34739–34750. [Google Scholar] [CrossRef] [Green Version]




| Carbon Source | Capacitance (F g−1) | Cycling Rate | Electrode Mass (mg cm−2) | Solvent | Reference |
|---|---|---|---|---|---|
| Cellulose, starch, eucalyptus wood | 236 | 1 mV s−1 | - | ACN | [23] |
| Herbaceous biomass waste | 127 | 0.5 mA cm−2 | 11 | ACN | [10] |
| Wood sawdust and tannic acid | 140 | 0.2 A g−1 | 9–11 | ACN | [11] |
| Birch wood sawdust | 160 | 0.01 A g−1 | 4 | ACN | [25] |
| Sucrose | 120 | 1 A g−1 | 1.5–3 | ACN | [12] |
| Leonardite fulvic acid | 170 | 0.05 A g−1 | - | PC | [26] |
| Carbon microfibers | 172 | 1 A g−1 | 3 | ACN | [27] |
| Willow bark | 147 | 1 mV s−1 | 4 | ACN | This work |
| Willow wood | 394 | 1 A g−1 | 1.5–2 | Aqu. | [20] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobisch, M.A.; Phiri, J.; Dou, J.; Gane, P.; Vuorinen, T.; Bauer, W.; Prehal, C.; Maloney, T.; Spirk, S. Willow Bark for Sustainable Energy Storage Systems. Materials 2020, 13, 1016. https://doi.org/10.3390/ma13041016
Hobisch MA, Phiri J, Dou J, Gane P, Vuorinen T, Bauer W, Prehal C, Maloney T, Spirk S. Willow Bark for Sustainable Energy Storage Systems. Materials. 2020; 13(4):1016. https://doi.org/10.3390/ma13041016
Chicago/Turabian StyleHobisch, Mathias Andreas, Josphat Phiri, Jinze Dou, Patrick Gane, Tapani Vuorinen, Wolfgang Bauer, Christian Prehal, Thaddeus Maloney, and Stefan Spirk. 2020. "Willow Bark for Sustainable Energy Storage Systems" Materials 13, no. 4: 1016. https://doi.org/10.3390/ma13041016