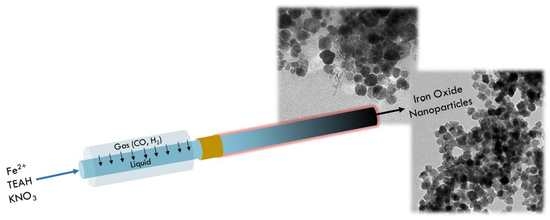
A Modular Millifluidic Platform for the Synthesis of Iron Oxide Nanoparticles with Control over Dissolved Gas and Flow Configuration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Particle Synthesis
- Reactor System 1: this configuration comprised a liquid–liquid segmented flow reactor, where the organic phase (heptane) wets the reactor wall and segments the aqueous stream. A segmented flow pattern was achieved (size of slugs/droplets ~4 mm) by mixing the streams containing the iron precursor with the base solution and the organic stream in a polyether ether ketone (PEEK) cross junction (1 mm ID, Upchurch Scientific, Oak Harbor, WA, USA). The flow rate of the organic stream was set to 1 mL/min, while equal flow rates of 0.5 mL/min were used for the iron precursor and the base solutions. In this system, the gas–liquid contactor was not used, as the solutions were preventively bubbled with (inert gas). A schematic of the setup is shown in Figure 1b.
- Reactor System 2: this configuration comprised the tube-in-tube gas–liquid contactor upstream of the reaction coil. In the tube-in-tube contactor, an organic stream (heptane) was saturated with . The heptane stream was used to segment the aqueous one before entering the reactor. The two water solutions (base and iron precursor) were mixed and segmented in a PEEK cross junction (1 mm ID, Upchurch Scientific, Oak Harbor, WA, USA). The flow rate of the organic stream was set to 1 mL/min, while equal flow rates of 0.5 mL/min were used for the iron precursor and the base solutions. A schematic of the setup is shown in Figure 1c.
- Reactor System 3: this configuration consisted of a single-phase reactor where the two reactant streams containing the iron precursor and the base were mixed in a PEEK T-junction (1 mm ID, Upchurch Scientific, Oak Harbor, WA, USA) before entering the reaction stage. The overall flow rate was set to 2 mL/min, and each aqueous stream was pumped at 1 mL/min. In this case, the gas–liquid contactor was not used, as the solutions were preventively bubbled with (inert gas). A schematic of the setup is shown in Figure 1d.
- Reactor System 4: this configuration consisted of a single-phase reactor. Here the base solution passed through the tube-in-tube contactor at a flow rate of 0.5 mL/min, where it was saturated with , and then mixed with the solution of iron precursor (1.5 mL/min). The two solutions were mixed in a PEEK T-junction (1 mm ID, Upchurch Scientific, Oak Harbor, WA, USA) before entering the reaction stage. A schematic of the setup is shown in Figure 1e.
2.3. Reactor Scale Up
2.4. Particle Characterization
3. Results and Discussion
3.1. Two-Phase Flow Reactor Systems
3.2. Single-Phase Flow Reactor Systems
3.3. Magnetic and Heating Properties of the Nanoparticles
3.4. Scaled-up Reactor System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D. Appl. Phys. 2009, 42, 224001–224016. [Google Scholar] [CrossRef]
- Thanh, N.T.K. Magnetic Nanoparticles: From Fabrication to Clinical Applications; Taylor & Francis: Milton Park, UK, 2012. [Google Scholar]
- Thanh, N.T.K. Clinical Applications of Magnetic Nanoparticles; Taylor & Francis: Milton Park, UK, 2018. [Google Scholar]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Hervault, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef] [PubMed]
- Ranzoni, A.; Sabatte, G.; Van Ijzendoorn, L.J.; Prins, M.W.J. One-step homogeneous magnetic nanoparticle immunoassay for biomarker detection directly in blood plasma. ACS Nano 2012, 6, 3134–3141. [Google Scholar] [CrossRef]
- Huy, T.Q.; Van Chung, P.; Thuy, N.T.; Blanco-Andujar, C.; Thanh, N.T.K. Protein A-conjugated iron oxide nanoparticles for separation of Vibrio cholerae from water samples. Faraday Discuss. 2014, 175, 73–82. [Google Scholar] [CrossRef]
- Hachani, R.; Birchall, M.A.; Lowdell, M.W.; Kasparis, G.; Tung, L.D.; Manshian, B.B.; Soenen, S.J.; Gsell, W.; Himmelreich, U.; Gharagouzloo, C.A.; et al. Assessing cell-nanoparticle interactions by high content imaging of biocompatible iron oxide nanoparticles as potential contrast agents for magnetic resonance imaging. Sci. Rep. 2017, 7, 7850–7864. [Google Scholar] [CrossRef]
- Hachani, R.; Lowdell, M.; Birchall, M.; Hervault, A.; Mertz, D.; Begin-Colin, S.; Thanh, N.T.K. Polyol synthesis, functionalisation, and biocompatibility studies of superparamagnetic iron oxide nanoparticles as potential MRI contrast agents. Nanoscale 2016, 8, 3278–3287. [Google Scholar] [CrossRef]
- Hobson, N.J.; Weng, X.; Ashford, M.; Thanh, N.T.K.; Schätzlein, A.G.; Uchegbu, I.F. Facile aqueous, room temperature preparation of high transverse relaxivity clustered iron oxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 165–171. [Google Scholar] [CrossRef]
- Hobson, N.J.; Weng, X.; Siow, B.; Veiga, C.; Ashford, M.; Thanh, N.T.K.; Schätzlein, A.G.; Uchegbu, I.F. Clustering superparamagnetic iron oxide nanoparticles produces organ-targeted high-contrast magnetic resonance images. Nanomedicine 2019, 14, 1135–1152. [Google Scholar] [CrossRef]
- Eberbeck, D.; Dennis, C.L.; Huls, N.F.; Krycka, K.L.; Gruttner, C.; Westphal, F. Multicore magnetic nanoparticles for magnetic particle imaging. IEEE Trans. Magn. 2013, 49, 269–274. [Google Scholar] [CrossRef]
- Satarkar, N.S.; Hilt, J.Z. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. J. Control. Release 2008, 130, 246–251. [Google Scholar] [CrossRef]
- Hoare, T.; Santamaria, J.; Goya, G.F.; Irusta, S.; Lin, D.; Lau, S.; Padera, R.; Langer, R.; Kohane, D.S. A magnetically triggered composite membrane for on-demand drug delivery. Nano Lett. 2009, 9, 3651–3657. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Hervault, A.; Dunn, A.E.; Lim, M.; Boyer, C.; Mott, D.; Maenosono, S.; Thanh, N.T.K. Doxorubicin loaded dual pH- and thermo-responsive magnetic nanocarrier for combined magnetic hyperthermia and targeted controlled drug delivery applications. Nanoscale 2016, 8, 12152–12161. [Google Scholar] [CrossRef] [PubMed]
- Fornara, A.; Johansson, P.; Petersson, K.; Gustafsson, S.; Jian, Q.; Olsson, E.; Iiver, D.; Krozer, A.; Muhammed, M.; Johansson, C. Tailored magnetic nanoparticles for direct and sensitive detection of biomolecules in biological samples. Nano Lett. 2008, 8, 3423–3428. [Google Scholar] [CrossRef]
- Meddahi-Pellé, A.; Legrand, A.; Marcellan, A.; Louedec, L.; Letourneur, D.; Leibler, L. Organ repair, hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew. Chemie Int. Ed. 2014, 53, 6369–6373. [Google Scholar] [CrossRef]
- Lagrow, A.P.; Besenhard, M.O.; Hodzic, A.; Sergides, A.; Bogart, L.K.; Gavriilidis, A.; Thanh, N.T.K. Unravelling the growth mechanism of the co-precipitation of iron oxide nanoparticles with the aid of synchrotron X-Ray diffraction in solution. Nanoscale 2019, 11, 6620–6628. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Ortega, D.; Pankhurst, Q.A.; Thanh, N.T.K. Elucidating the morphological and structural evolution of iron oxide nanoparticles formed by sodium carbonate in aqueous medium. J. Mater. Chem. 2012, 22, 12498–12506. [Google Scholar] [CrossRef]
- Ahn, T.; Kim, J.H.; Yang, H.M.; Lee, J.W.; Kim, J.D. Formation pathways of magnetite nanoparticles by coprecipitation method. J. Phys. Chem. C 2012, 116, 6069–6076. [Google Scholar] [CrossRef]
- Gutiérrez, L.; Costo, R.; Grüttner, C.; Westphal, F.; Gehrke, N.; Heinke, D.; Fornara, A.; Pankhurst, Q.A.; Johansson, C.; Veintemillas-Verdaguer, S.; et al. Synthesis methods to prepare single- and multi-core iron oxide nanoparticles for biomedical applications. Dalt. Trans. 2015, 44, 2943–2952. [Google Scholar] [CrossRef]
- Abou Hassan, A.; Sandre, O.; Cabuil, V.; Tabeling, P. Synthesis of iron oxide nanoparticles in a microfluidic device: preliminary results in a coaxial flow millichannel. Chem. Commun. 2008, 15, 1783–1785. [Google Scholar] [CrossRef]
- Abou-Hassan, A.; Dufrêchfer, J.F.; Sandre, O.; Mériguet, G.; Bernard, O.; Cabuil, V. Fluorescence confocal laser scanning microscopy for pH mapping in a coaxial flow microreactor: Application in the synthesis of superparamagnetic nanoparticles. J. Phys. Chem. C 2009, 113, 18097–18105. [Google Scholar] [CrossRef]
- Norfolk, L.; Rawlings, A.E.; Bramble, J.P.; Ward, K.; Francis, N.; Waller, R.; Bailey, A.; Staniland, S.S. Macrofluidic coaxial flow platforms to produce tunable magnetite nanoparticles: A study of the effect of reaction conditions and biomineralisation protein mms6. Nanomaterials 2019, 9, 1729. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Nightingale, A.M.; Krishnadasan, S.H.; Kamaly, N.; Wylenzinska-Arridge, M.; Zeissler, K.; Branford, W.R.; Ware, E.; DeMello, A.J.; DeMello, J.C. Direct synthesis of dextran-coated superparamagnetic iron oxide nanoparticles in a capillary-based droplet reactor. J. Mater. Chem. 2012, 22, 4704–4708. [Google Scholar] [CrossRef]
- Tong, S.; Quinto, C.A.; Zhang, L.; Mohindra, P.; Bao, G. Size-dependent heating of magnetic iron oxide nanoparticles. ACS Nano 2017, 11, 6808–6816. [Google Scholar] [CrossRef] [PubMed]
- Santoyo Salazar, J.; Perez, L.; De Abril, O.; Truong Phuoc, L.; Ihiawakrim, D.; Vazquez, M.; Greneche, J.M.; Begin-Colin, S.; Pourroy, G. Magnetic iron oxide nanoparticles in 10–40 nm range: Composition in terms of magnetite/maghemite ratio and effect on the magnetic properties. Chem. Mater. 2011, 23, 1379–1386. [Google Scholar] [CrossRef]
- Sugimoto, T.; Matijević, E. Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels. J. Colloid Interface Sci. 1980, 74, 227–243. [Google Scholar] [CrossRef]
- Vergés, M.A.; Costo, R.; Roca, A.G.; Marco, J.F.; Goya, G.F.; Serna, C.J.; Morales, M.P. Uniform and water stable magnetite nanoparticles with diameters around the monodomain-multidomain limit. J. Phys. D. Appl. Phys. 2008, 41, 134003. [Google Scholar] [CrossRef]
- Larrea, A.; Sebastian, V.; Ibarra, A.; Arruebo, M.; Santamaria, J. Gas slug microfluidics: a unique tool for ultrafast, highly controlled growth of iron oxide nanostructures. Chem. Mater. 2015, 27, 4254–4260. [Google Scholar] [CrossRef]
- Hessel, V.; Angeli, P.; Gavriilidis, A.; Löwe, H. Gas-liquid and gas-liquid-solid microstructured reactors: Contacting principles and applications. Ind. Eng. Chem. Res. 2005, 44, 9750–9769. [Google Scholar] [CrossRef]
- Kashid, M.N.; Renken, A.; Kiwi-Minsker, L. Microstructured Reactors for Fluid-Fluid Reactions; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Dencic, I.; Hessel, V. Gas-Liquid Reactions. In Microreactors in Organic Chemistry and Catalysis; Wirth, T., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 221–288. [Google Scholar]
- Yang, L.; Jensen, K.F. Mass transport and reactions in the tube-in-tube reactor. Org. Process Res. Dev. 2013, 17, 927–933. [Google Scholar] [CrossRef]
- Khan, S.A.; Günther, A.; Schmidt, M.A.; Jensen, K.F. Microfluidic synthesis of colloidal silica. Langmuir 2004, 20, 8604–8611. [Google Scholar] [CrossRef] [PubMed]
- Sebastian Cabeza, V.; Kuhn, S.; Kulkarni, A.A.; Jensen, K.F. Size-controlled flow synthesis of gold nanoparticles using a segmented flow microfluidic platform. Langmuir 2012, 28, 7007–7013. [Google Scholar] [CrossRef] [PubMed]
- Correa, J.R.; Canetti, D.; Castillo, R.; Llópiz, J.C.; Dufour, J. Influence of the precipitation pH of magnetite in the oxidation process to maghemite. Mater. Res. Bull. 2006, 41, 703–713. [Google Scholar] [CrossRef]
- Larrea, A.; Eguizabal, A.; Sebastián, V. Gas-directed production of noble metal-magnetic heteronanostructures in continuous fashion: application in catalysis. ACS Appl. Mater. Interfaces 2019, 11, 43520–43532. [Google Scholar] [CrossRef]
- Sebastian, V.; Smith, C.D.; Jensen, K.F. Shape-controlled continuous synthesis of metal nanostructures. Nanoscale 2016, 8, 7534–7543. [Google Scholar] [CrossRef]
- Rahman, M.T.; Krishnamurthy, P.G.; Parthiban, P.; Jain, A.; Park, C.P.; Kim, D.P.; Khan, S.A. Dynamically tunable nanoparticle engineering enabled by short contact-time microfluidic synthesis with a reactive gas. RSC Adv. 2013, 3, 2987–2990. [Google Scholar] [CrossRef]
- Huang, H.; Hwang, G.B.; Wu, G.; Karu, K.; Du Toit, H.; Wu, H.; Callison, J.; Parkin, I.P.; Gavriilidis, A. Rapid synthesis of [Au25(Cys)18] nanoclusters via carbon monoxide in microfluidic liquid-liquid segmented flow system and their antimicrobial performance. Chem. Eng. J. 2019. [Google Scholar] [CrossRef]
- Falk, L.; Commenge, J.M. Performance comparison of micromixers. Chem. Eng. Sci. 2010, 65, 405–411. [Google Scholar] [CrossRef]
- Glasnov, T.N.; Kappe, C.O. The microwave-to-flow paradigm: Translating high-temperature batch microwave chemistry to scalable continuous-flow processes. Chem. A Eur. J. 2011, 17, 11956–11968. [Google Scholar] [CrossRef]
- Sebastian, V.; Pilar Calatayud, M.; Goya, G.F.; Santamaria, J. Magnetically-driven selective synthesis of Au clusters on Fe3O4 nanoparticles. Chem. Commun. 2013, 49, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Skaff, H.; Emrick, T.; Dinsmore, A.D.; Russell, T.P. Nanoparticle assembly and transport at liquid-liquid interfaces. Science 2003, 299, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Tong, L.; Xia, Y. Synthesis of colloidal metal nanocrystals in droplet reactors: The pros and cons of interfacial adsorption. Nano Lett. 2014, 14, 4189–4194. [Google Scholar] [CrossRef] [PubMed]
- Uson, L.; Arruebo, M.; Sebastian, V.; Santamaria, J. Single phase microreactor for the continuous, high-temperature synthesis of <4 nm superparamagnetic iron oxide nanoparticles. Chem. Eng. J. 2018, 340, 66–72. [Google Scholar]
- Tandon, V.; Bhagavatula, S.K.; Nelson, W.C.; Kirby, B.J. Zeta potential and electroosmotic mobility in microfluidic devices fabricated from hydrophobic polymers: 1. The origins of charge. Electrophoresis 2008, 29, 1092–1101. [Google Scholar] [CrossRef]
- Chen, M.; Wu, B.; Yang, J.; Zheng, N. Small adsorbate-assisted shape control of Pd and Pt nanocrystals. Adv. Mater. 2012, 24, 862–879. [Google Scholar] [CrossRef]
- Li, P.; Miser, D.E.; Rabiei, S.; Yadav, R.T.; Hajaligol, M.R. The removal of carbon monoxide by iron oxide nanoparticles. Appl. Catal. B Environ. 2003, 43, 151–162. [Google Scholar] [CrossRef]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D. Appl. Phys. 2014, 47, 495003–495017. [Google Scholar] [CrossRef]
- Marciello, M.; Connord, V.; Veintemillas-Verdaguer, S.; Vergés, M.A.; Carrey, J.; Respaud, M.; Serna, C.J.; Morales, M.P. Large scale production of biocompatible magnetite nanocrystals with high saturation magnetization values through green aqueous synthesis. J. Mater. Chem. B 2013, 1, 5995–6004. [Google Scholar] [CrossRef]
- León Félix, L.; Sanz, B.; Sebastián, V.; Torres, T.E.; Sousa, M.H.; Coaquira, J.A.H.; Ibarra, M.R.; Goya, G.F. Gold-decorated magnetic nanoparticles design for hyperthermia applications and as a potential platform for their surface-functionalization. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Ortega, D.; Southern, P.; Pankhurst, Q.A.; Thanh, N.T.K. High performance multi-core iron oxide nanoparticles for magnetic hyperthermia: microwave synthesis, and the role of core-to-core interactions. Nanoscale 2015, 7, 1768–1775. [Google Scholar] [CrossRef]
- Famiani, S.; Lagrow, A.P.; Besenhard, M.O.; Maenosono, S.; Thanh, N.T.K. Synthesis of fine-tuning highly magnetic Fe@FexOy nanoparticles through continuous injection and a study of magnetic hyperthermia. Chem. Mater. 2018, 30, 8897–8904. [Google Scholar] [CrossRef]
- Habib, A.H.; Ondeck, C.L.; Chaudhary, P.; Bockstaller, M.R.; McHenry, M.E. Evaluation of iron-cobalt/ferrite core-shell nanoparticles for cancer thermotherapy. J. Appl. Phys. 2008, 103, 7–10. [Google Scholar] [CrossRef]
- Phong, P.T.; Nguyen, L.H.; Manh, D.H.; Lee, I.J.; Phuc, N.X. Computer simulations of contributions of Néel and Brown relaxation to specific loss power of magnetic fluids in hyperthermia. J. Electron. Mater. 2017, 46, 2393–2405. [Google Scholar] [CrossRef]
- Baumgartner, J.; Dey, A.; Bomans, P.H.H.; Le Coadou, C.; Fratzl, P.; Sommerdijk, N.A.J.M.; Faivre, D. Nucleation and growth of magnetite from solution. Nat. Mater. 2013, 12, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Panariello, L.; Mazzei, L.; Gavriilidis, A. Modelling the synthesis of nanoparticles in continuous microreactors: the role of diffusion and residence time distribution on nanoparticle characteristics. Chem. Eng. J. 2018, 350, 1144–1154. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Briens, C.L. Correlation for the direct calculation of the terminal velocity of spherical particles in newtonian and pseudoplastic (power-law) fluids. Powder Technol. 1991, 67, 87–91. [Google Scholar] [CrossRef]








| Reactor System | Particle Size (nm) | Saturation Magnetization () | SAR () | ILP () |
|---|---|---|---|---|
| 2 (two-phase flow with -saturated heptane) | 501 * 765 ** | 2.7 * 2.65 ** | ||
| 3 (single-phase flow with solutions preventively bubble with ) | 79 | 220 * 395 ** | 1.25 * 1.3 ** | |
| 4 (single-phase flow with CO-saturated base solution) | 80 | 215 * 382 ** | 1.2 * 1.3 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panariello, L.; Wu, G.; Besenhard, M.O.; Loizou, K.; Storozhuk, L.; Thanh, N.T.K.; Gavriilidis, A. A Modular Millifluidic Platform for the Synthesis of Iron Oxide Nanoparticles with Control over Dissolved Gas and Flow Configuration. Materials 2020, 13, 1019. https://doi.org/10.3390/ma13041019
Panariello L, Wu G, Besenhard MO, Loizou K, Storozhuk L, Thanh NTK, Gavriilidis A. A Modular Millifluidic Platform for the Synthesis of Iron Oxide Nanoparticles with Control over Dissolved Gas and Flow Configuration. Materials. 2020; 13(4):1019. https://doi.org/10.3390/ma13041019
Chicago/Turabian StylePanariello, Luca, Gaowei Wu, Maximilian O. Besenhard, Katerina Loizou, Liudmyla Storozhuk, Nguyen Thi Kim Thanh, and Asterios Gavriilidis. 2020. "A Modular Millifluidic Platform for the Synthesis of Iron Oxide Nanoparticles with Control over Dissolved Gas and Flow Configuration" Materials 13, no. 4: 1019. https://doi.org/10.3390/ma13041019
APA StylePanariello, L., Wu, G., Besenhard, M. O., Loizou, K., Storozhuk, L., Thanh, N. T. K., & Gavriilidis, A. (2020). A Modular Millifluidic Platform for the Synthesis of Iron Oxide Nanoparticles with Control over Dissolved Gas and Flow Configuration. Materials, 13(4), 1019. https://doi.org/10.3390/ma13041019