Study on CO2 Capture Characteristics and Kinetics of Modified Potassium-Based Adsorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Preparation
2.1.1. Ingredients
2.1.2. Preparation of Aerogel Support
2.1.3. K2CO3 Loading into Aerogel Support
2.2. Experimental Methods
2.3. Kinetic Models
3. Results and Discussion
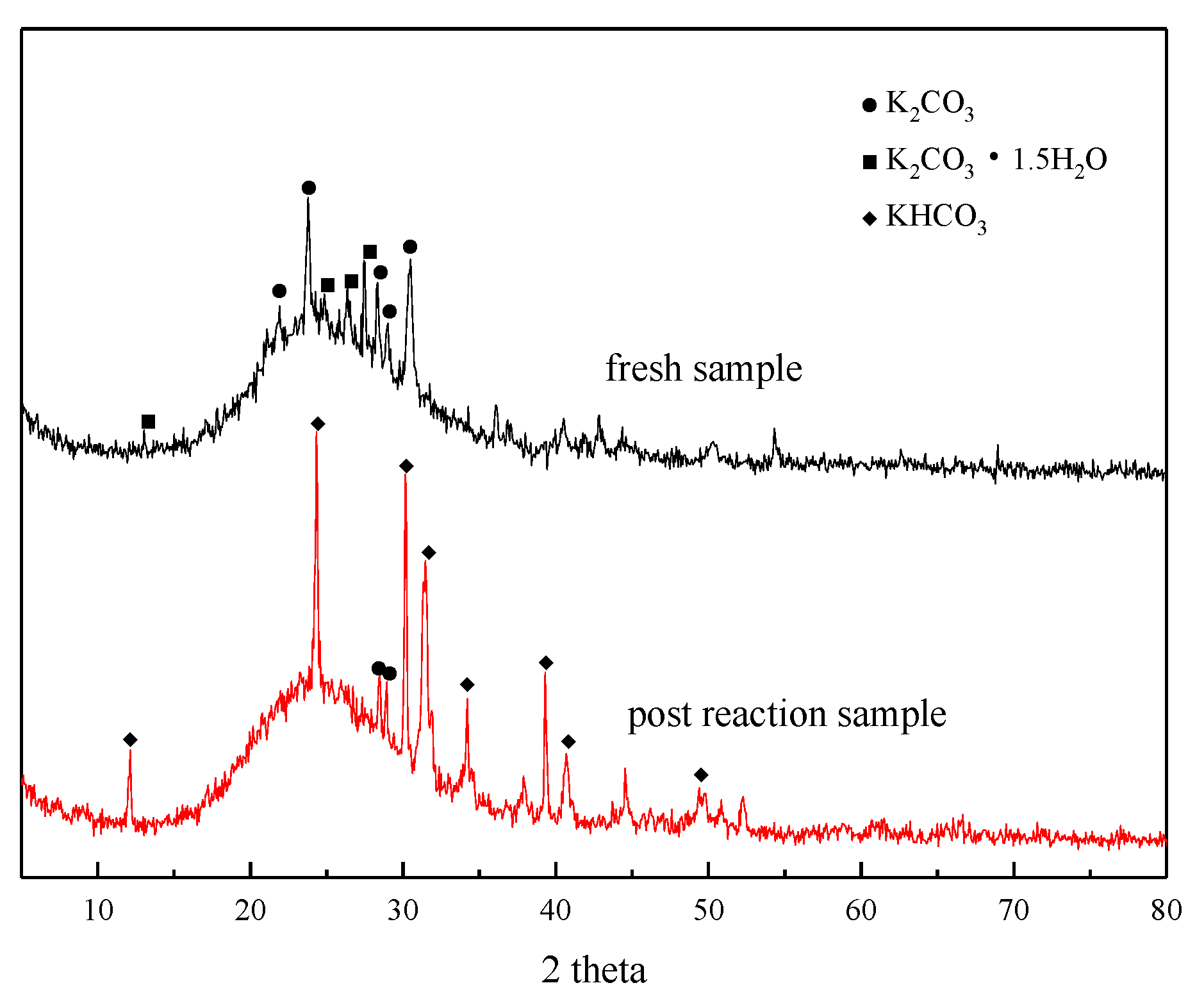
3.1. Adsorbent Characterization
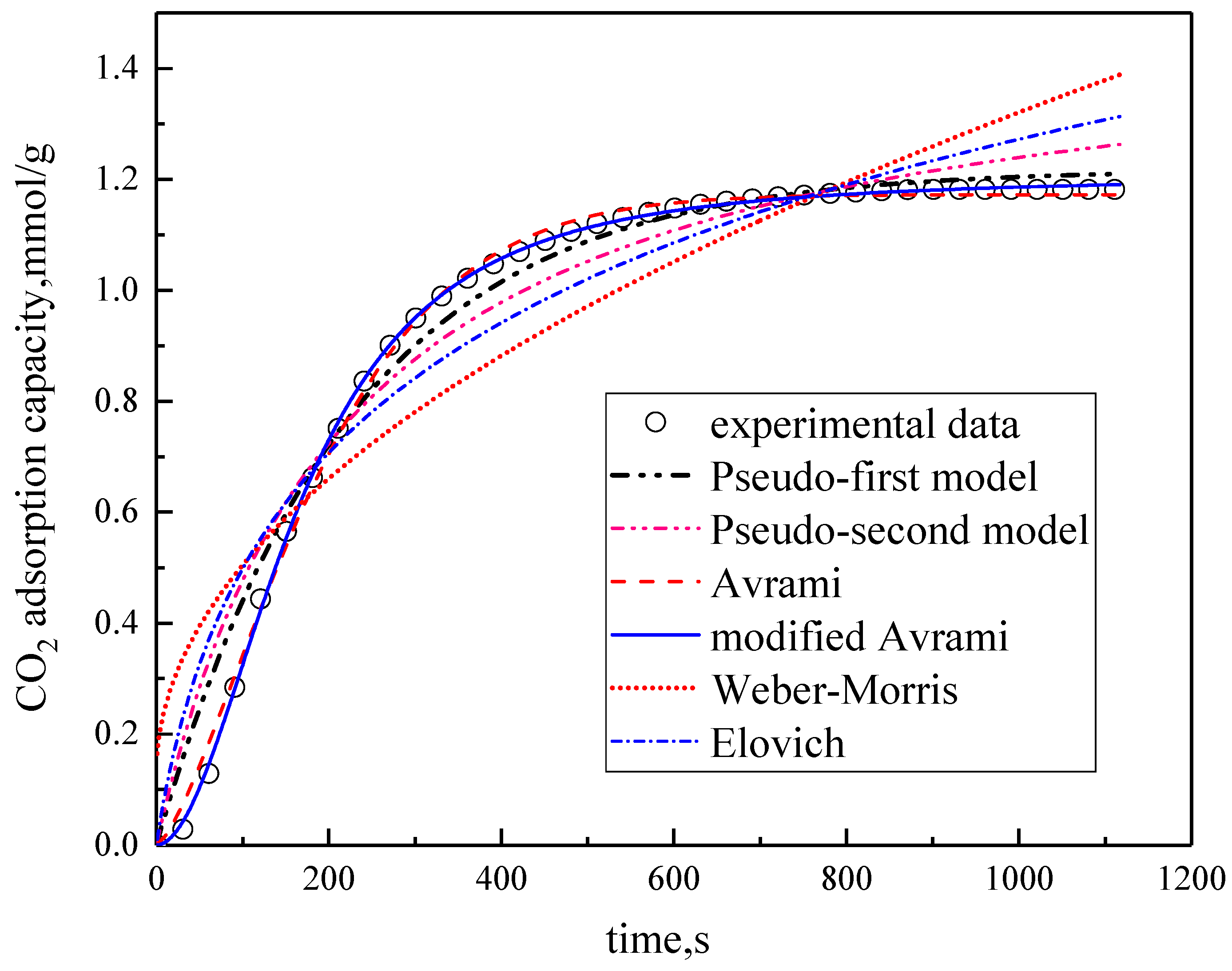
3.2. Comparison of Kinetic Models
3.3. Effects of Reaction Conditions on Adsorption Characteristics
3.3.1. Reaction Temperature
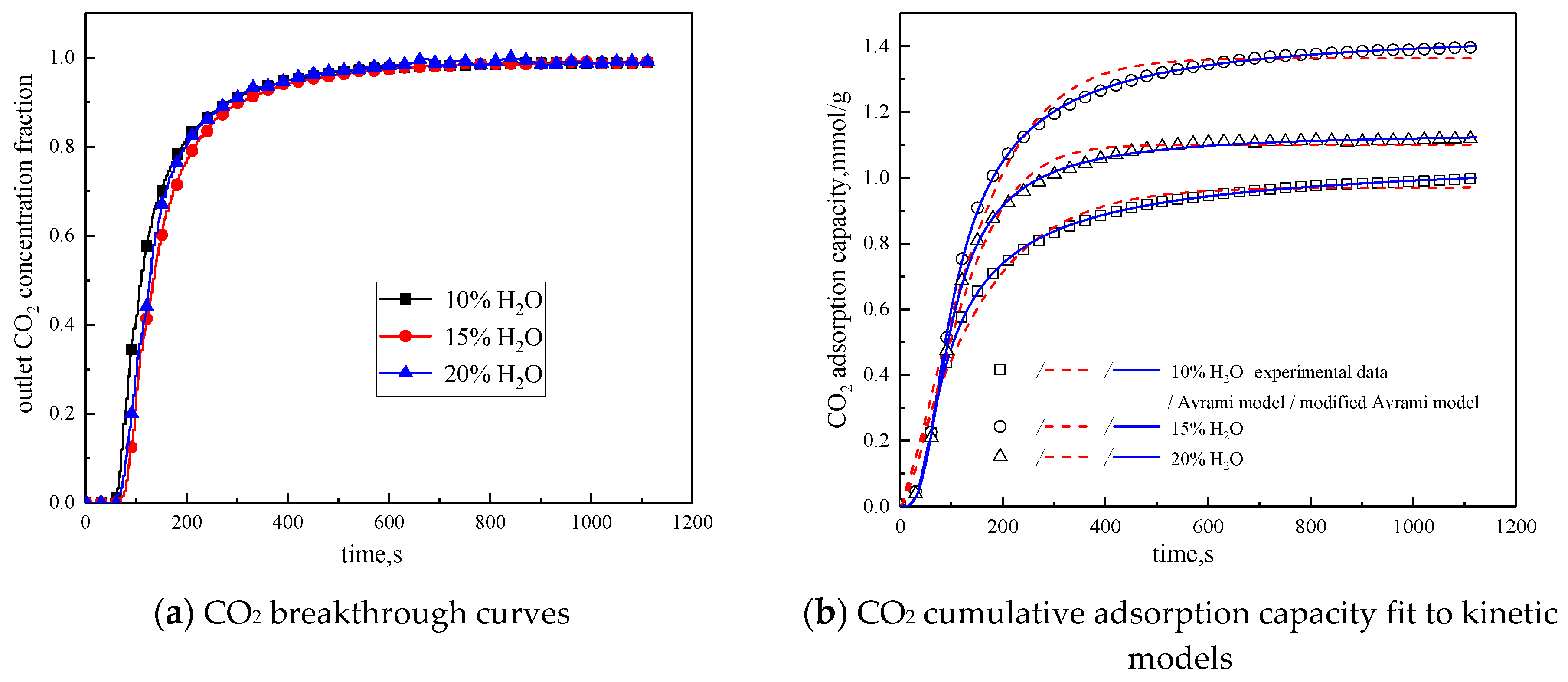
3.3.2. Water Vapor Concentration
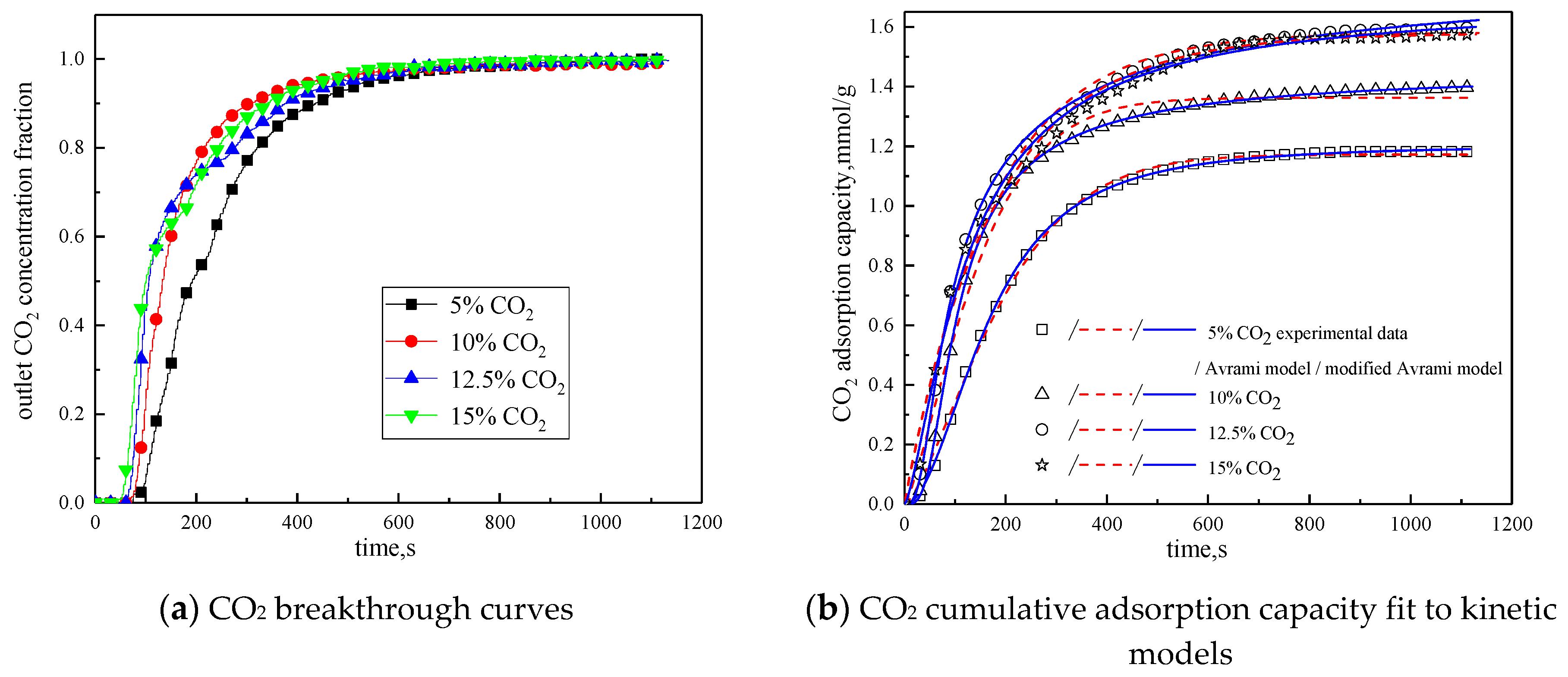
3.3.3. CO2 Concentration
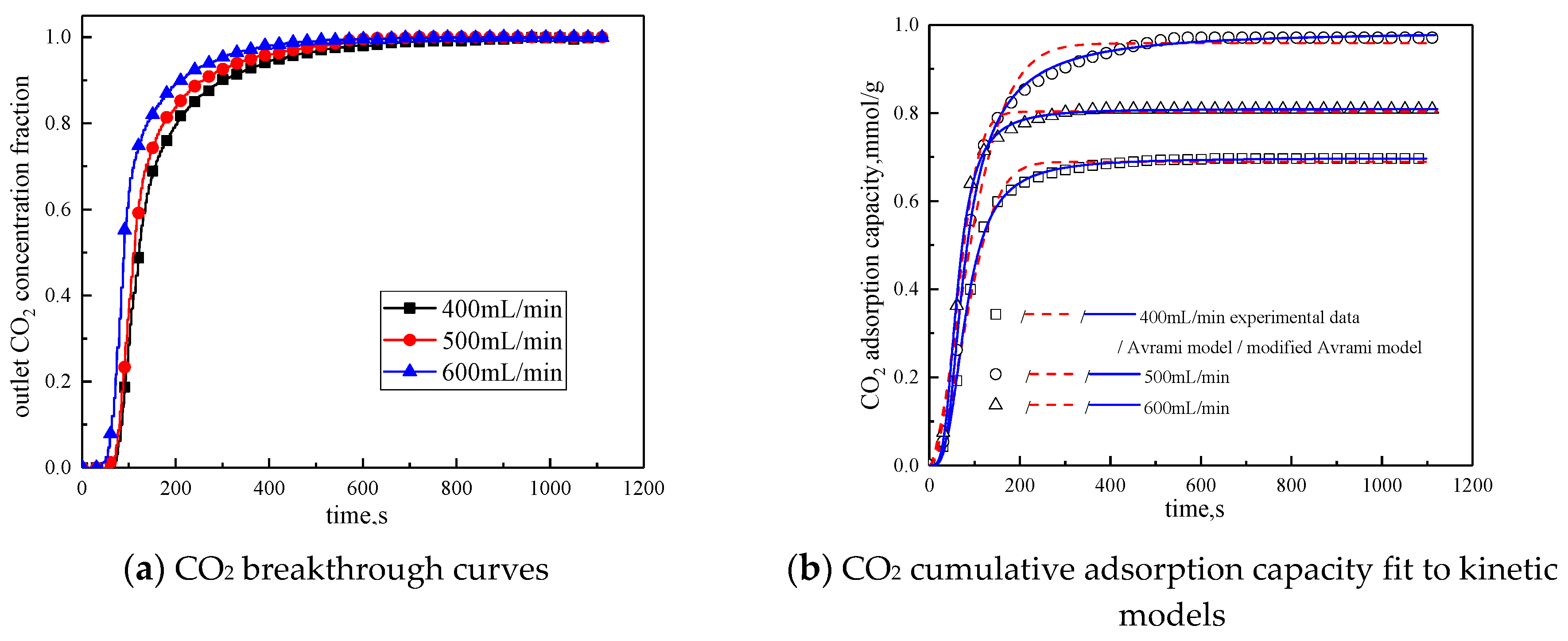
3.3.4. Gas Flow Rate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Le Quéré, C.; Moriarty, R.; Andrew, R.M.; Canadell, J.; Sitch, S.; Korsbakken, J.I.; Friedlingstein, P.; Peters, G.P.; Andres, R.J.; Boden, T.A.; et al. Global Carbon Budget 2015. Earth Syst. Sci. Data 2015, 7, 349–396. [Google Scholar] [CrossRef] [Green Version]
- Michael, J. IPCC fifth assessment synthesis report: “Climate change 2014: Longer report”: Critical analysis. Technol. Forecast. Soc. Chang. 2015, 92, 362–363. [Google Scholar] [CrossRef]
- Douglas, A.; Costas, T. Separation of CO2 from Flue Gas: A Review. Sep. Sci. Technol. 2011, 40, 321–348. [Google Scholar] [CrossRef]
- Pardemann, R.; Meyer, B. Pre-Combustion Carbon Capture[M]// Handbook of Clean Energy Systems; John Wiley & Sons, Ltd.: Hongkong, China, 2015. [Google Scholar]
- Buhre, B.; Elliott, L.; Sheng, C.; Gupta, R.; Wall, T. Oxy-fuel combustion technology for coal-fired power generation. Prog. Energy Combust. Sci. 2005, 31, 283–307. [Google Scholar] [CrossRef]
- Bu, C.; Gómez-Barea, A.; Leckner, B.; Chen, X.; Pallarès, D.; Liu, D.; Lu, P. Oxy-fuel conversion of sub-bituminous coal particles in fluidized bed and pulverized combustors. Proc. Combust. Inst. 2017, 36, 3331–3339. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Su, S.; An, H.; Yu, X.X. Post combustion CO2 capture by carbon fibre monolithic adsorbents. Prog. Energy Combust. Sci. 2009, 35, 438–455. [Google Scholar] [CrossRef]
- Bhown, A.S.; Freeman, B.C. Analysis and Status of Post-Combustion Carbon Dioxide Capture Technologies. Environ. Sci. Technol. 2011, 45, 8624–8632. [Google Scholar] [CrossRef]
- Bai, H.; Yeh, A.C. Removal of CO2 Greenhouse Gas by Ammonia Scrubbing. Ind. Eng. Chem. Res. 1997, 36, 2490–2493. [Google Scholar] [CrossRef]
- Kawabuchi, Y.; Oka, H.; Kawano, S.; Mochida, I.; Yoshizawa, N. The modification of pore size in activated carbon fibers by chemical vapor deposition and its effects on molecular sieve selectivity. Carbon 1998, 36, 377–382. [Google Scholar] [CrossRef]
- Ahmed, A.; Paitoon, T.; Amit, C.; Raphael, I. Kinetics of the reactive absorption of carbon dioxide in high CO2-loaded, concentrated aqueous monoethanolamine solutions. Chem. Eng. Sci. 2003, 58, 23–24. [Google Scholar] [CrossRef]
- Zhonglin, Z.; Xiaoping, C.; Daoyin, L.; Wei, D.; Ye, W.; Qingmin, M. Reaction Mechanism Model of the CO2 Absorption with K2CO3 Sorbents. Chin. Soc. Electr. Eng. 2014, 23, 3865–3873. [Google Scholar] [CrossRef]
- Yi, C.-K.; Jo, S.-H.; Seo, Y.; Lee, J.-B.; Ryu, C.-K. Continuous operation of the potassium-based dry sorbent CO2 capture process with two fluidized-bed reactors. Int. J. Greenh. Gas Control. 2007, 1, 31–36. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, Y.; Li, C.; Lu, S. Removal of low concentration CO2 at ambient temperature using several potassium-based sorbents. Appl. Energy 2014, 124, 241–247. [Google Scholar] [CrossRef]
- Chuanwen, Z.; Xiaoping, C.; Changsui, Z. Carbonation Reaction Characteristics of Dry Potassium-based Sorbent for CO2 Capture. J. Chem. Ind. Eng. 2008, 59, 2328–2333. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, X.; Zhao, C. K2CO3/Al2O3 for Capturing CO2 in Flue Gas from Power Plants. Part 2: Regeneration Behaviors of K2CO3/Al2O3. Energy Fuels 2012, 26, 1406–1411. [Google Scholar] [CrossRef]
- Lee, S.C.; Choi, B.; Lee, S.J.; Jung, S.Y.; Ryu, C.K.; Kim, J.C. CO2 Absorption and Regeneration using Na and K Based Sorbents. Adv. Pharmacol. 2004, 153, 527–530. [Google Scholar]
- Martín, C.; Sweatman, M.; Brandani, S.; Fan, X. Wet impregnation of a commercial low cost silica using DETA for a fast post-combustion CO2 capture process. Appl. Energy 2016, 183, 1705–1721. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.; Shen, X.; Cui, S.; Fan, M. Development of monolithic adsorbent via polymeric sol–gel process for low-concentration CO2 capture. Appl. Energy 2015, 147, 308–317. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, X.; Fan, M.; Yang, M.; Cui, S. Dynamic capture of low-concentration CO2 on amine hybrid silsesquioxane aerogel. Chem. Eng. J. 2016, 283, 1059–1068. [Google Scholar] [CrossRef]
- Sheng, C.; Weiwei, C.; Xiaodong, S.; Maohong, F.; Armistead (Ted), R.; Zhanwu, W. Xibin, Mesoporous amine-modified SiO2 aerogel: A potential CO2 sorbent. Energy Environ. Sci. 2011, 4, 2070–2074. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, Y.; Li, W.; Bu, C.; Wang, X.; Lu, P. Experimental and modeling investigation on CO2 sorption kinetics over K2CO3-modified silica aerogels. Chem. Eng. J. 2017, 312, 50–58. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, C.; Sun, J.; Li, W.; Lu, P. Facile synthesis of silica aerogel supported K2CO3 sorbents with enhanced CO2 capture capacity for ultra-dilute flue gas treatment. Fuel 2018, 215, 735–743. [Google Scholar] [CrossRef]
- Shu Panxiang, C.; Xiaoyu, S.; Xiao, L.; Zhiwu, L. CO2 Adsorption on Premodified Li/Al Hydrotalcite Impregnated with Polyethylenimine. Ind. Eng. Chem. Res. 2019, 58, 1177–1189. [Google Scholar] [CrossRef]
- Raganati, F.; Alfe, M.; Gargiulo, V.; Chirone, R.; Ammendola, P. Kinetic study and breakthrough analysis of the hybrid physical/chemical CO2 adsorption/desorption behavior of a magnetite-based sorbent. Chem. Eng. J. 2019, 372, 526–535. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, H.; Li, W.; Li, J.; Xu, R.; Zhang, K.; He, G.; Shearing, P.R.; Brett, D.J.L. Core–shell TiO2@C ultralong nanotubes with enhanced adsorption of antibiotics. J. Mater. Chem. A 2019, 7, 19081–19086. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Li, W.; Jiang, H.; Lin, R.; Wang, Z.; Wu, J.; He, G.; Shearing, P.R.; Brett, D.J.L. ZIF-8-Derived Hollow Carbon for Efficient Adsorption of Antibiotics. Nanomaterials 2019, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.-G.; Jia, L.; Wang, Y.-L.; Zhao, R.; Mei, X.-S.; Liu, Y.-Y.; Jin, Y. Study on Adsorption Mechanism and Failure Characteristics of CO2 Adsorption by Potassium-Based Adsorbents with Different Supports. Materials 2018, 11, 2424. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Jia, L.; Yao, Y.-X.; Huo, R.-P.; Qiao, X.-L.; Fan, B.-G. Study of the Effect of Adsorption Temperature on Elemental Mercury Removal Performance of Iron-Based Modified Biochar. Energy Fuels 2019, 33, 11408–11419. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Sayari, A. Modeling adsorption of CO2 on amine-functionalized mesoporous silica. 2: Kinetics and breakthrough curves. Chem. Eng. J. 2010, 161, 182–190. [Google Scholar] [CrossRef]
- Heydari-Gorji, A.; Sayari, A. CO2 capture on polyethylenimine-impregnated hydrophobic mesoporous silica: Experimental and kinetic modeling. Chem. Eng. J. 2011, 173, 72–79. [Google Scholar] [CrossRef]





| Designed Loading | Designed K Content | Experimental K Content | Experimental Loading |
|---|---|---|---|
| 25% | 14.13% | 10.29% | 18.21% |
| BET Specific Surface Area m2/g | Cumulative Pore Volume cm3/g | Relative Specific Pore Volume % | |||
|---|---|---|---|---|---|
| Micropore | Mesopore | Macropore | |||
| K2CO3 | 2.28 | 0.0050 | 1.46 | 65.01 | 33.52 |
| Silica aerogel support | 838.94 | 0.8482 | 8.54 | 91.40 | 0.06 |
| Modified adsorbent | 110.46 | 0.0857 | 0 | 98.95 | 1.05 |
| Models | Parameter Calculation Results | |||||
|---|---|---|---|---|---|---|
| Pseudo–first order | SSE% | R2 | q mmol/g | k1 min−1 | ||
| 2.5372 | 0.9866 | 1.2181 | 0.0448 | |||
| Pseudo–second order | SSE% | R2 | q mmol/g | k2 mmol/(g·min) | ||
| 5.7308 | 0.9605 | 1.5075 | 0.0306 | |||
| Weber–Morris | SSE% | R2 | kWB mmol/(g·min1/2) | C | ||
| 22.1566 | 0.8164 | 0.0377 | 0.1267 | |||
| Elovich | SSE% | R2 | kE mmol/(g·min1/2) | β | t0 | |
| 10.454 | 0.9176 | 0.3832 | 2.6095 | 37.4943 | ||
| Avrami | SSE% | R2 | q mmol/g | ka min−1 | n | |
| 0.3128 | 0.9977 | 1.1724 | 0.0602 | 1.4161 | ||
| Modified Avrami | SSE% | R2 | q mmol/g | kma gn−1min−mmmol1−n | m | n |
| 0.0483 | 0.9998 | 1.204 | 0.0196 | 1.937 | 0.7586 | |
| t °C | 50 | 60 | 70 | 80 | |
|---|---|---|---|---|---|
| Experimental result | q mmol/g | 1.0990 | 1.6283 | 1.4513 | 0.9226 |
| Avrami | SSE% | 2.2402 | 3.6465 | 1.5201 | 1.0813 |
| R2 | 0.9820 | 0.9862 | 0.9759 | 0.9750 | |
| q mmol/g | 1.4170 | 1.9525 | 1.0766 | 0.9230 | |
| ka min−1 | 0.0667 | 0.0813 | 0.0874 | 0.0960 | |
| n | 1.5529 | 1.5343 | 1.4824 | 1.6509 | |
| Modified Avrami | SSE% | 0.0510 | 0.1293 | 0.0617 | 0.1468 |
| R2 | 0.9995 | 0.9994 | 0.9989 | 0.9964 | |
| q mmol/g | 1.6396 | 2.2355 | 1.2131 | 1.2212 | |
| kma gn−1min−mmmol1−n | 0.0283 | 0.0315 | 0.0385 | 0.0402 | |
| m | 1.9583 | 1.7364 | 1.9234 | 1.8759 | |
| n | 0.3127 | 0.2401 | 0.3168 | 0.5719 | |
| Models | Ea kJ/mol | R2 |
|---|---|---|
| Avrami | 11.3877 | 0.9316 |
| Modified Avrami | 16.0697 | 0.9535 |
| Water Vapor Concentration | 10% | 15% | 20% | |
|---|---|---|---|---|
| Experimental result | q mmol/g | 0.9969 | 1.3966 | 1.1189 |
| Avrami | SSE% | 0.9713 | 1.7977 | 0.9782 |
| R2 | 0.9855 | 0.9877 | 0.9891 | |
| q mmol/g | 0.9707 | 1.3629 | 1.0999 | |
| ka min−1 | 0.0707 | 0.0778 | 0.0948 | |
| n | 1.1095 | 1.3203 | 1.4804 | |
| Modified Avrami | SSE% | 0.0169 | 0.0307 | 0.0514 |
| R2 | 0.9997 | 0.9997 | 0.9993 | |
| q mmol/g | 1.0808 | 1.4537 | 1.1379 | |
| kma gn−1min−mmmol1−n | 0.0206 | 0.0289 | 0.0498 | |
| m | 1.8344 | 1.9202 | 1.8419 | |
| n | 0.6152 | 0.9896 | 0.2135 |
| CO2 Concentration | 5% | 10% | 12.5% | 15% | |
|---|---|---|---|---|---|
| Experimental result | q mmol/g | 1.1815 | 1.3966 | 1.5979 | 1.5733 |
| Avrami | SSE% | 0.3128 | 1.7977 | 1.8463 | 1.1013 |
| R2 | 0.9977 | 0.9877 | 0.9894 | 0.9932 | |
| q mmol/g | 1.1725 | 1.3629 | 1.5813 | 1.5783 | |
| ka min−1 | 0.0603 | 0.0778 | 0.0819 | 0.0845 | |
| n | 1.4162 | 1.3203 | 1.5156 | 1.4740 | |
| Modified Avrami | SSE% | 0.0483 | 0.0307 | 0.2801 | 0.8862 |
| R2 | 0.9998 | 0.9997 | 0.9982 | 0.9943 | |
| q mmol/g | 1.2040 | 1.4537 | 1.8253 | 1.6894 | |
| kma gn−1min−mmmol1−n | 0.0217 | 0.0289 | 0.0326 | 0.0381 | |
| m | 1.9370 | 1.9202 | 2.0355 | 1.8372 | |
| n | 0.7586 | 0.9896 | 0.3425 | 0.1390 |
| Gas Flow Rate mL/min | 400 | 500 | 600 | |
|---|---|---|---|---|
| Experimental result | q mmol/g | 0.6967 | 0.9711 | 0.8083 |
| Avrami | SSE% | 0.2159 | 0.2076 | 0.2453 |
| R2 | 0.9924 | 0.9931 | 0.9921 | |
| q mmol/g | 0.6892 | 0.9584 | 0.8030 | |
| ka min−1 | 0.0412 | 0.0637 | 0.0841 | |
| n | 1.4149 | 1.5550 | 1.5372 | |
| Modified Avrami | SSE% | 0.0231 | 0.0206 | 0.0339 |
| R2 | 0.9991 | 0.9991 | 0.9989 | |
| q mmol/g | 0.6978 | 0.9858 | 0.8091 | |
| kma gn−1min−mmmol1−n | 0.0160 | 0.0176 | 0.0291 | |
| m | 1.8447 | 1.7000 | 1.9117 | |
| n | 0.4539 | 0.6902 | 0.5180 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; Wang, Y.; Shen, X.; Qiao, X.; Jia, L.; Xiang, J.; Jin, Y. Study on CO2 Capture Characteristics and Kinetics of Modified Potassium-Based Adsorbents. Materials 2020, 13, 877. https://doi.org/10.3390/ma13040877
Guo B, Wang Y, Shen X, Qiao X, Jia L, Xiang J, Jin Y. Study on CO2 Capture Characteristics and Kinetics of Modified Potassium-Based Adsorbents. Materials. 2020; 13(4):877. https://doi.org/10.3390/ma13040877
Chicago/Turabian StyleGuo, Baihe, Yanlin Wang, Xin Shen, Xiaolei Qiao, Li Jia, Jun Xiang, and Yan Jin. 2020. "Study on CO2 Capture Characteristics and Kinetics of Modified Potassium-Based Adsorbents" Materials 13, no. 4: 877. https://doi.org/10.3390/ma13040877
APA StyleGuo, B., Wang, Y., Shen, X., Qiao, X., Jia, L., Xiang, J., & Jin, Y. (2020). Study on CO2 Capture Characteristics and Kinetics of Modified Potassium-Based Adsorbents. Materials, 13(4), 877. https://doi.org/10.3390/ma13040877





