1. Introduction
Cementitious materials are some of the most essential and reliable construction materials used in a range of applications, from building, bridges, and underground structures to special structures, such as nuclear power plants, chimneys, and reactors. The cement-based structures are normally exposed to room temperature but can sometimes be exposed to high temperature environments. When cementitious materials and structures are exposed to high temperatures, the material and mechanical properties can deteriorate, such as decreases in compressive and tensile strength, elastic modulus, cracking, and spalling [
1,
2]. In addition, complex physical and chemical changes occur when exposed to elevated temperatures [
3,
4]. Primarily, the physically combined water begins to evaporate, and the C-S-H (Calcium-Silicate-Hydrate) starts to dehydrate at approximately 100 °C [
5,
6]. The C-S-H decomposes gradually between around 100 °C to 300 °C [
6]. From approximately 500 °C, the portlandite begins to disintegrate, and the calcite begins to decompose [
3,
6]. These phenomena lead to a deterioration of the material, such as a decay of strength and elastic modulus, as well as cracking and spalling of concrete.
After the cement-based structures are damaged by high temperatures, most of the structures can work perfectly after proper repair and maintenance instead of rebuilding the structures. To ensure that a structure can be repaired or replaced, an appropriate evaluation method is needed to define the damaged part of the structure due to the high temperature. At the same time, the evaluation should be fast and easy after an event. Typically, core strength and rebound hammer tests are performed to define the strength decay of the high-temperature damaged structures. Furthermore, the ultrasonic pulse velocity and the impact echo method can be used to detect the degree of damage. Similarly, some techniques, such as infrared thermal imaging, drilling resistance, petrographic examination, thermoluminescence, and the Windsor probe test, also detect damage to concrete [
7,
8,
9]. On the other hand, these non-destructive techniques provide an indirect indication of damage to the structure and have limited application to the wide and large area of a real structure in practice.
The evaluation of high-temperature damaged structures also uses color changes. Normal cementitious materials containing siliceous aggregate tend to change color to pink or red at 300–600 °C, to whitish gray at 600–900 °C, and to buff at 900–1000 °C [
10,
11,
12,
13,
14]. Moreover, the strength of concrete begins to decrease from 300 °C and drops by approximately 50% to 60% at approximately 500 °C [
15,
16]. Therefore, a color change to pink or red can indicate a deterioration of the material and mechanical properties. However, the degree of color change is difficult to define and recognize, particularly by the naked eye. Moreover, the color changes are less prominent for calcareous and igneous aggregates. Therefore, some studies based on color changes roughly estimated the temperature of the cementitious materials and were lack in the application to define the temperature information.
Color is the most immediate visible aspect in terms of exposure to high temperatures. To define the temperature information more precisely, a color change pigment was essentially mixed with cementitious materials. Some materials have a property to change color in response to the exterior environment, such as temperature and light [
17]. The materials, which were recently recognized to exhibit a distinct color change in response to change in temperature, are found in different forms, such as metal oxides, polymers, pigments, and leuco dyes. Reversible thermochromic materials are used in the applications of smart windows, ultra-thin films, temperature sensors, cool colored, and protective coatings [
18,
19,
20,
21,
22,
23,
24,
25,
26]. The irreversible thermochromic materials experience a permanent color change at certain temperatures and are widely used in applications, such as aeronautical components, furnaces, damage warnings, and thermal flow sensors [
27,
28,
29]. Previous studies focused on metal structures but not on cementitious materials and structures widely used in the construction field.
In this study, manganese violet pigment was identified in the furnace test to undergo an efficient color change from dark violet to grayish yellow with temperatures above 400 °C. The manganese violet pigment, which is composed of manganese dioxide, ammonium dihydrogen phosphate, and phosphoric acid, presents a dark violet color due to the presence of phosphate and ammonia [
30,
31]. The color change to grayish yellow results from the evaporation of water and the liberation of ammonia from the pigment particles. The manganese violet pigment was then applied to white cement at a ratio of 1%, 3%, and 5% of the total mass. The white cement is similar to ordinary Portland cement except for the color. The white color was attributed to the decrease in the amount of chromium, manganese, iron, copper, vanadium, nickel, and titanium in the ordinary Portland cement. The white cement confers an advantage for the excellent exposure of color when mixed, and the material properties are unaffected due to a decrease in the above components [
32]. The pigment mixed samples were heated in a furnace from room temperature to 450 °C. The mixed samples underwent a typical thermochromic change to a grayish green color. The thermochromic change was observed visually by the naked eye and was evaluated using digital images captured at each temperature interval.
Digital images are typically recorded in
RGB color space, which is widely used in cameras and monitors by the percentage of red, green, and blue constituents. The
RGB values of each component are in the range of zero to 255 [
33]. The disadvantage of
RGB color space is dependent on each device, which has its own different color sensor characteristics and produces different
RGB output responses. Unlike the
RGB,
Lab color space can be used as a device-independent model when representing color. The color information in both
RGB and
Lab color spaces obtained from the digital images were analyzed, and the color information with temperatures was quantified. From the above techniques, a reliable method for temperature detection can be obtained by integrating the color change data and with those of the microstructural and the compositional changes. The microstructure changes caused by the high temperatures were studied by scanning electron microscopy (SEM). In addition, the changes in their composition due to the high temperature were examined using energy dispersive X-ray spectroscopy (EDX). Therefore, this study analyzed the color information visually, the changes in the
RGB and
Lab color spaces obtained from the digital images, and the microstructure and compositional changes with the application of high temperatures.
4. Application of MV Pigment with Cementitious Material
4.1. Thermochromic Change of White Cement Mixed Samples with MV Pigment
White Portland cement is widely used in cement composites for the good exposure of color with pigments. Primarily, white cement remains a stable silver or pale white under high temperature conditions. Therefore, this study manufactured the white cement composite samples with the MV pigment. The MV pigment was separately added in 1%, 3%, and 5% of the weight of white cement. The mixed samples were heated individually in the furnace from room temperature to 450 °C. The photographs were recorded to examine the color of the samples at 100 °C, 200 °C, 300 °C, 350 °C, and 370 °C, and after that, every 10 °C up to 450 °C.
Figure 6 shows the original and fully changed colors of the mixed samples at room temperature and 440 °C, respectively.
With the addition of white cement, the color of the pigment changed from violet to blue at room temperature. When heated, the mixed samples exhibited similar thermochromic changes as the pure MV pigment. Between room temperature to 410 °C, the mixed samples underwent a process of water removal from the pigment, but the color change was insufficient to be observed with the naked eye. Subsequently, the surface color of the mixed samples tended to fade away. When the temperature reached 440 °C, the mixed samples with 1%, 3%, and 5% of the MV pigment completely changed to a grayish green color. The color change was attributed to both the evaporation of water and the decomposition of ammonia from the pigment particles. The mixed samples did not recover their original color upon cooling to room temperature, indicating an irreversible color change. The color change occurred at 410 °C in the pure pigment but at 440 °C for the mixed samples. This might be due to the reduced temperature intrusion between the layers due to the mixing of white cement.
4.2. SEM Micrograph and EDX Analysis of White Cement Mixed Samples
Figure 7 shows SEM images of the white cement to characterize the changes in the morphology associated with high temperatures. At room temperature, the particles of the white cement have a structural diversity with globular shapes, including hexagonal, spherical, and some irregular tiny particles. When heated to 440 °C, a slight change was found due to the breakage of particles, and the white cement was relatively stable.
Figure 8 presents SEM micrographs of the mixed sample at room temperature and at 440 °C, respectively. From the figure, the mixed sample at room temperature exhibits triangular, hexagonal, and spherical particles with a small cluster of rod-shaped structures due to the presence of MV pigment. At 440 °C, the hexagonal and rod-shaped cluster structures disappeared due to the dehydration of MV particles, which changed the mixed sample to irregular bundled structures.
Table 2 presents the results of EDX analysis for the white cement and the mixed samples. In the white cement, the samples are predominantly composed of C, O, and Ca, with the mass contents of 14.9%, 47.35%, and 25.09%, respectively. At 440 °C, the C, O, and Ca contents were 15.35%, 48.03%, and 26.85%, which correspond to an increase of approximately 3%, 1.5%, and 7%, respectively.
The mixed sample consists of high peaks of C, O, and Ca with the mass components of 17.18%, 40.43%, and 19.19%, respectively, and low peaks of Mg, Al, Si, P, S, Mn, Sn, Sb, I, and Fe. The mixed sample with MV pigment included new elements of P, Mn, Sn, Sb, and I, which consisted of 2.05%, 1.78%, 1.24%, 8.03%, and 2.21%, respectively. When the temperature reached 440 °C, the C increased by 41.4%, and the O and Ca contents decreased by 3.3 and 11%, respectively. The mass of the new elements of P, Mn, Sn, Sb, and I by the addition of MV at 440 °C changed to 1.54%, 1.72%, 0.89%, 6.67%, and 2.07%, which correspond to a 24%, 3%, 28%, 16%, and 6% decrease, respectively.
4.3. Thermochromic Analysis in the RGB Color Spaces
The color changes of the white cement mixed samples were evaluated using
RGB and
Lab color spaces.
Figure 9 shows the changes in
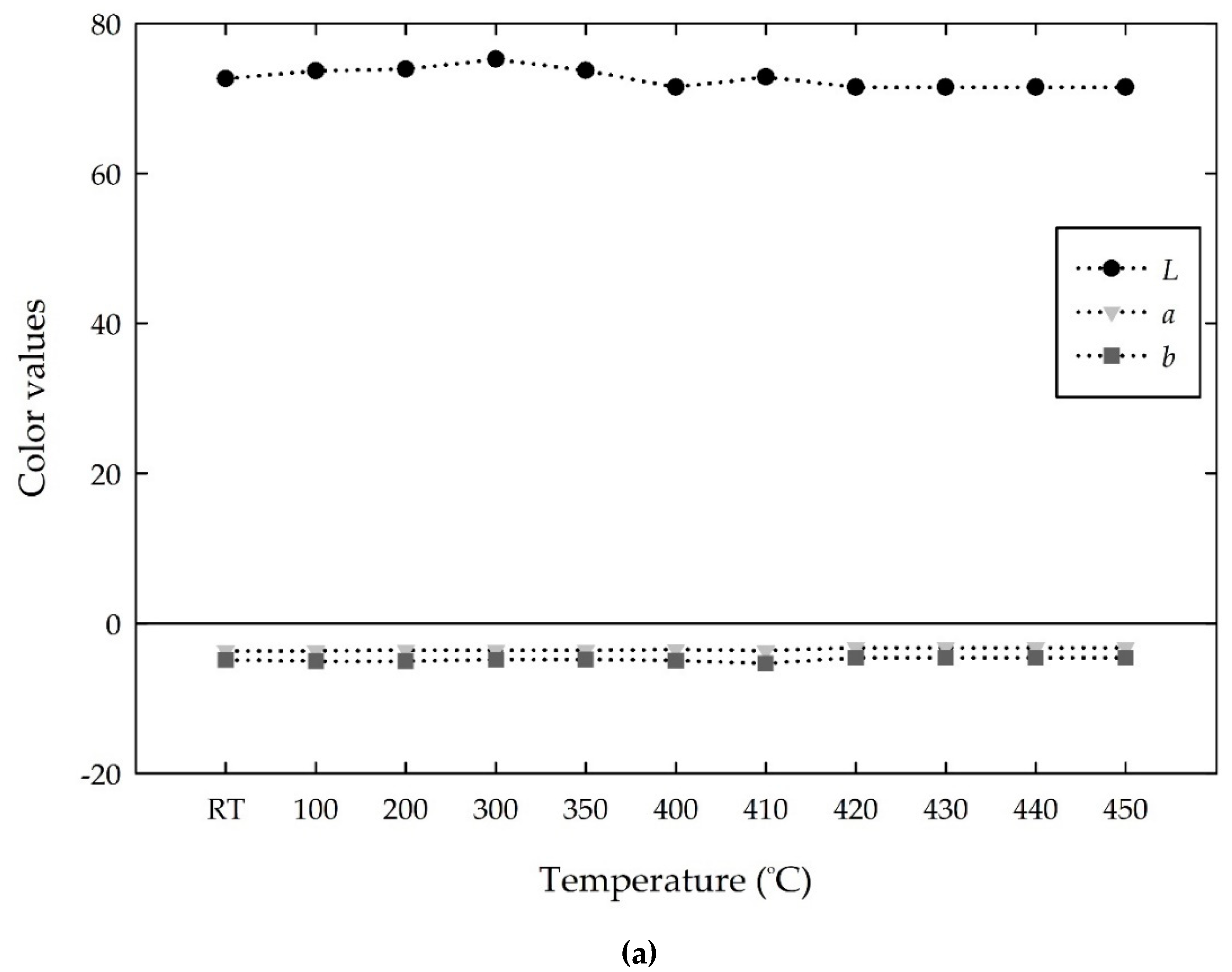
RGB values with increasing temperature. The white cement showed stable and high
RGB values from room temperature to 450 °C, as shown in
Figure 9a. At room temperature, the
RGB color intensities of white cement were (165, 179, 186), which represents a grayish blue color. Heating of the white cement barely changed the color and thus displayed stable values with the mean
RGB values of (164, 177, 183) from room temperature to 450 °C.
Figure 9b–d show the changes in the
RGB values of the mixed samples with increasing temperature. The addition of 1% pigment to white cement decreased the
RGB values at room temperature from approximately (165, 179, 186) to (127, 125, 134), which corresponds to a 22% to 30% (R: 22%, G: 30%, B: 27%) decrease. In the mixed samples with 3 % and 5% pigments, the
RGB values decreased by approximately 30% to 42% (
R: 30%,
G: 42%,
B: 32%) and 33% to 47% (
R: 34%,
G: 47%,
B: 33%), respectively. The decrease in the
RGB values in the mixed samples indicates the disappearance of the whiteness intensity and the development of a mild violet color with the addition of 1%, 3%, and 5% pigments. With increasing temperature from room temperature to 410 °C, the mixed samples with 1%, 3%, and 5% pigments were almost stable with mean
RGB values of approximately (127, 131, 133), (108, 108, 120), and (99, 97, 116), respectively, which are very close to those at room temperature. At higher temperatures, the
RGB increased gradually toward the whiteness intensity, and the color completely changed at 440 °C. The
RGB values of the mixed samples with 1%, 3%, and 5% MV at 440 °C were similarly (156, 168, 144), (145, 155, 133), and (157, 164, 146), respectively. Considering the reference
RGB values of gray and green colors, the color obtained in the mixed samples at 440 °C can be defined as a grayish green color. Above 440 °C, the
RGB values were stable and irreversible.
With increasing pigment content, the mean
RGB values of the mixed samples from room temperature to 410 °C, in which the color of the samples was stable, were compared with those at a critical temperature of 440 °C, as shown in
Figure 10. Compared to
R values in between room temperature and 410 °C, those at 440 °C increased by approximately 22%, 33%, and 55% in the WC + 1% MV, WC + 3% MV, and WC +5 % MV specimens, respectively, as shown in
Figure 10a. At a critical temperature of 440 °C, which completely changed to a grayish green color, the mixed samples with 1%, 3%, and 5% pigment showed
R values in the range of 144 to 155. This indicates that the
R value decreased due to the inclusion of pigment at room temperature, but increased to a similar range at a critical temperature of 440 °C.
Figure 10b shows the change in
G values for the white cement mixed samples. With increasing MV pigment, the
G values decreased at room temperature. At 440 °C, the
G values of the white cement with 1%, 3%, and 5% of MV pigment increased by 28%, 42%, and 68%, respectively, and showed a similar range of 155 to 168, irrespective of the content of MV pigment.
Figure 10c shows the change in the
B value for the white cement mixed samples. The
B intensities, which were also stable between room temperature and 410 °C, decreased from approximately 183 to 116 with increasing content of MV pigment from zero to 5%. The
B value at the critical temperature of 440 °C increased to 144, 133, and 146, which corresponds to a 7%, 10%, and 25% increase for the white cement samples with 1%, 3%, and 5%, respectively. The addition of pigments decreased the
B values, but the amount of the increase is relatively small compared to that of the
R and
G values.
In the
RGB color intensities, the incorporation of MV pigment particles in 1%, 3%, and 5% decreased the
RGB intensities, which were stable between room temperature and 410 °C. The color intensities began to increase from 410 °C and completely changed to grayish green at 440 °C, at which point little difference of the
RGB values was found among the white cement mixed samples. That is, with increasing pigment content, the
RGB intensities decreased at room temperature but obtained relatively constant values at 440 °C. The
RGB values and color intensities after 440 °C exhibited a slight difference, irrespective of the amount of MV pigment added to the white cement. Therefore, the magnitude of the change in the
RGB values at 440 °C is also discussed. The changing magnitude of the
RGB values mainly depends on the pigment to white cement ratio, as shown in
Figure 11. With increasing addition of MV pigment from 1% to 5%, the magnitude of the change in the
RGB almost linearly increased. According to the linear trend analysis, the
R and
G values showed a 10.9 and 13.0 increase at 440 °C per 1% addition of MV pigment to white cement, respectively. The approximately 6.5 increase in the
B value per 1% MV pigment was relatively small. This suggests that the
R and
G values, which show a higher change at 440 °C than the
B value, would be an index to instantly identify and evaluate cementitious materials subjected to a critical high temperature.
The color changes of the white cement mixed samples are related to the increasing number of
RGB values. Therefore, the total color changes in the
RGB color intensities are defined as the Euclidean distance
using the following equation:
where Δ
R, Δ
G, and Δ
B represent the differences of the
R,
G, and
B values, respectively, 440 °C and those between room temperature to 410 °C. These also show a linear increase with increasing MV pigment ratio, as shown in
Figure 12. The increasing rate of
is approximately 14.7 per 1% addition of MV pigment to white cement. As a result, the total color changes of white cement mixed with MV pigment can be effectively determined using the increases in
RGB color values and
.
4.4. Thermochromic Analysis in the Lab Color Spaces
Figure 13 shows the changes in the
Lab values of the white cement mixed samples with increasing temperature. The white cement exhibited a constant high
L value of approximately 72, and the
a and
b values present negative constant values of approximately −3 and −4, respectively, with increasing temperature from room temperature to 450 °C, as shown in
Figure 13a. This suggests that the white cement is stationary with increasing temperature. The addition of MV pigment decreased the
L and
b values, whereas it increased the
a value at room temperature, as shown in
Figure 13b–d. The mixed samples with 1%, 3%, and 5% of the MV pigment showed a decrease in
L value from 72 to 52, 45, and 40, corresponding to a 28%, 36%, and 43% decrease, respectively. The
a value, which was −3 at room temperature for white cement, changed to positive values (redness degrees) of 2, 8, and 10 for the mixed samples with 1%, 3%, and 5% of MV pigment, respectively. In contrast, the
b intensity, which was negative for the white cement, showed a further decrease to a negative value (blueness degree). The change in the
Lab values influences the development of color on the mixed samples. That is, the WC + 1% MV sample with the Lab of (52, 2, −4) represents a dark grayish blue color, and WC + 3% MV and WC + 5% MV samples with the
Lab of (45, 8, −10) and (40, 10, −13), respectively, illustrate dark grayish violet color at room temperature. As the temperature increased from room temperature to 410 °C, the
L value was relatively constant, the
a value gradually decreased from positive to negative, and the b value increased from negative to positive. However, the changes in the
Lab values were too low to observe the color change of the mixed samples until 410 °C.
After 410 °C, the color of the samples started to show a grayish green color with increasing L value and changing a from positive to negative and b from negative to positive values. The increase in L value increases the lightness intensity, and the decrease in a and increase in b indicate the greenness and yellowness intensities, respectively. At 440 °C, the mixed samples completely changed to a grayish green color and showed Lab values of (64, −7, 9) for 1% MV, (62, −7, 10) for 3% MV, and (64, −6, 9) for 5% MV samples. As discussed in the RGB color spaces, the Lab values were also similar at the critical temperature of 440 °C, irrespective of the amount of MV pigment added to the white cement. Above the critical temperature, the mixed samples exhibited stable and invariable Lab values.
Figure 14 compares the
Lab values of the mixed samples averaged from room temperature to 410 °C with those at a critical temperature of 440 °C. As shown in
Figure 14a, the
L value (or, the lightness intensity) decreased when the pigment concentration was increased from zero to 5%. However, the
L value at 440 °C showed a similar level, ranging from 62 to 64, which indicates a grayish color.
Figure 14b shows the change in
a value. Compared to those between room temperature and 410 °C, the
a values at the critical temperature of 440 °C decreased from positive to negative, resulting in a very similar range of −7 to −6 for all three mixed samples. In contrast, the
b value increased from negative to positive, ranging from 9 to 10, which illustrates the increase in yellowness intensity, when the mixed samples reached the critical temperature of 440 °C, as shown in
Figure 14c.
Based on the mean
Lab values between room temperature and 410 °C, in which no major change was obtained in all the white cement mixed samples,
Figure 15 shows the magnitude of the change in the
Lab values at 440 °C. The
L and
b values changed to positive values, and the
a values changed to negative values. That is, with increasing MV pigment from zero to 5%, the
L and
b values increased almost linearly from −1.9 to 23.8 and from 0.3 to 20.6, respectively, whereas the
a value decreased from 0.4 to −11.1. A linear trend analysis showed that the magnitude of the
L and
b values at 440 °C increased by approximately 4.7 and 3.6, respectively, and the
a value, the change of which was relatively small, decreased by approximately 2.1 per 1% increase in MV pigment.
In addition, the total changes in the
Lab intensities at the critical temperature of 440 °C were calculated, as shown in
Figure 16. Similar to Equation (2), the total change was defined as the Euclidean distance (
), using the difference between the mean
Lab values from room temperature to 410 °C and those at 440 °C. As the content of the MV pigment added to white cement increased from zero to 5%,
tended to increase almost linearly at a rate of approximately 5.8 per 1% addition of MV pigment to white cement. Along with
RGB intensities, the
Lab changes can be used to determine the color change at a critical temperature with the white cement-mixed samples containing MV pigment.
5. Conclusions
This study proposed an irreversible thermochromic cementitious material using MV pigment. According to the thermochromic tests, the MV pigment underwent a reversible change from violet to blue at temperatures lower than 400 °C, but the color change was barely discernable by the naked eye. When the temperature reached 410 °C, the MV pigment completely changed from violet to grayish yellow, which is associated with the evaporation of water and the liberation of ammonia from the pigment particles. The changed color was maintained at temperatures higher than 410 °C and when the temperature was returned to room temperature.
In the analysis of MV pigment in the RGB and Lab color spaces, the values were relatively constant from room temperature to 370 °C. At higher temperatures, the RGB values increased gradually toward the whiteness color intensity until 400 °C. The L and b values also gradually increased after 370 °C, while the a value decreased. At 410 °C, when the color completely changed from dark violet to grayish yellow, the RGB and Lab provided a sudden change and remained constant after the critical temperature of 410 °C. In particular, the a value changed from positive to negative, and the b value changed from negative to positive.
The MV pigment was then mixed with white cement at ratios of 1%, 3%, and 5% of the mass of the white cement. The color of the mixed samples was dark grayish blue and violet, depending on the content of MV pigment. The colors at room temperature were retained until 410 °C. Hence, the RGB and Lab intensities were relatively stable and constant. The color started to change at temperatures higher than 410 °C and completely turned to grayish green at 440 °C. In accordance with the change in color, the RGB increased toward the whiteness intensity. Compare to those before the thermochromic change occurred, the RGB values at 440 °C increased by approximately 22%–55%, 28%–68%, and 7%–25%, respectively. The RGB values almost linearly increased with the increasing content of MV pigment.
Similarly, the L value increased by approximately 23%–60% at 440 °C. The a value changed from positive to negative, and the b value changed from negative to positive. The changes in the Lab values also increased almost linearly with increasing MV pigment content. According to the linear trend analyses, the magnitude of the Lab values at 440 °C changed by approximately 4.7, 3.6, and −2.1, respectively, and the RGB values increased by approximately 10.9, 13.0, and 6.5, respectively, per 1% increase in MV pigment. In addition, the total changes in the RGB and Lab color intensities at the critical temperature of 440 °C, defined as the Euclidean distance, showed the increasing rates of approximately 14.7 and 5.8 per 1% addition of MV pigment to white cement.
However, the level of the RGB and Lab values and the color intensity of the mixed samples at 440 °C were in very similar ranges, irrespective of the amount of MV pigment added to white cement. Above a critical temperature of 440 °C and back to room temperature, the mixed samples exhibited stable and irreversible RGB and Lab values. Therefore, with a direct visual inspection of the distinct color change, the changes in the RGB and Lab values can provide an instant and promising index to identify the magnitude and dispersion of critical temperatures in cementitious materials and structures with MV pigment.
SEM images showed that the rod-shaped and hexagonal crystal structures of the MV pigment at room temperature were modified to a cluster of void structures at 410 °C. The EDX analysis results of the MV pigment, comprised of 11.36%, 48.91%, 16.30%, and 14.56% of C, O, P and Mn, respectively, changed to 6.89%, 46.37%, 17.05%, and 14.37%, after exposure to 410 °C, respectively. In the SEM images, the white cement, composed of hexagonal and spherical shapes with some irregular tiny particles, was relatively stable with slight changes due to the breakage of particles at 440 °C. The mixed samples consisted of triangular, hexagonal, and spherical particles with a small cluster of rod-shaped structures at room temperature. At 440 °C, the hexagonal and rod-shaped cluster structures disappeared due to the dehydration of MV particles, which changed into irregular bundled structures. In the EDX analysis, the white cement, which is mainly composed of C, O, and Ca with the mass contents of 14.9%, 47.35%, and 25.09% at room temperature, increased by 3%, 1.5%, and 7%, respectively, at 440 °C. The mixed sample exhibited C, O, and Ca with the mass components of 17.18%, 40.43%, and 19.19%, respectively, and P, Mn, Sn, Sb, and I with the mass of 2.05%, 1.78%, 1.24%, 8.03%, and 2.21%, respectively, due to the addition of MV pigment. At 440 °C, the mass of C, O, and Ca changed to 24.29%, 39.09%, and 17.05%, respectively, and that of P, Mn, Sn, Sb, and I decreased by 24%, 3%, 28%, 16%, and 6%, respectively.