Investigation on Low-temperature Annealing Process of Solution-processed TiO2 Electron Transport Layer for Flexible Perovskite Solar Cell
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Device Fabrication
2.2.1. Cleaning of FTO Substrate
2.2.2. Formation of Electron Transport Layer
2.2.3. Formation of Perovskite Light-absorption Layer
2.2.4. Formation of Hole Transport Layer
2.2.5. Formation of Counter Electrode
2.3. Characterization
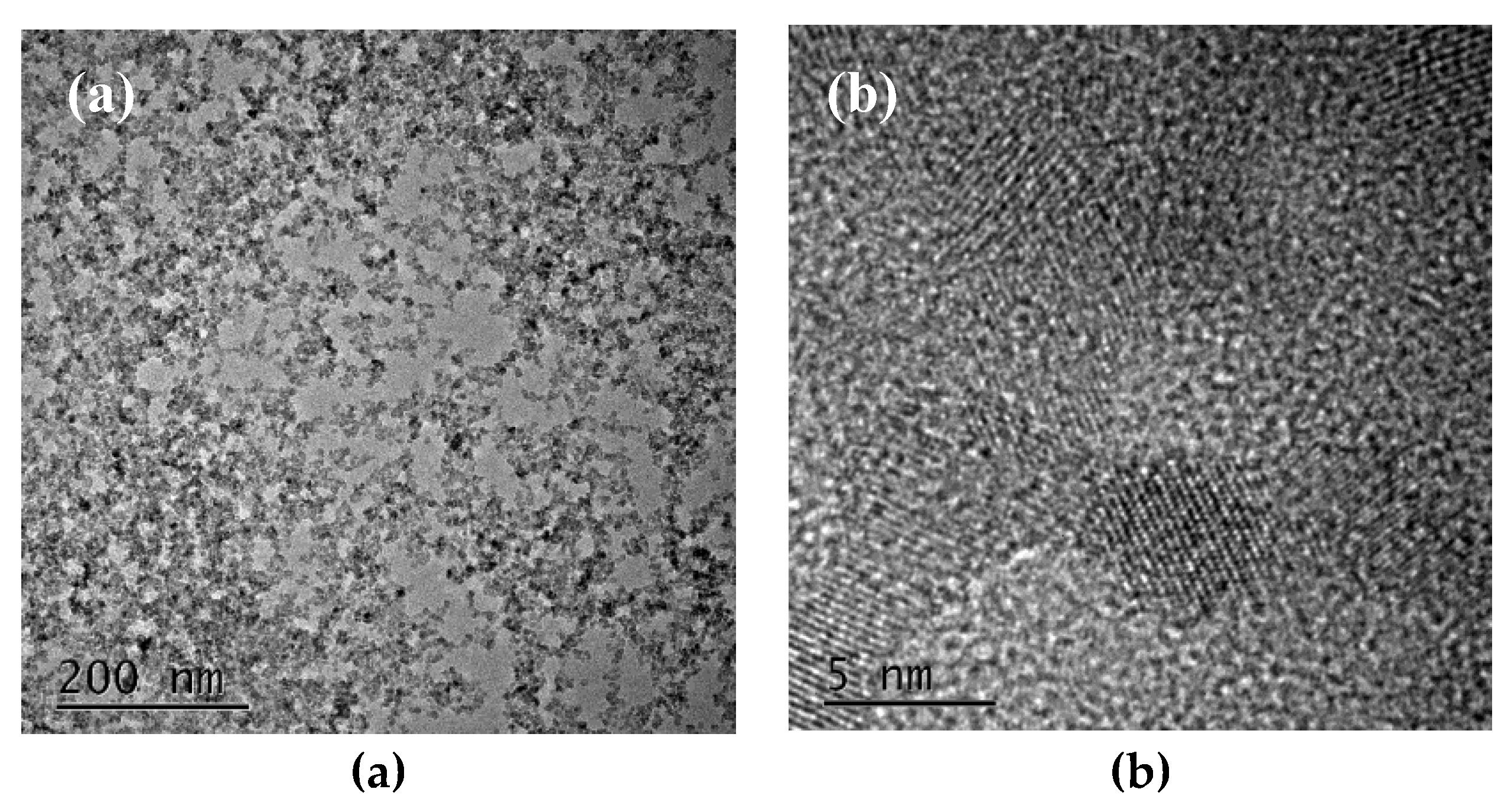
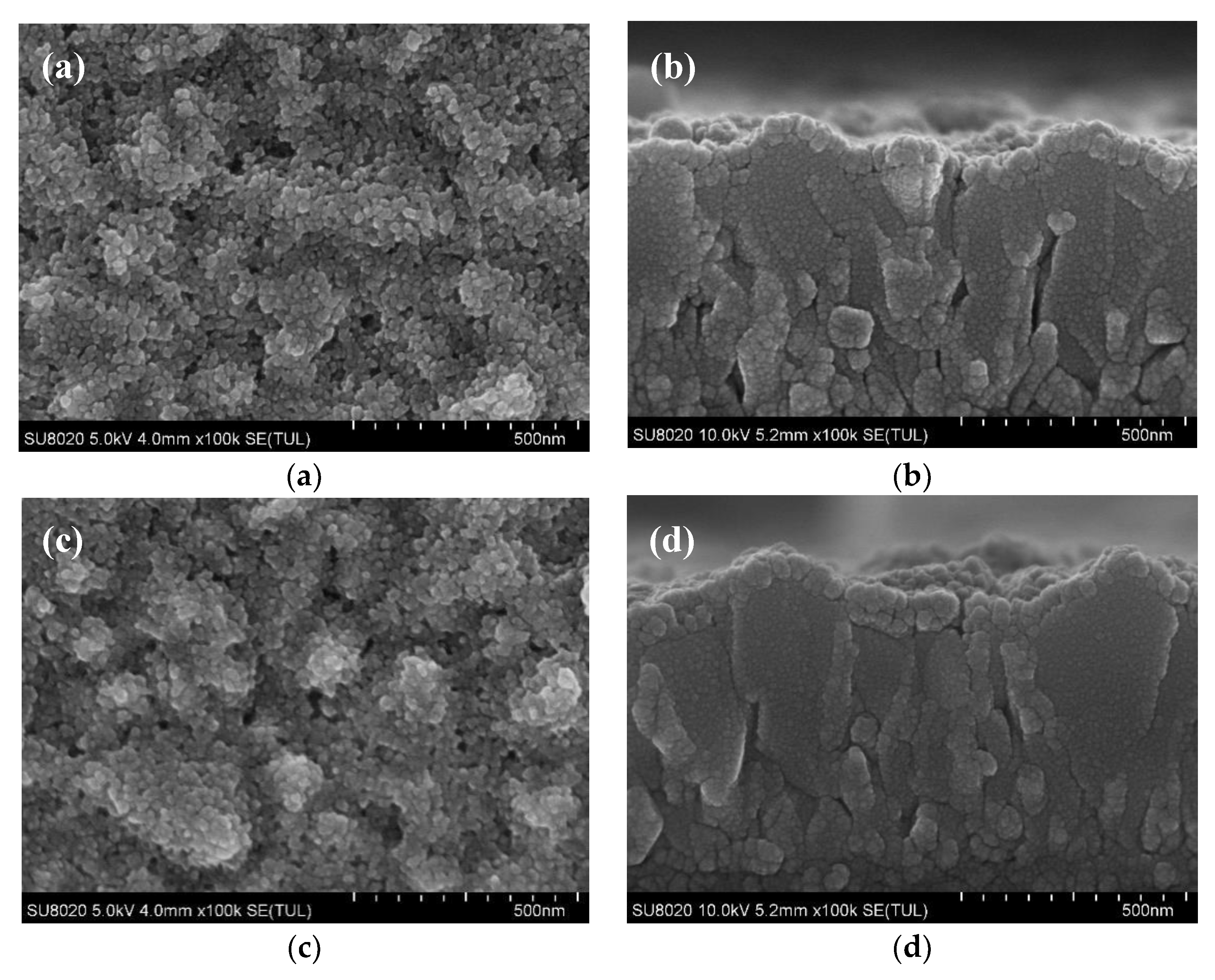
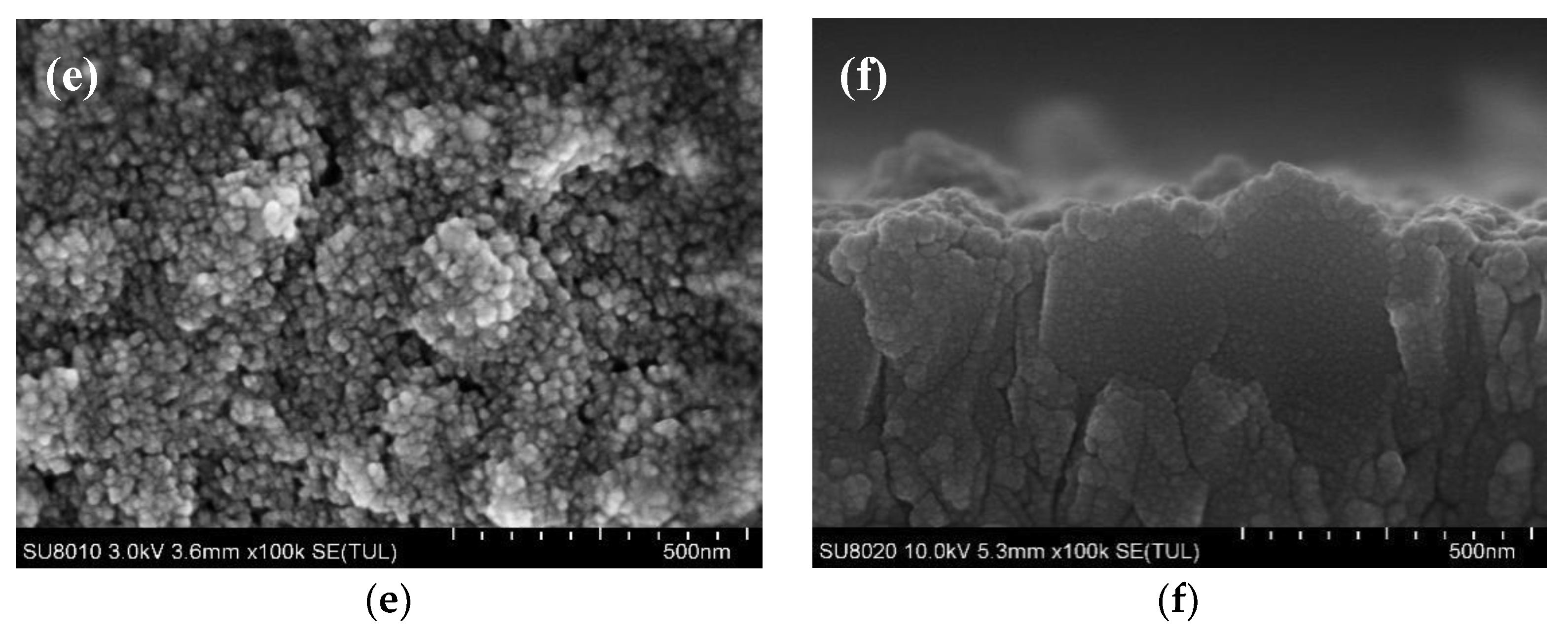
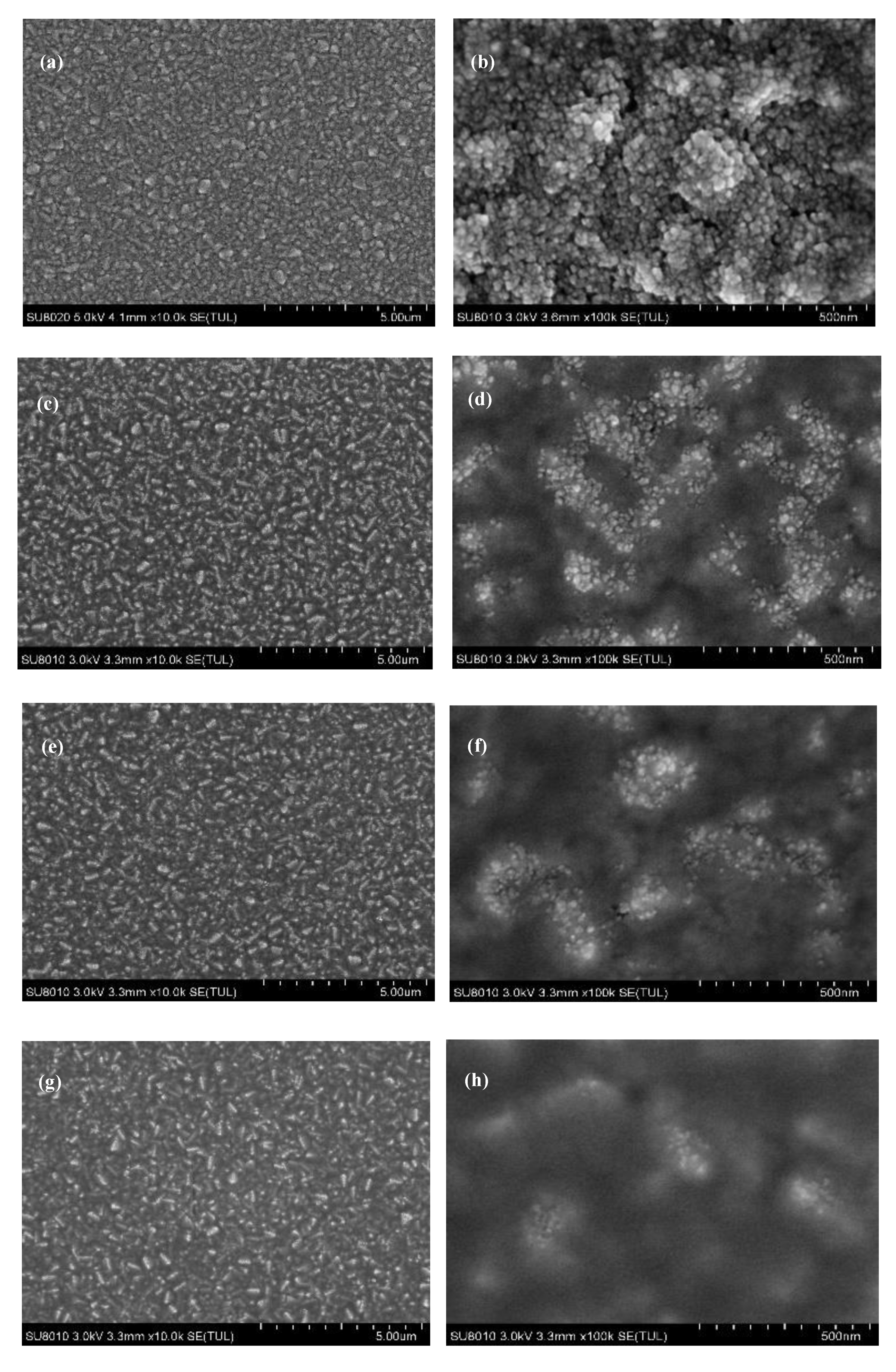
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Shi, Y.; Dong, Q.; Li, Y.; Wang, S.; Yu, X.; Wu, M.; Ma, T. Low temperature and solution processed amorphous WOx as electron selective layer for perovskite solar cells. J. Phys. Chem. Lett. 2015, 6, 755. [Google Scholar] [CrossRef]
- Eze, V.O.; Seike, Y.; Mori, T. Efficient planar perovskite solar cells using solution-processed amorphous WOx/fullerene C60 as electron extraction layers. Org. Electron. 2017, 46, 253–262. [Google Scholar] [CrossRef]
- Huang, K.; Peng, Y.; Gao, Y.; Shi, J.; Li, H.; Mo, X.; Huang, H.; Gao, Y.; Ding, L.; Yang, J. High-performance flexible perovskite solar cells via precise control of electron transport layer. Adv. Energy Mater. 2019, 9, 1901419. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Zhou, X.; Gao, J.; Chen, W.; Wang, X.; Xu, B. Hydrothermally treated SnO2 as the electron transport layer in high-efficiency flexible perovskite solar cells with a certificated efficiency of 17.3%. Adv. Funct. Mater. 2019, 29, 1807604. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, Y.; Chen, C.; Wu, X.; Kong, X.; Li, Z.; Gao, X.; Wang, Q.; Lu, X.; Zhou, G.; et al. A non-destructive transfer strategy for high-efficiency flexible perovskite solar cells. ACS Appl. Mater. Interfaces 2019, 11, 47003–47007. [Google Scholar] [CrossRef]
- Maranghi, S.; Parisi, M.L.; Basosi, R.; Sinicropi, A. Environmental profile of the manufacturing process of perovskite photovoltaics: Harmonization of life cycle assessment studies. Energies 2019, 12, 3746. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Meng, L.; Yang, Y.M.; Xu, G.; Hong, Z.; Chen, Q.; You, J.; Li, G.; Yang, Y.; Li, Y. High-efficiency robust perovskite solar cells on ultra-thin flexible substrates. Nat. Commun. 2016, 7, 10214. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Hu, X.; Liu, C.; Meng, X.; Huang, Z.; Yang, J.; Duan, X.; Long, J.; Zhao, Z.; Tian, L.; et al. Water-resistant and flexible perovskite solar cells via a glued interfacial layer. Adv. Funct. Mater. 2019, 29, 1902629. [Google Scholar] [CrossRef]
- Liu, Z.; Li, S.; Wang, X.; Cui, Y.; Qin, Y.; Leng, S.; Xu, Y.; Yao, K.; Huang, H. Interfacial engineering of front-contact with finely tuned polymer interlayers for high-performance large-area flexible perovskite solar cells. Nano Energy 2019, 62, 734–744. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, X.; Yang, Z.; Zhang, X.; Niu, J.; Wang, Z.; Zou, S.; Priya, S.; Liu, S.; Yang, D. Record efficiency stable flexible perovskite solar cell using effective additive assistant strategy. Adv. Mater. 2018, 30, 1801418. [Google Scholar] [CrossRef]
- Zhang, W.; Saliba, M.; Moore, D.T.; Pathak, S.K.; Hörantner, M.T.; Stergiopoulos, T.; Stranks, S.D.; Eperon, G.E.; Alexander-Webber, J.A.; Abate, A.; et al. Ultrasmooth organic–inorganic perovskite thin-film formation and crystallization for efficient planar heterojunction solar cells. Nat. Commun. 2015, 6, 6142. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Jang, I.H.; Pellet, N.; Grätzel, M.; Park, N.G. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat. Nanotechnol. 2014, 9, 927–932. [Google Scholar] [CrossRef]
- Lee, J.W.; Seol, D.J.; Cho, A.N.; Park, N.G. High-efficiency perovskite solar cells based on the black polymorph of HC(NH2)2 PbI3. Adv. Mater. 2014, 26, 4991–4998. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Zhang, J.; Yang, Z.; Liu, S.; Li, C. High efficiency flexible perovskite solar cells using superior low temperature TiO2. Energy Environ. Sci. 2015, 8, 3208–3214. [Google Scholar] [CrossRef]
- Qiu, W.; Paetzold, U.W.; Gehlhaar, R.; Smirnov, V.; Boyen, H.G.; Tait, J.G.; Conings, B.; Zhang, W.; Nielsen, C.B.; McCulloch, L.; et al. An electron beam evaporated TiO2 layer for high efficiency planar perovskite solar cells on flexible polyethylene terephthalate substrates. Electron beam evaporated TiO2 layer for high efficiency planar perovskite solar cells on polyethylene terephthalate substrates. J. Mater. Chem. 2015, 3, 22824–22829. [Google Scholar]
- Jeong, I.; Jung, H.; Park, M.; Park, J.S.; Son, H.J.; Joo, J.; Lee, J.; Ko, M.J. A tailored TiO2 electron selective layer for high-performance flexible perovskite solar cells via low temperature UV process. Nano Energy 2016, 28, 380–389. [Google Scholar] [CrossRef]
- Chu, Y.; Cai, H.; Huang, L.; Xing, Z.; Du, Y.; Ni, J.; Li, J.; Zhang, J. High-efficient flexible perovskite solar cells with low temperature TiO2 layer via UV/Ozone photo-annealing treatment. Phys. Status Solidi A 2019, 216, 1800669. [Google Scholar] [CrossRef]
- You, M.S.; Heo, J.H.; Park, J.K.; Moon, S.H.; Park, B.J.; Im, S.H. Low temperature solution processable TiO2 nano-sol for electron transporting layer of flexible perovskite solar cells. Sol. Energy Mater. Sol. Cells 2019, 194, 1–6. [Google Scholar] [CrossRef]
- Shao, Y.; Xiao, Z.; Bi, C.; Yuan, Y.; Huang, J. Origin and elimination of photo-current hysteresis by fullerene passivation in CH3NH3PbI3 planar heterojunction solar cells. Nat. Commun. 2014, 5, 5784–5791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Guo, Y.; Yin, X.; He, M.; Zou, X. Spongy carbon film deposited on a separated substrate as a counter electrode for perovskite-based solar cell. Mater. Lett. 2016, 182, 248–252. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Wang, J.; Qin, P.; Tao, H.; Lei, H.; Liu, Q.; Dai, X.; Zhao, X. Perovskite solar cell with an efficient TiO2 compact film. Appl. Mater. Interfaces 2014, 6, 15959–15965. [Google Scholar] [CrossRef] [PubMed]
- Abdi-Jalebi, M.; Dar, M.I.; Sadhanala, A.; Senanayak, S.P.; Giordano, F.; Zakeeruddin, S.M.; Grätzel, M.; Friend, R.H. Impact of mesoporous titania-perovskite interface on the performance of hybrid organic-inorganic perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 3264–3269. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zou, X.; Cheng, J.; Chen, D.; Yao, Y.; Chang, C.; Yu, X.; Zhou, Z.; Wang, J.; Liu, B. Impact of perovskite composition on film formation quality and photophysical properties for flexible perovskite solar cells. Molecules 2020, 25, 732. [Google Scholar] [CrossRef] [Green Version]


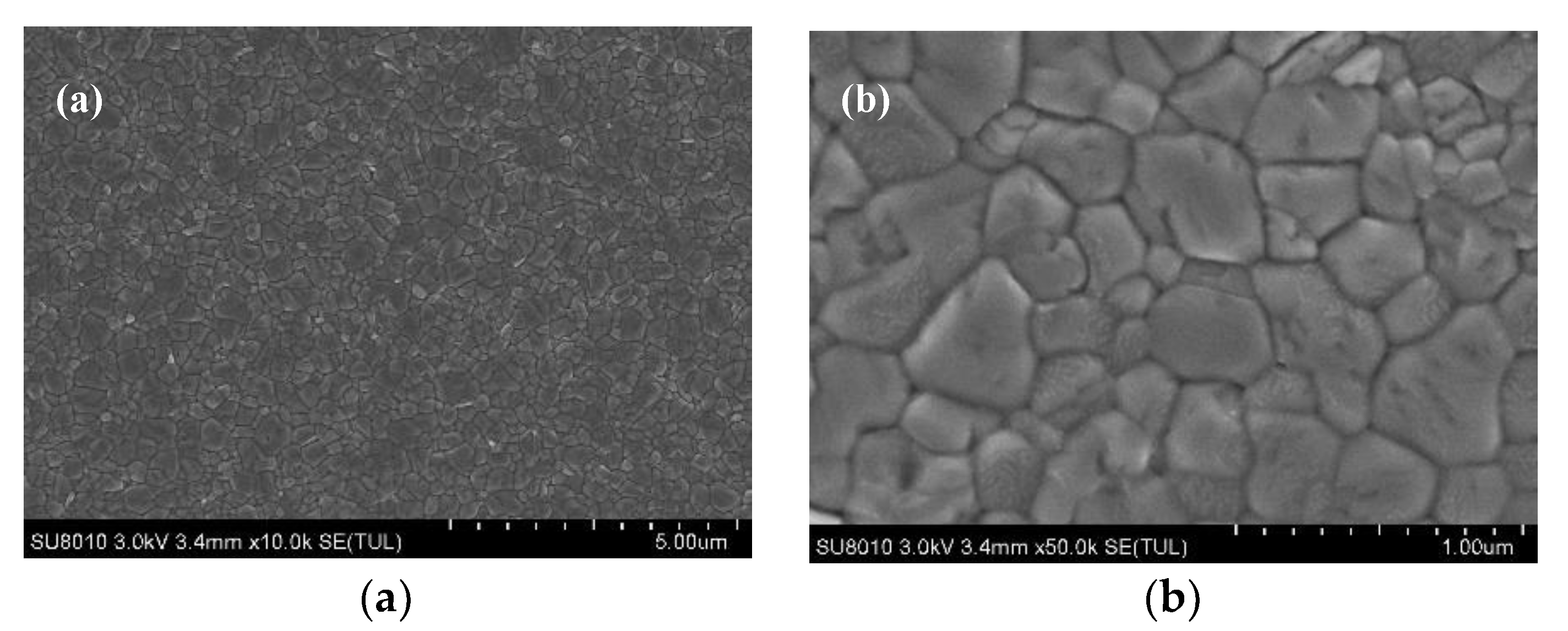
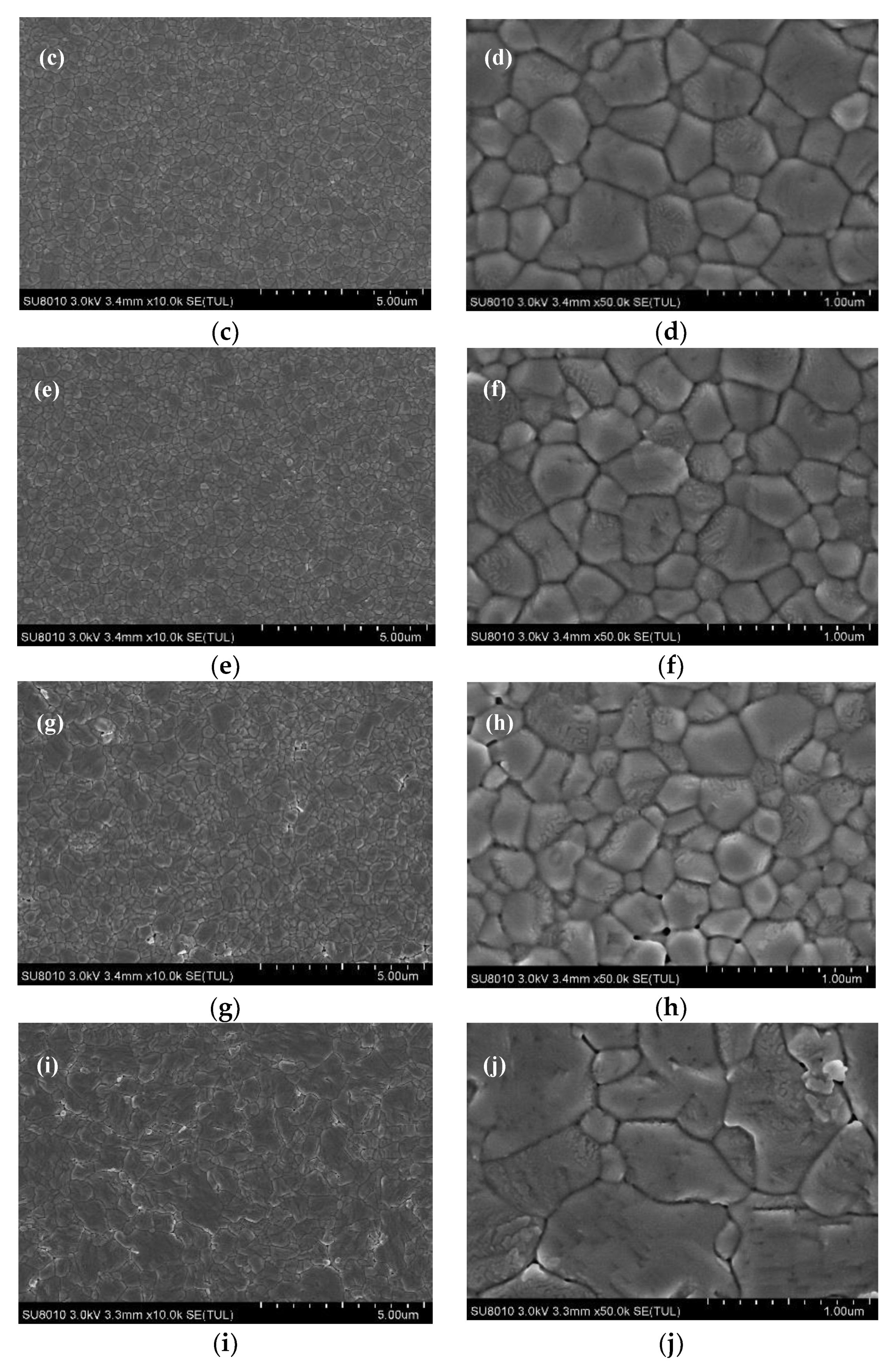

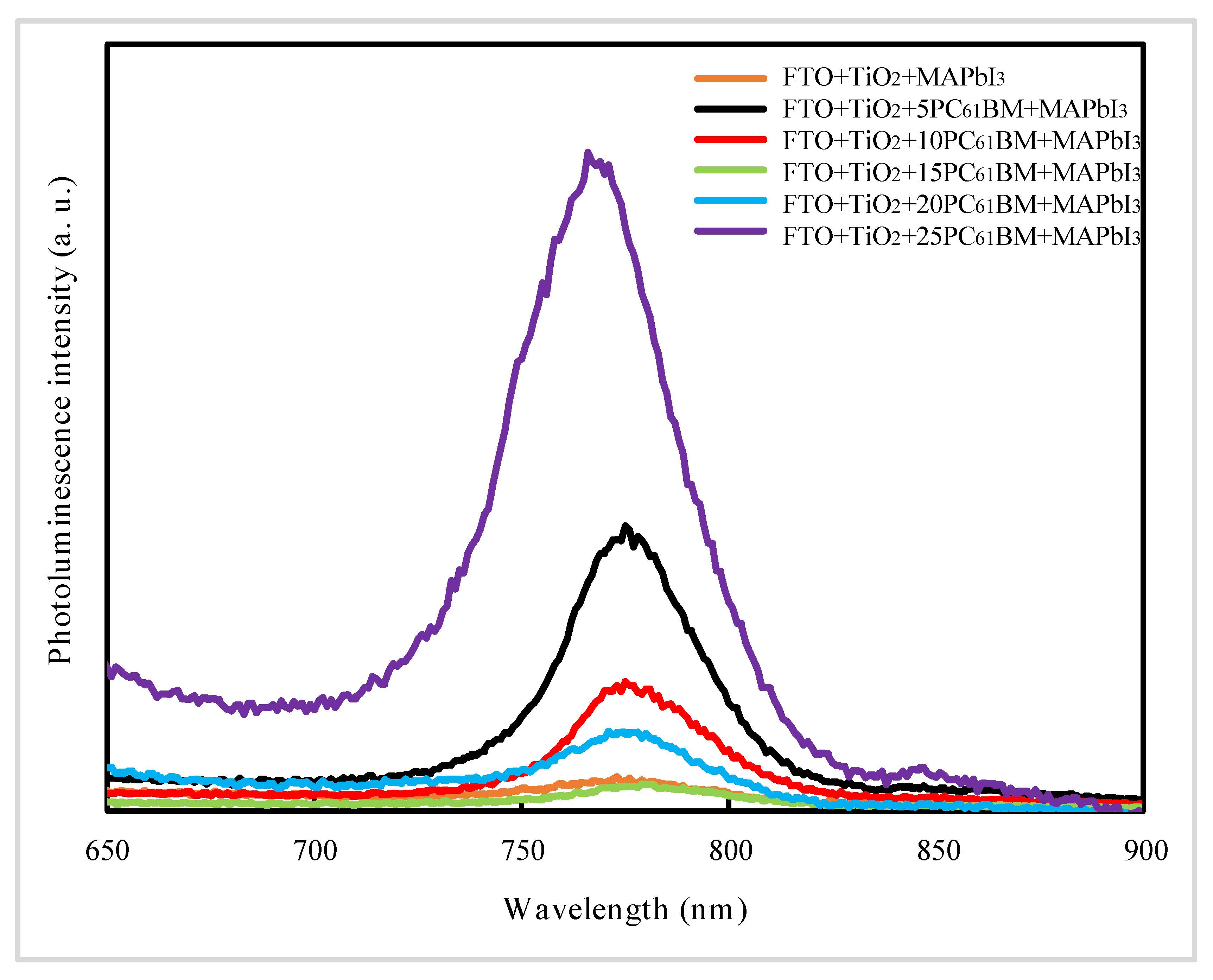
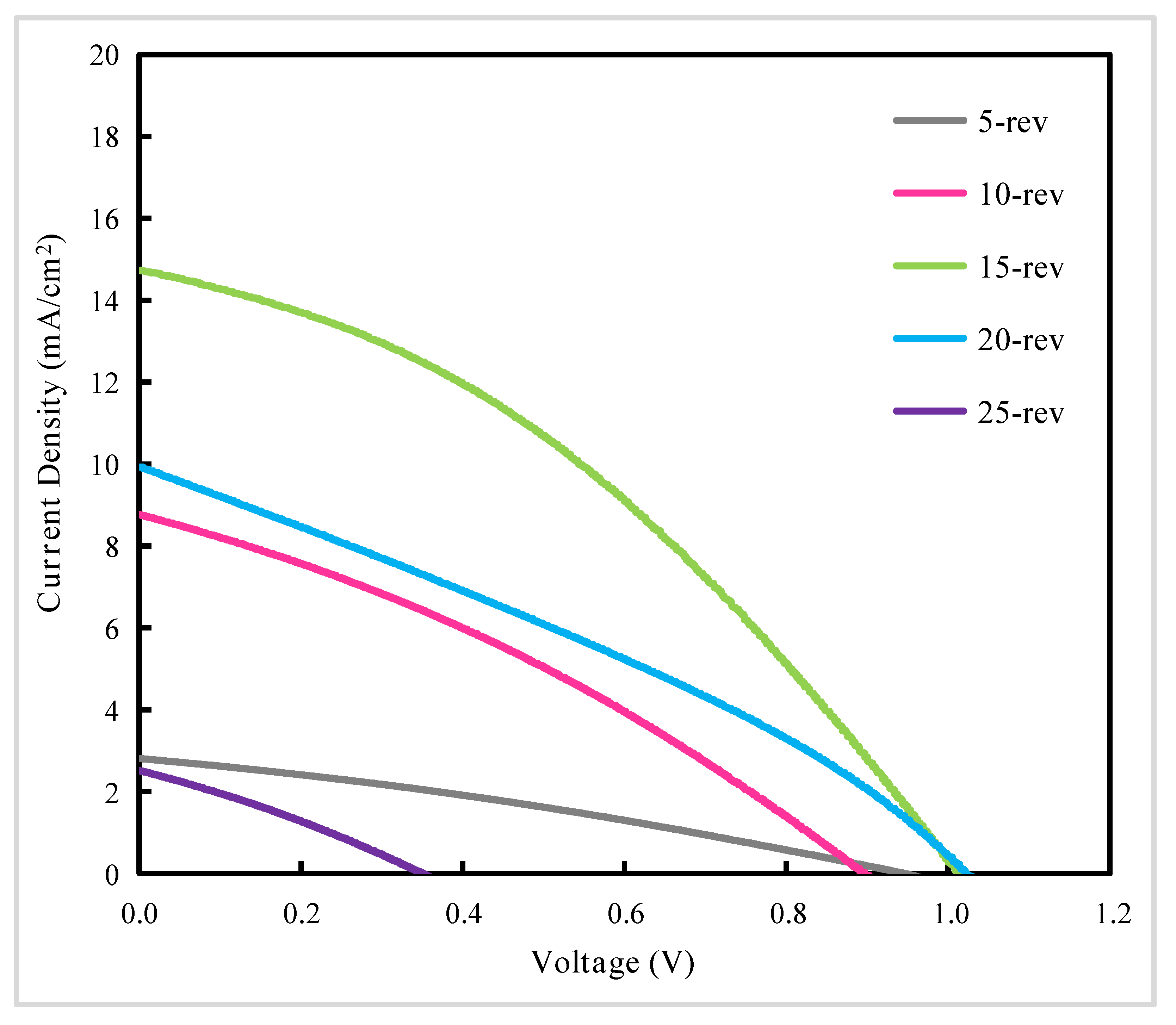
| Samples | PCE a (%) | Voc b (V) | Jsc c (mA/cm2) | FF d |
|---|---|---|---|---|
| Di | 0.26 | 0.55 | 1.94 | 0.27 |
| Pr | 0.11 | 0.47 | 0.93 | 0.25 |
| De | 0.96 | 0.66 | 4.82 | 0.30 |
| Samples | PCE a (%) | Voc b (V) | Jsc c (mA/cm2) | FF d |
|---|---|---|---|---|
| 5-rev | 0.81 | 0.94 | 2.81 | 0.31 |
| 10-rev | 2.52 | 0.90 | 8.76 | 0.32 |
| 15-rev | 5.48 | 1.01 | 14.74 | 0.37 |
| 20-rev | 3.14 | 1.02 | 9.94 | 0.31 |
| 25-rev | 0.26 | 0.35 | 2.52 | 0.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Zou, X.; Cheng, J.; Chen, D.; Yao, Y.; Chang, C.; Liu, B.; Wang, J.; Zhou, Z.; Li, G. Investigation on Low-temperature Annealing Process of Solution-processed TiO2 Electron Transport Layer for Flexible Perovskite Solar Cell. Materials 2020, 13, 1031. https://doi.org/10.3390/ma13051031
Yu X, Zou X, Cheng J, Chen D, Yao Y, Chang C, Liu B, Wang J, Zhou Z, Li G. Investigation on Low-temperature Annealing Process of Solution-processed TiO2 Electron Transport Layer for Flexible Perovskite Solar Cell. Materials. 2020; 13(5):1031. https://doi.org/10.3390/ma13051031
Chicago/Turabian StyleYu, Xing, Xiaoping Zou, Jin Cheng, Dan Chen, Yujun Yao, Chuangchuang Chang, Baoyu Liu, Junqi Wang, Zixiao Zhou, and Guangdong Li. 2020. "Investigation on Low-temperature Annealing Process of Solution-processed TiO2 Electron Transport Layer for Flexible Perovskite Solar Cell" Materials 13, no. 5: 1031. https://doi.org/10.3390/ma13051031
APA StyleYu, X., Zou, X., Cheng, J., Chen, D., Yao, Y., Chang, C., Liu, B., Wang, J., Zhou, Z., & Li, G. (2020). Investigation on Low-temperature Annealing Process of Solution-processed TiO2 Electron Transport Layer for Flexible Perovskite Solar Cell. Materials, 13(5), 1031. https://doi.org/10.3390/ma13051031




