Planetary Milling and Self-Propagating High-Temperature Synthesis of Al-TiB2 Composites
Abstract
:1. Introduction
2. Materials and Methods
3. Results
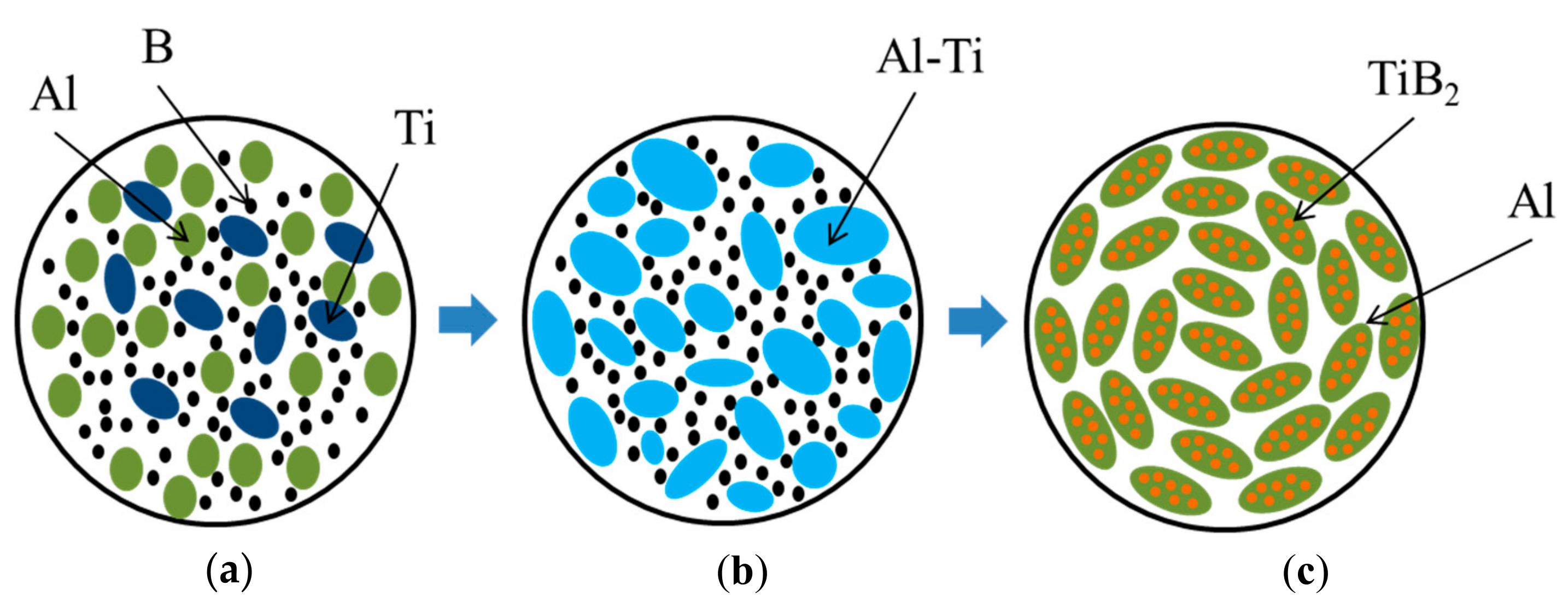
3.1. Planetary Milling Time vs. Structure and Density of Al-Ti-B Powder Mixture
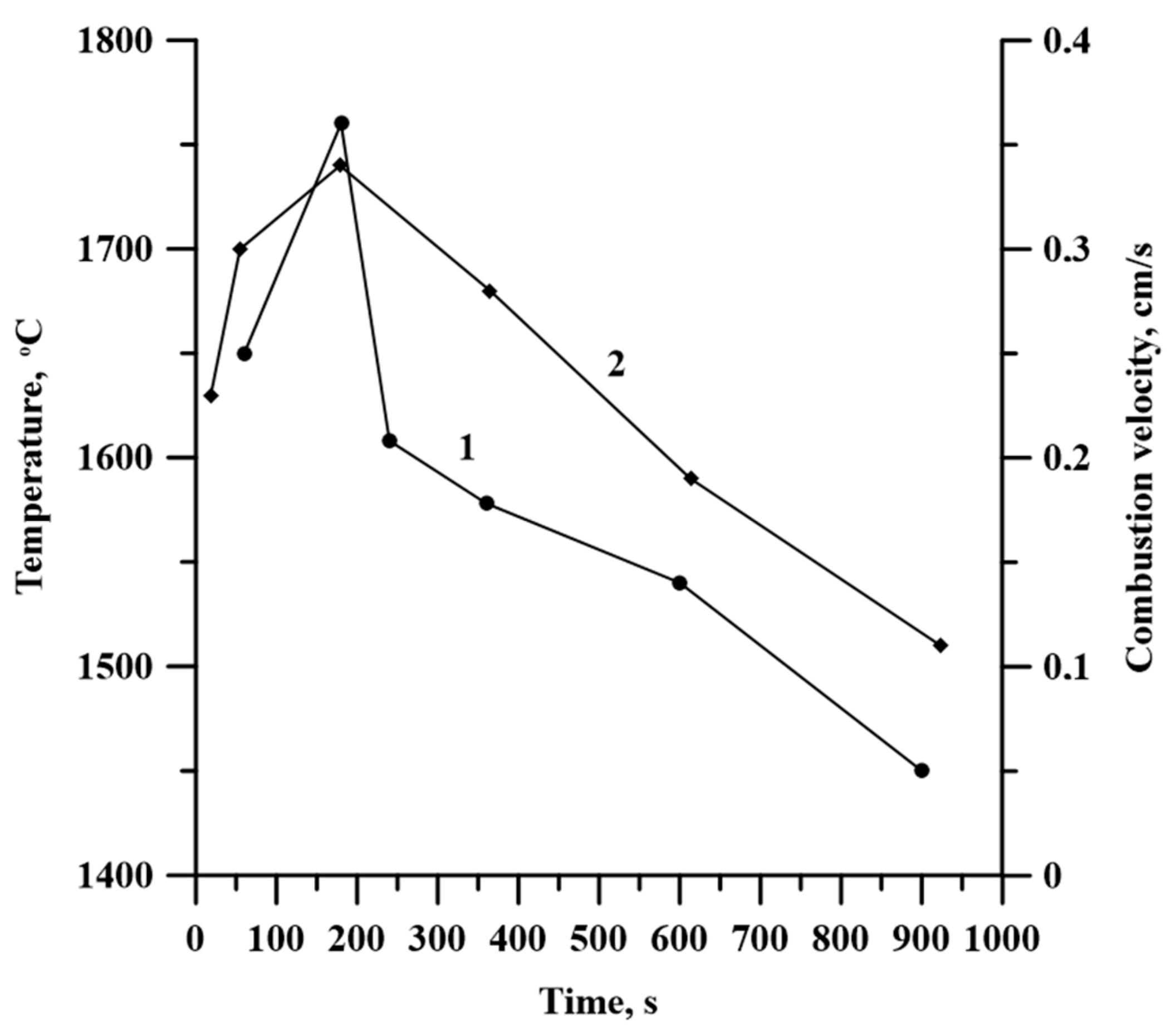
3.2. Time Dependence of Planetary Milling of the Initial Al-Ti-B Powder Misxture on Its Combustion Temparature and Velocity
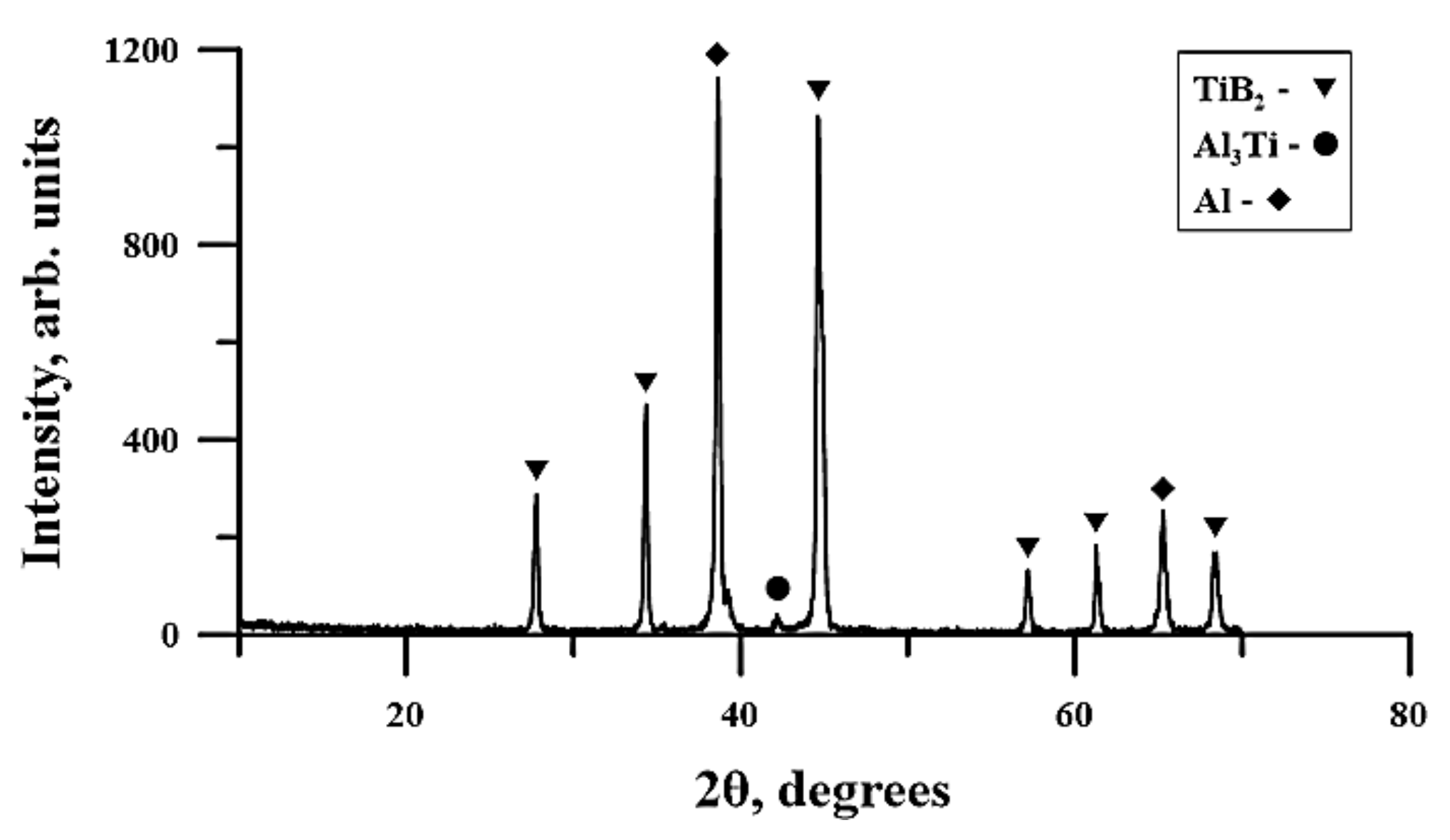
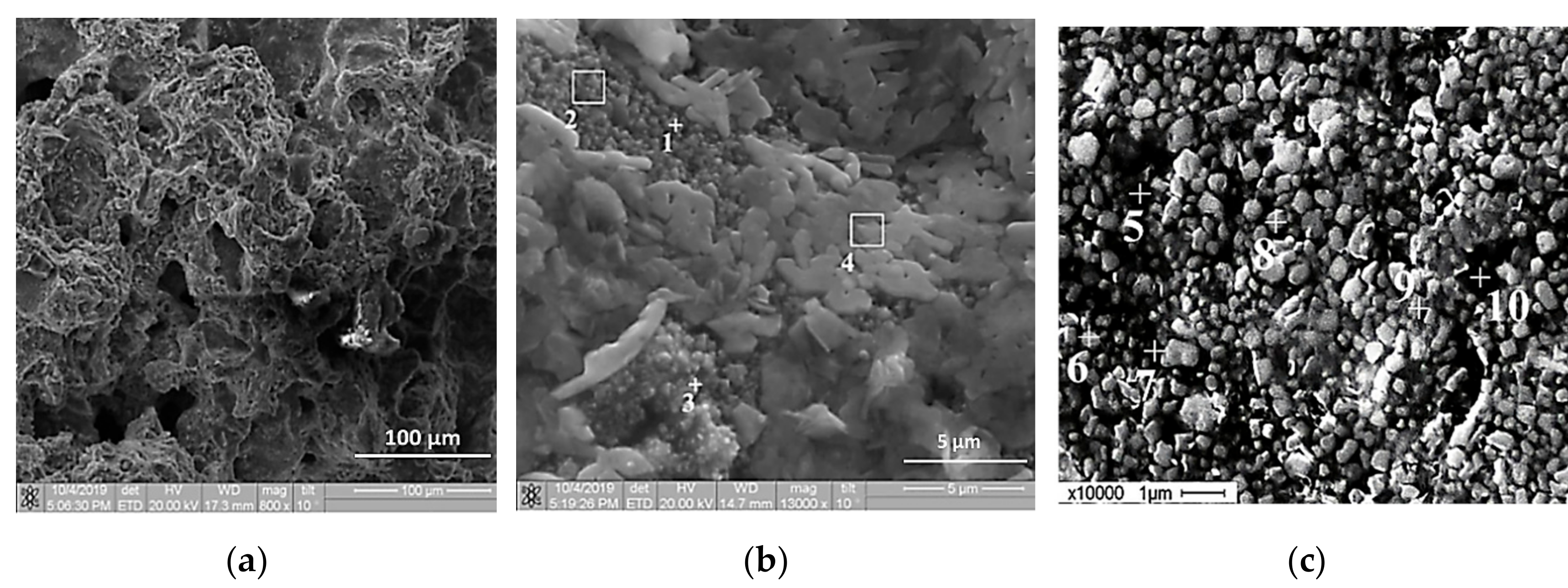
3.3. Time Dependence of Planetary Milling of the Al-Ti-B Powder Misxture on the Phase Composition and Structure of Combustion Products
4. Discussion
5. Conclusions
- Lamellar composites were formed after planetary milling of the Al-Ti-B mixture. These composites were composed of Al, Ti, and B particles less than 1 µm. An increase in the planetary milling time from 180 to 2400 s led to an increase in the average size of the lamellar composite from 33.9 to 51.2 μm.
- An increase in the time of planetary milling led to a change in the density of the compacted samples, as well as the temperature and their combustion velocity. These time dependences were extreme, with maximum values during 180 s of planetary milling. An increase in the planetary milling time of more than 180 s led to a decrease in the density of compacted samples, as well as the temperature and their combustion velocity. A change in the combustion rate and temperature was associated with a change in the particles structure of the mixture after its planetary milling. The combustion of samples obtained after planetary milling of the mixture was carried out in the spin combustion mode.
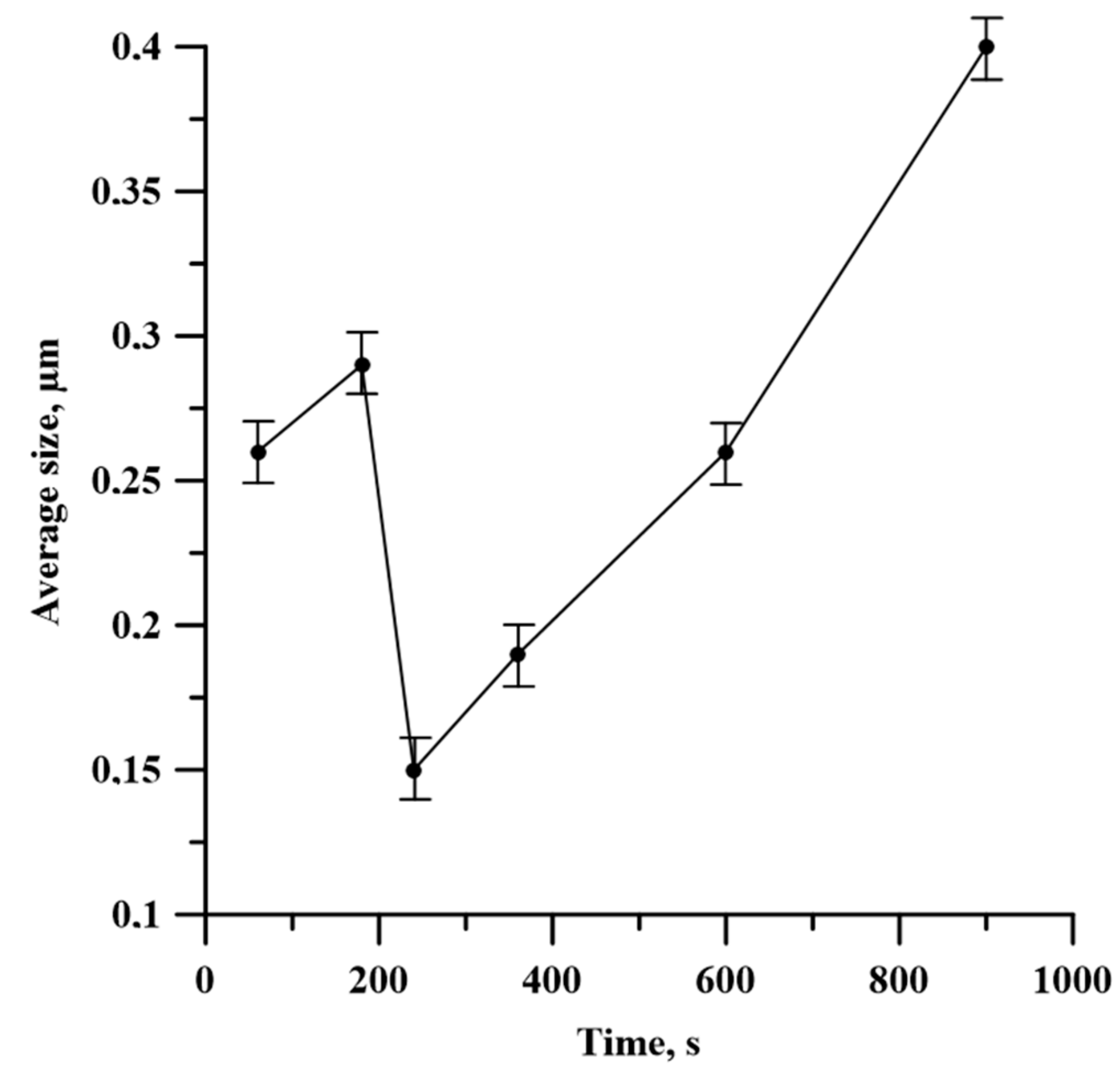
- The structure of SHS-materials obtained from Al-Ti-B powder systems after planetary milling was a metal matrix Al with inclusions of TiB2 particles. The Al3Ti layer was discovered between the TiB2 and the aluminum matrix. The TiB2 particle size was less than 1 μm in all samples. The dependence of the average size of TiB2 particles in SHS products on the duration of planetary milling of the initial mixture was established. The increasing planetary milling time from 60 to 180 s, the average particle size in the obtained SHS products increased from 0.26 to 0.29 μm. After further planetary milling of the powder mixture in the range from 180 to 240 s, the average particle size decreased from 0.29 to 0.15 µm. Finally, an increasing in planetary milling from 240 to 900 s ensured an increase in the average particle size to 0.4 μm.
- The formation mechanism of Al-Al3Ti-TiB2 composites during combustion of the Al-Ti-B powder mixture in the mode of self-propagating high-temperature synthesis was established.
Author Contributions
Funding
Conflicts of Interest
References
- Suresh, S.; Shenbag, N.; Moorthi, V. Aluminium-titanium diboride (Al-TiB2) metal matrix composites: Challenges and opportunities. Procedia. Eng. 2012, 38, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, S.; Narayanasamy, R.; Babu, S.K.; Dinesh, G.; Kumar, B.A.; Sivaprasad, K. Sliding wear behaviour of Al 6063/TiB2 in situ composites at elevated temperatures. Mater. Des. 2009, 30, 2521–2531. [Google Scholar] [CrossRef]
- Xiao, P.; Yimin, G.; Xirong, Y.; Feixing, X.; Cuicui, Y.; Bo, L.; Yefei, L.; Zhiwei, L.; Qiaoling, Z. Processing, microstructure and ageing behavior of in-situ submicron TiB2 particles reinforced AZ91 Mg matrix composites. J. Alloys Compd. 2018, 764, 96–106. [Google Scholar] [CrossRef]
- Birol, Y. Production of Al-Ti-B grain refining master alloys from B2O3 and K2TiF6. J. Alloys Compd. 2007, 443, 94–98. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Zhang, Y.; Qin, T.; Zhou, X.R.; Thompson, G.E.; Pennycook, T.; Hashimoto, T. Grain refining mechanism in the Al/Al-Ti-B system. Acta Mater. 2015, 84, 292–304. [Google Scholar] [CrossRef]
- Vicario, I.; Poulon-Quintin, A.; Lagos, M.; Silvain, J.F. Effect of material and process atmosphere in the preparation of Al-Ti-B grain refiner by SHS. Metals 2015, 5, 1387–1396. [Google Scholar] [CrossRef] [Green Version]
- Zaviar, S.; Shijil, V.R.; Raphael, S.; Sreeram, R.; Sudarsh, C.K.; Rajeev, V.R.; Manesh, K.K. Effect of Grain Refiner (0 to 1 wt% Al-5Ti-B) Addition on the Microstructural and Hardness Characteristics of Al 336 Alloy. In Recent Advances in Materials, Mechanics and Management. In Proceedings of the 3rd International Conference on Materials, Mechanics and Management, Trivandrum, Kerala, India, 13–15 July 2017; pp. 13–15. [Google Scholar]
- Promakhov, V.V.; Ziatdinov, M.K.; Zhukov, I.A.; Vorozhtsov, S.A.; Matveev, A.E.; Titov, S.S. Self-propagating high-temperature synthesis of new class of master alloys for aluminum alloys. Polzunovskii vestnik 2016, 1, 76–79. [Google Scholar]
- Zhukov, I.A.; Promakhov, V.V.; Matveev, A.E.; Platov, V.V.; Khrustalev, A.P.; Dubkova, Y.A.; Vorozhtsov, S.A.; Potekaev, A.I. Principles of structure and phase composition formation in composite master alloys of the Al-Ti-B/B4C systems used for aluminum alloy modification. Russ Phys J. 2018, 60, 2025–2031. [Google Scholar] [CrossRef]
- Zhukov, I.; Promakhov, V.; Dubkova, Y.; Matveev, A.; Ziatdinov, M.; Zhukov, А. Al-Ti-B4C Materials Obtained by High-Temperature Synthesis and Used as a Master-Alloy for Aluminum. MATEC Web Conf 2018, 243, 00010. [Google Scholar] [CrossRef]
- Mursalat, M.; Schoenitz, M.; Dreizin, E.L. Composite Al∙ Ti powders prepared by high-energy milling with different process controls agents. Adv. Powder Technol. 2019, 30, 1319–1328. [Google Scholar] [CrossRef]
- Maglia, F.; Anselmi-Tamburini, A.; Deidda, C.; Delogu, F.; Cocco, G.; Munir, Z.A. Role of mechanical activation in SHS synthesis of TiC. J. Mater. Sci. 2004, 39, 5227–5230. [Google Scholar] [CrossRef]
- Kochetov, N.A.; Vadchenko, S.G. Effect of the time of mechanical activation of a Ti + 2B mixture on combustion of cylindrical samples and thin foils. Combust Explo. Shock 2015, 51, 467–471. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Grigor’eva, T.F.; Bokhonov, B.B.; Sharafutdinov, M.R.; Barinova, A.P.; Lyakhov, N.Z. Solid-state combustion in mechanically activated SHS systems. I. Effect of activation time on process parameters and combustion product composition. Combust Explo. Shock 2003, 39, 43–50. [Google Scholar] [CrossRef]
- Zhukov, I.A.; Ziatdinov, M.K.; Vorozhtsov, A.B.; Zhukov, A.S.; Vorozhtsov, S.A.; Promakhov, V.V. Self-propagating high-temperature synthesis of Al and Ti borides. Russ. Phy. J. 2016, 59, 1324–1326. [Google Scholar]
- Maslov, V.M.; Borovinskaya, I.P.; Merzhanov, A.G. Problem of the mechanism of gasless combustion. Combust Explo Shock 1976, 12, 631–636. [Google Scholar] [CrossRef]
- Saltykov, S.A. Stereometric Metallography. Metallurgiya: Moscow, Russia, 1970; p. 267. [Google Scholar]
- Gavrilova, N.N.; Nazarov, V.V.; Yarovaya, O.V. Microscopic Methods of Measuring Particle Size of Dispersion Materials; D. Mendeleev University of Chemical Technology of Russia: Moscow, Russia, 2012. (In Russian) [Google Scholar]
- Lee, J.H.; Lee, A.Y.; Chen, C.C. Reverse burning phenomenon in self-propagating high-temperature synthesis. J. Mater. Res. 1998, 13, 1626–1630. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Mukas’yan, A.S. Solid-Flame Combustion; Torus Press:: Moscow, Russia, 2007. (In Russian) [Google Scholar]
- Avakumov, E.G. Mechanical Methods of Activating Chemical Processes. Nauka:: Novosibirsk, Russia, 1986; p. 305. (In Russian) [Google Scholar]
- Poluboyarov, V.A.; Lapin, A.E.; Korotaeva, Z.A.; Cherepanov, A.N.; Solonenko, O.P.; Kobotaeva, N.S.; Sirotkina, E.E.; Michael, A. The effect of mechanical activation of metal powders on their reactivity and properties of plasma-deposited coatings. CSD 2002, 10, 173–178. [Google Scholar]
- Kosenko, N.F.; Smirnova, M.A. Performance assessment of mechanical activation of aluminum oxide based on thermochemical data. Izv vuzov. Khimiya i khim tekhnologiya 2008, 51, 122–124. [Google Scholar]
- Sasikumar, C.; Srikanth, S.; Mukhopadhyay, N.K.; Mehrotra, S.P. Energetics of mechanical activation–Application to ilmenite. Miner. Eng. 2009, 22, 572–574. [Google Scholar] [CrossRef]
- Nakashima, P.N. The Crystallography of Aluminum and Its Alloys. arXiv preprint arXiv 2002, 01562, 2020. [Google Scholar] [CrossRef] [Green Version]
- Amador, C.; Hoyt, J.J.; Chakoumakos, B.C.; de Fontaine, D. Theoretical and experimental study of relaxations in Al3Ti and Al3Zr ordered phases. Phys. Rev. Lett. 1995, 74, 4955. [Google Scholar] [CrossRef] [PubMed]
- Pauling File Multinaries Edition Database: Inorganic Solid Phases. Springer & Material Phases Data System, National Institute for Materials Science: Tsukuba, Japan, 2016.
- Žitko, R.; Van Midden, H.J.; Zupanič, E.; Prodan, A.; Makridis, S.S.; Niarchos, D.; Stubos, A.K. Hydrogenation properties of the TiBx structures. Int. J. Hydrogen Energy 2011, 36, 12268–12278. [Google Scholar] [CrossRef] [Green Version]
- Gulyaev, A.P. Metallurgy; Metallurgiya:: Moscow, Russia, 1977; p. 648. [Google Scholar]
- Holloway, S.R. Metallic Matrix Composite with High Strength Titanium Aluminide Alloy Matrix and In Situ Formed Aluminum Oxide Reinforcement. US Patent N 16098538, 28 March 2019. [Google Scholar]
- Schuster, J.C.; Palm, M. Reassessment of the binary aluminum-titanium phase diagram. J. Phase Equilib. Diff. 2006, 27, 255–277. [Google Scholar] [CrossRef]
- Carlson, O.N. The Al-B (aluminum-boron) system. J. Phase Equilib. 1994, 15, 543–552. [Google Scholar] [CrossRef]
- Emamy, M.; Mahta, M.; Rasizadeh, J. Formation of TiB2 particles during dissolution of TiAl3 in Al–TiB2 metal matrix composite using an in situ technique. Compos. Sci. Technol. 2006, 66, 1063–1066. [Google Scholar] [CrossRef]
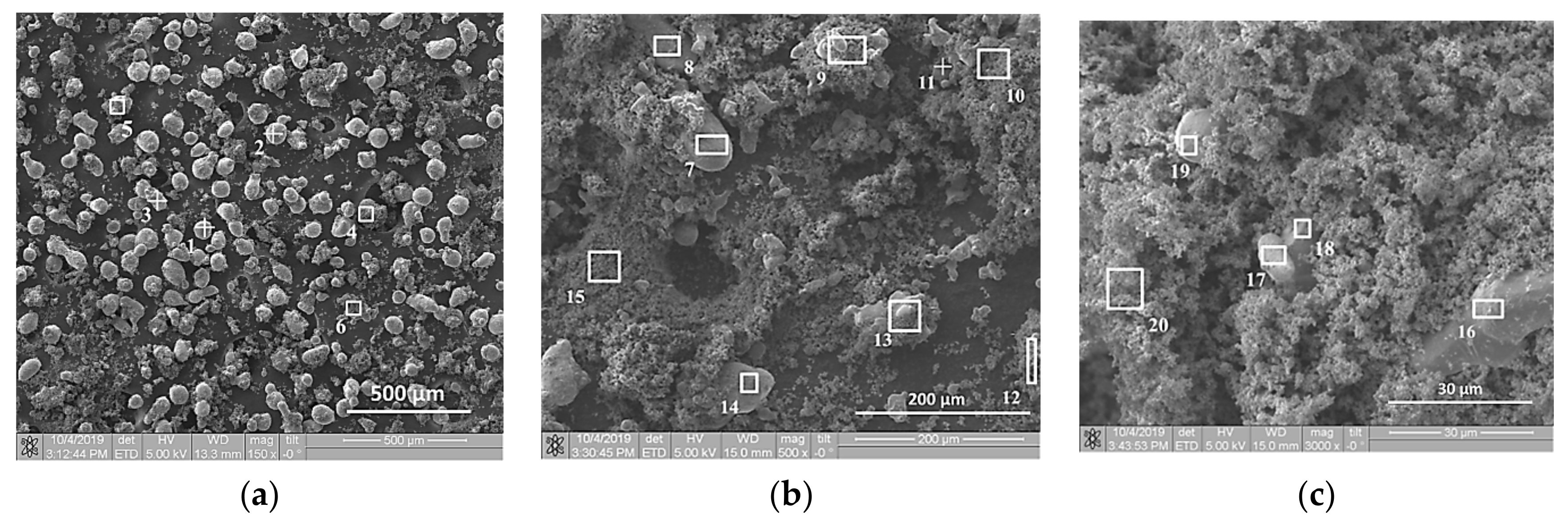


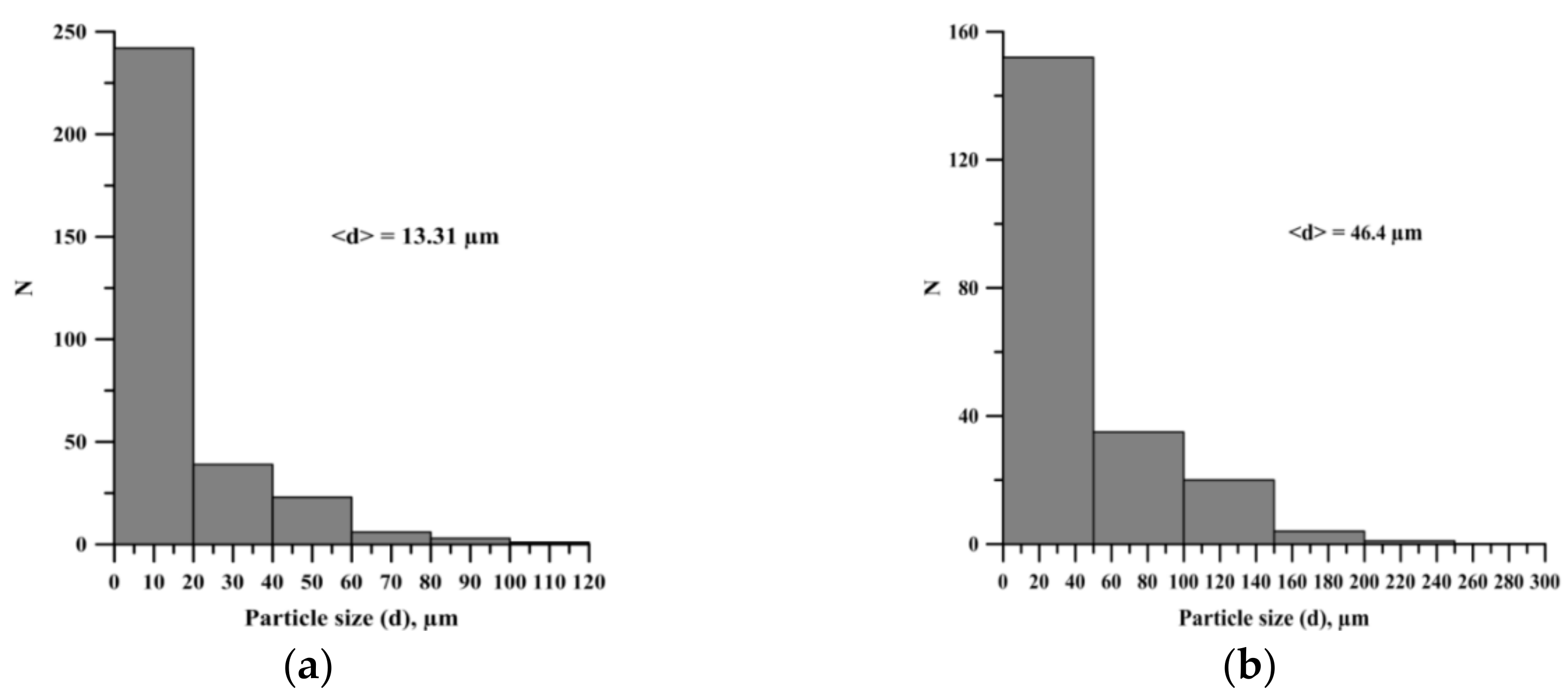
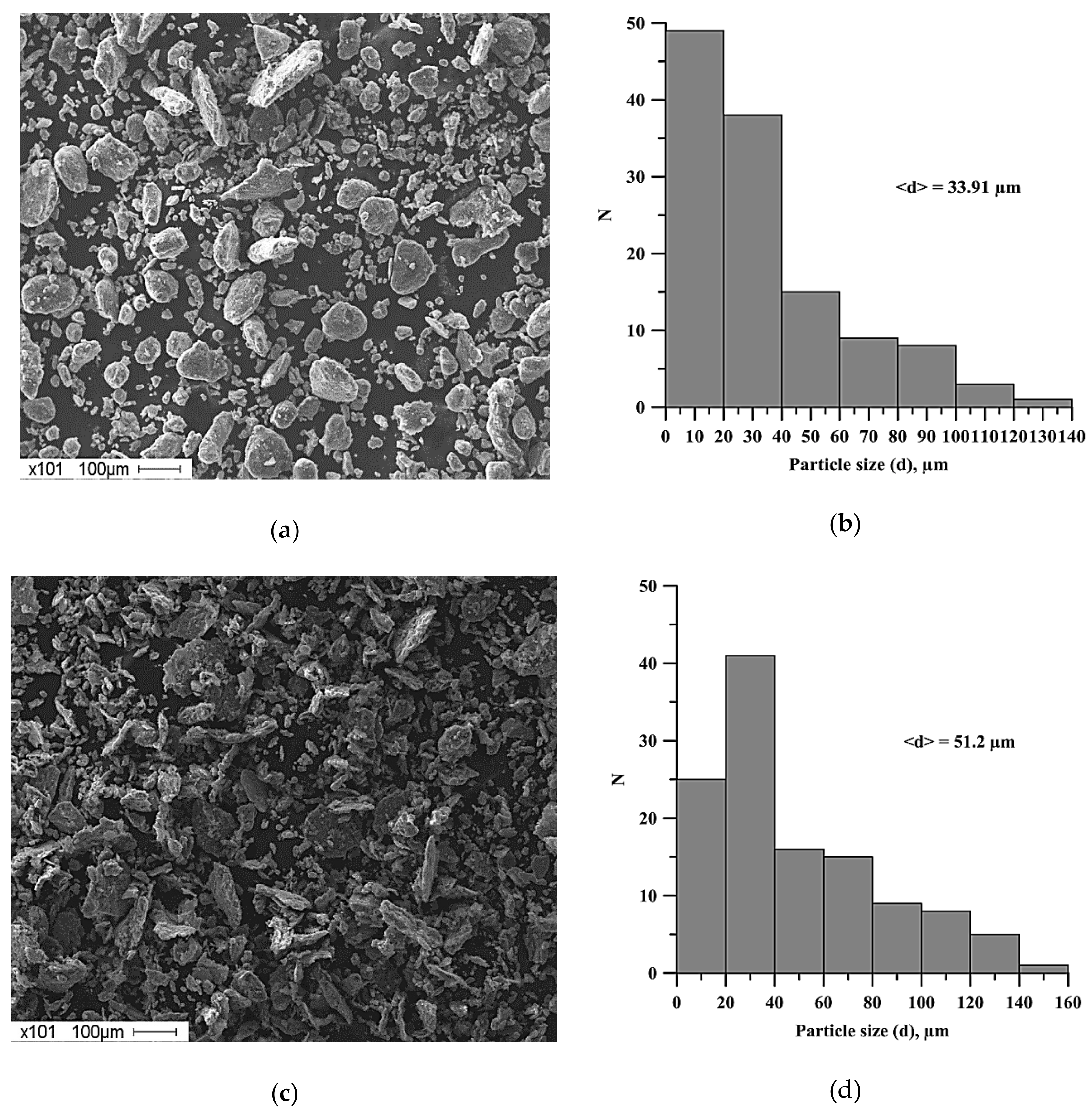
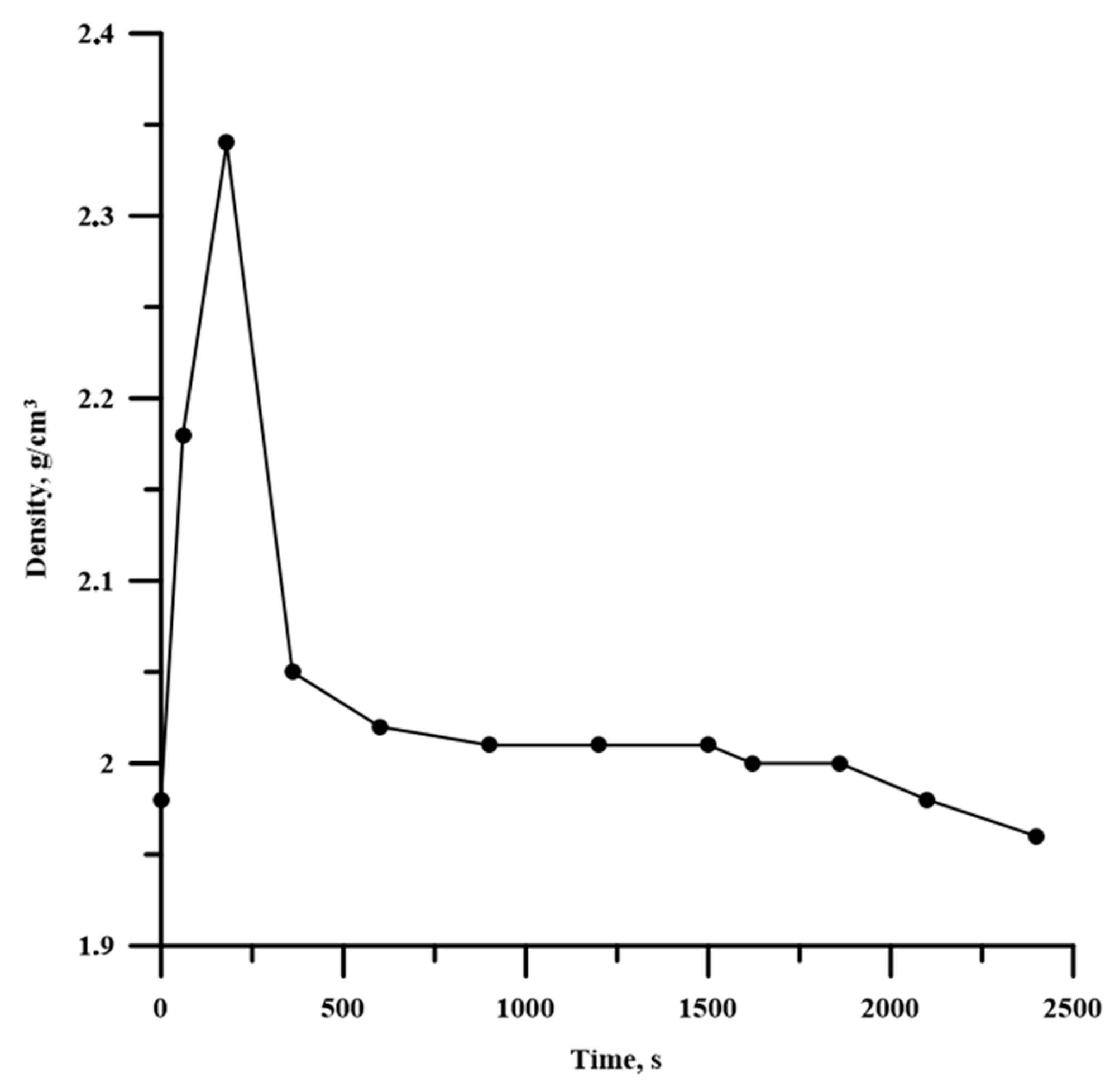


| Powder | Average Particle Size, µm | Purity, wt. % |
|---|---|---|
| Titanium (Ti) (OJSC «Polema») | 140 | ⩾99 |
| Boron (B) (OJSC «Aviabor») | 0.6 | ⩾99.2 |
| Aluminum (Al) (Ltd «Sual-PM») | 100 | ⩾98.4 |
| Element | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Al | + | + | + | + | + | + | + | + | + | + |
| Ti | - | - | + | + | + | + | + | - | - | + |
| B | - | - | - | + | + | - | + | + | - | + |
| Element | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 1 |
| Al | + | - | - | + | + | + | - | + | - | + |
| Ti | + | + | + | - | + | + | + | - | + | + |
| B | + | + | + | - | + | + | - | - | - | + |
| Element | 1–2 | 3 | 4–15 1 |
|---|---|---|---|
| Al | + | + | + |
| Ti | + | - | + |
| B | + | + | + |
| Element | 1 | 2 | 3 1 |
|---|---|---|---|
| Al | + | + | + |
| Ti | + | + | + |
| B | + | + | + |
| Specimens | Phases | Composition, wt.% | Lattice Parameters, Ǻ | CSR 2, nm |
|---|---|---|---|---|
| 60 s PM1 (Z1_306_19) | TiB2 | 44 | a = 3.0274, c = 3.2261 | 71 |
| Al | 55 | a = 4.0455 | 68 | |
| Al3Ti | Traces | a = 3.8462, c = 8.5904 | - | |
| 180 s PM (Z1_307_19) | TiB2 | 40 | a = 3.0290, c = 3.2285 | 97 |
| Al | 58 | a = 4.0487 | 100 | |
| Al3Ti | Traces | a = 3.8504, c = 8.5932 | - | |
| 360 s PM(Z1_308_19) | TiB2 | 42 | a = 3.0296, c = 3.2293 | 74 |
| Al | 55 | a = 4.0501 | 48 | |
| Al3Ti | Traces | a = 3.8528, c = 8.5979 | 47 | |
| 900 s PM (Z1_309_19) | TiB2 | 39 | a = 3.0256, c = 3.2264 | 43 |
| Al | 54 | a = 4,0447 | 38 | |
| Al3Ti | Traces | a = 3.8430, c = 8.5854 | 35 |
| Element | 1, 2, 3, 6, 8, 9 | 4, 5, 6, 7, 10 1 |
|---|---|---|
| Al | + | + |
| Ti | + | + |
| B | + | - |
| Compound | Lattice Parameters, Ǻ |
|---|---|
| Al | а = 4.040 |
| Al3Ti | а = 3.8467, c = 8.608 |
| TiB2 | а = 3.026, с = 3.212 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matveev, A.; Zhukov, I.; Ziatdinov, M.; Zhukov, A. Planetary Milling and Self-Propagating High-Temperature Synthesis of Al-TiB2 Composites. Materials 2020, 13, 1050. https://doi.org/10.3390/ma13051050
Matveev A, Zhukov I, Ziatdinov M, Zhukov A. Planetary Milling and Self-Propagating High-Temperature Synthesis of Al-TiB2 Composites. Materials. 2020; 13(5):1050. https://doi.org/10.3390/ma13051050
Chicago/Turabian StyleMatveev, Alexey, Ilya Zhukov, Mansur Ziatdinov, and Alexander Zhukov. 2020. "Planetary Milling and Self-Propagating High-Temperature Synthesis of Al-TiB2 Composites" Materials 13, no. 5: 1050. https://doi.org/10.3390/ma13051050
APA StyleMatveev, A., Zhukov, I., Ziatdinov, M., & Zhukov, A. (2020). Planetary Milling and Self-Propagating High-Temperature Synthesis of Al-TiB2 Composites. Materials, 13(5), 1050. https://doi.org/10.3390/ma13051050




