Effect of Nb Content on Cyclic Oxidation Behavior of As-Cast Ti-1100 Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
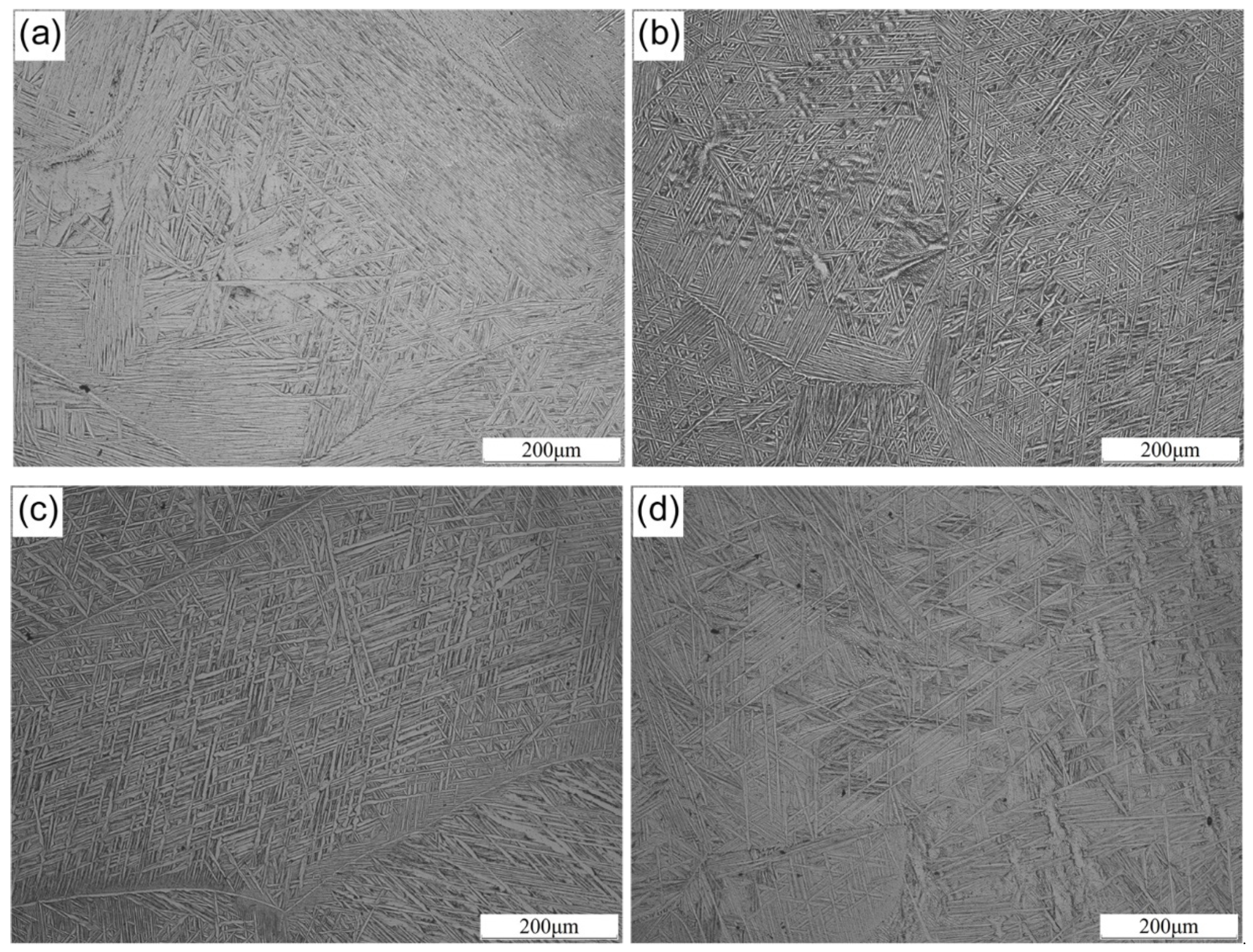
3.1. Microstructure
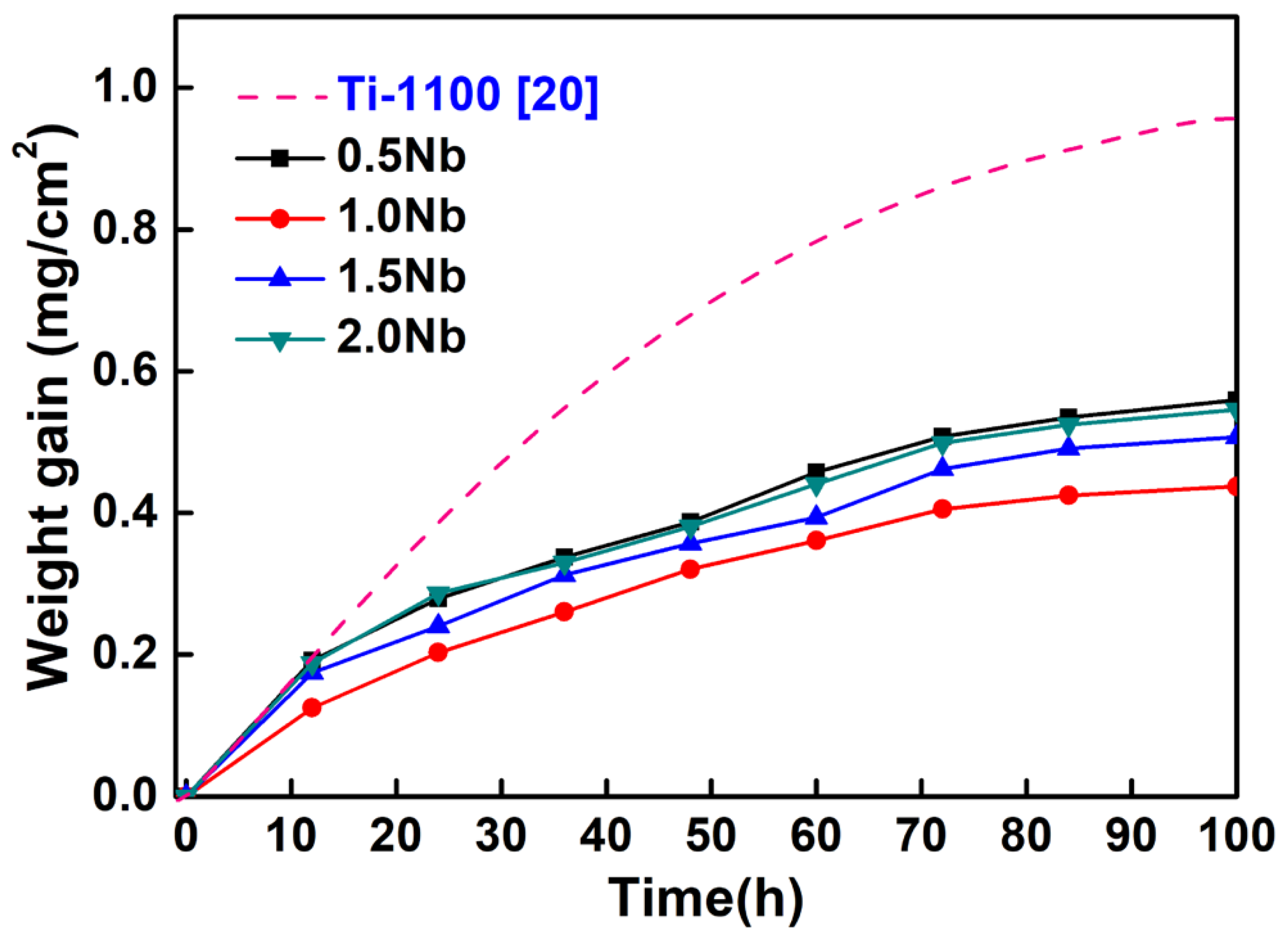
3.2. Analysis of Oxidation Kinetics
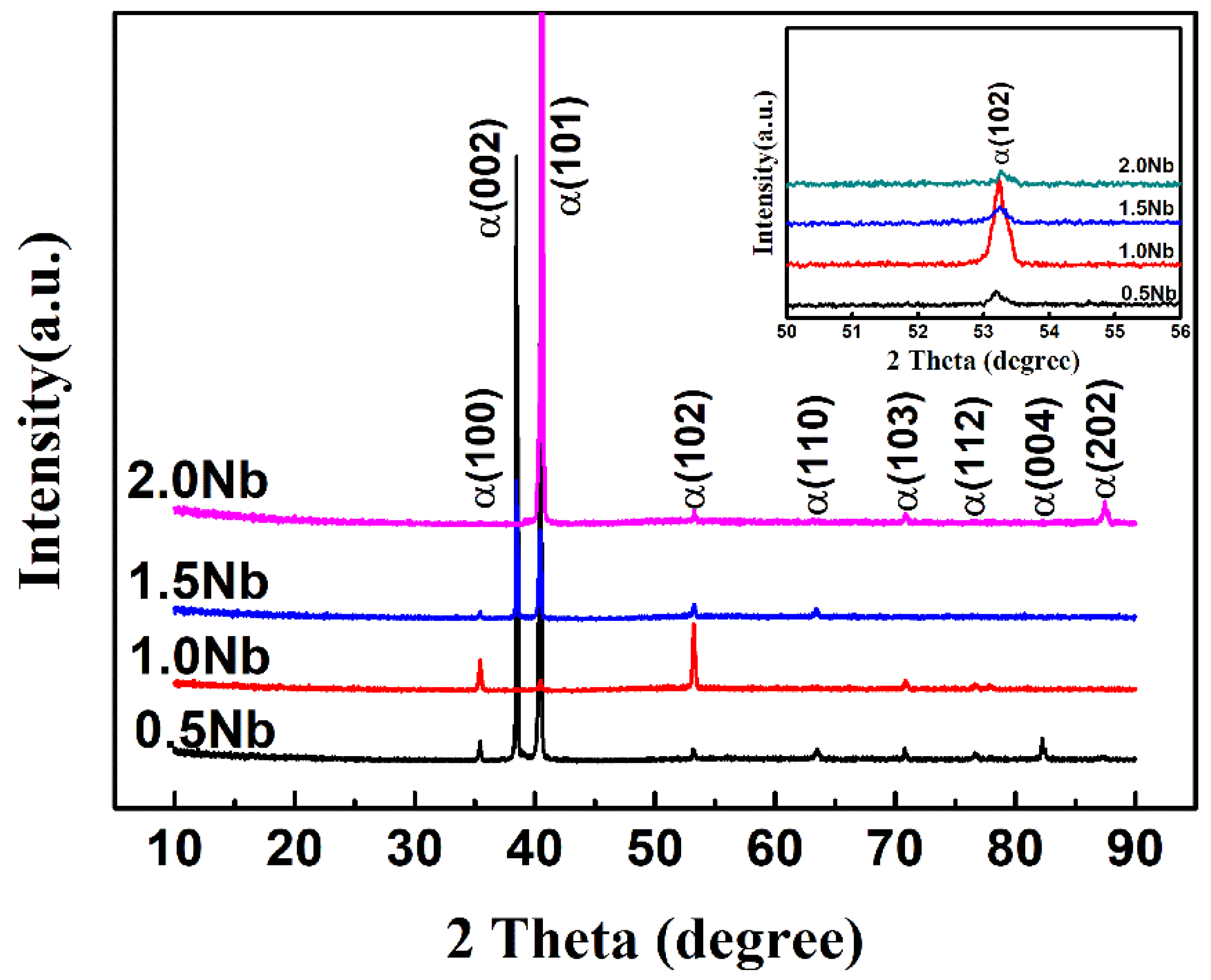
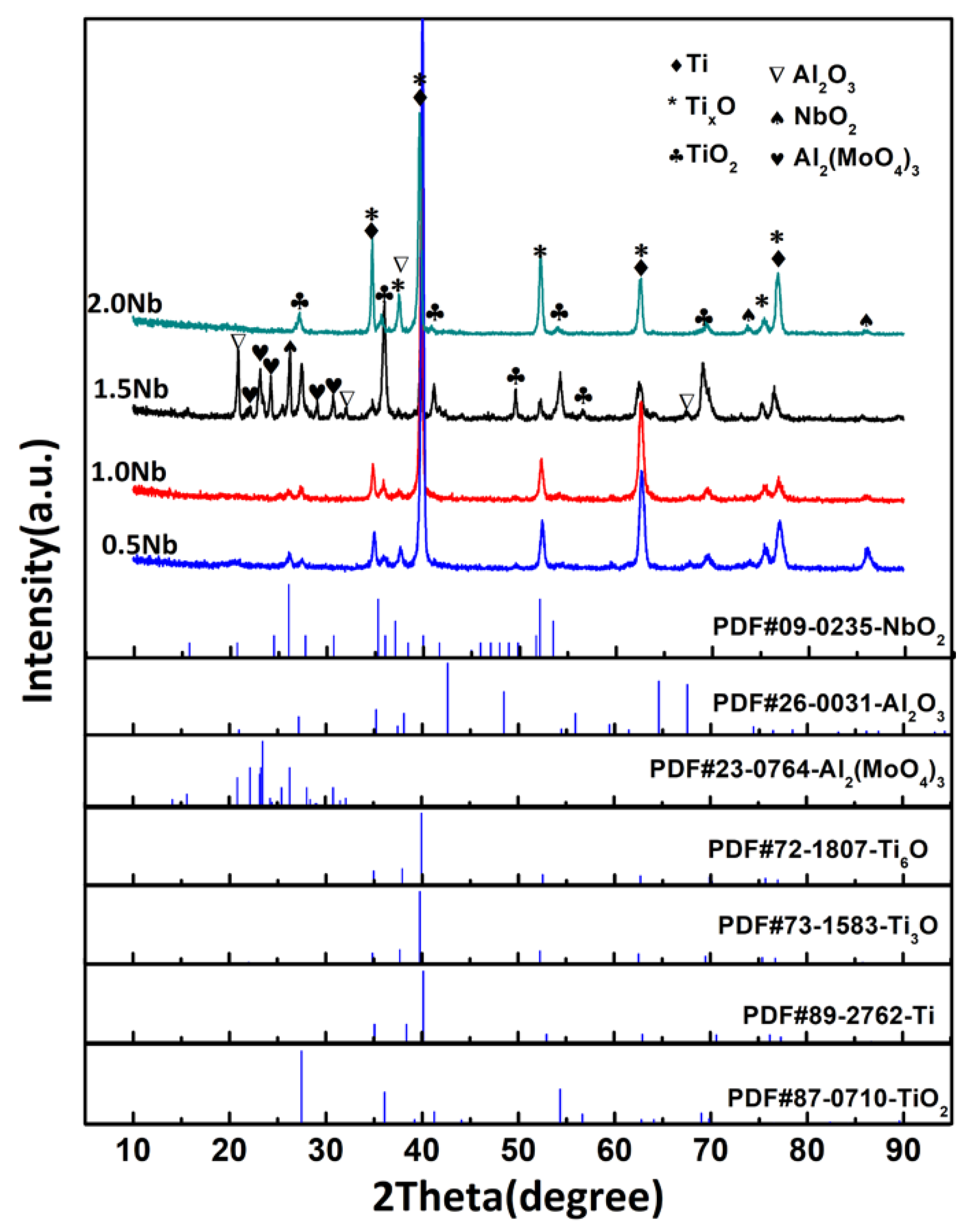
3.3. Phase Composition of the Oxidized Surface
3.4. Surface Morphology of the Oxidized Surface
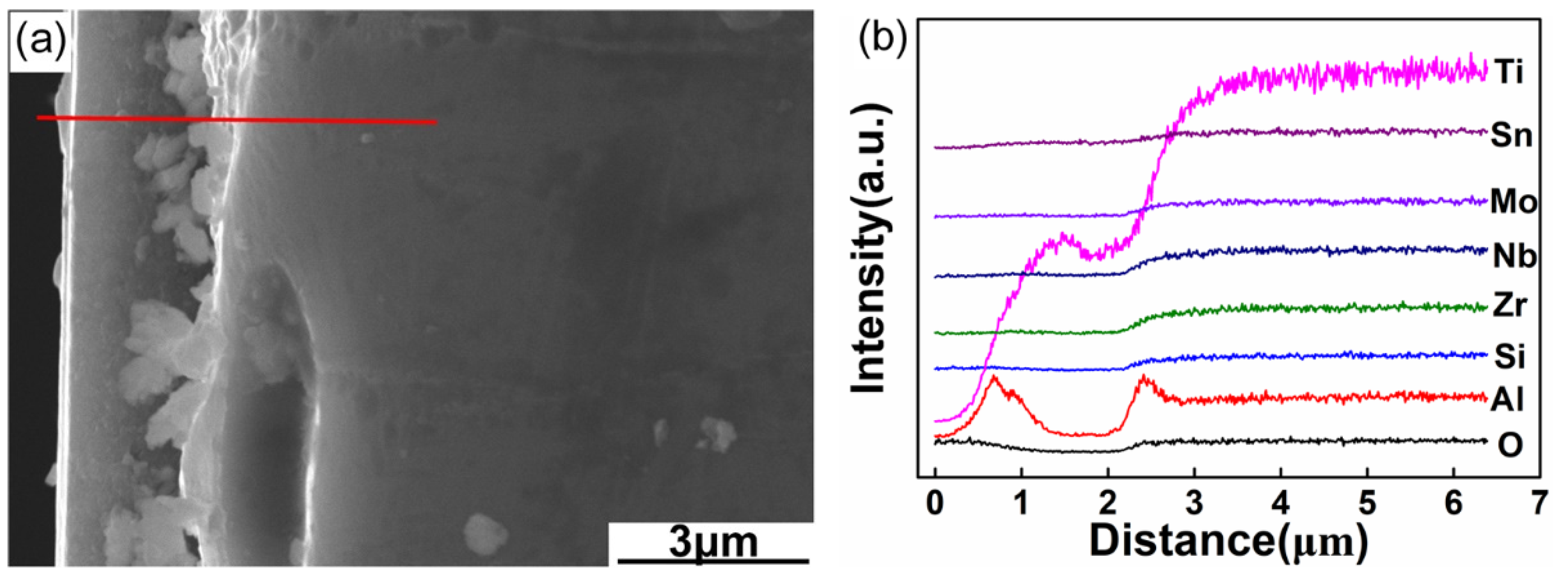
3.5. Cross-Section Morphologies and Elemental Profiles of the Oxide Layers
4. Conclusions
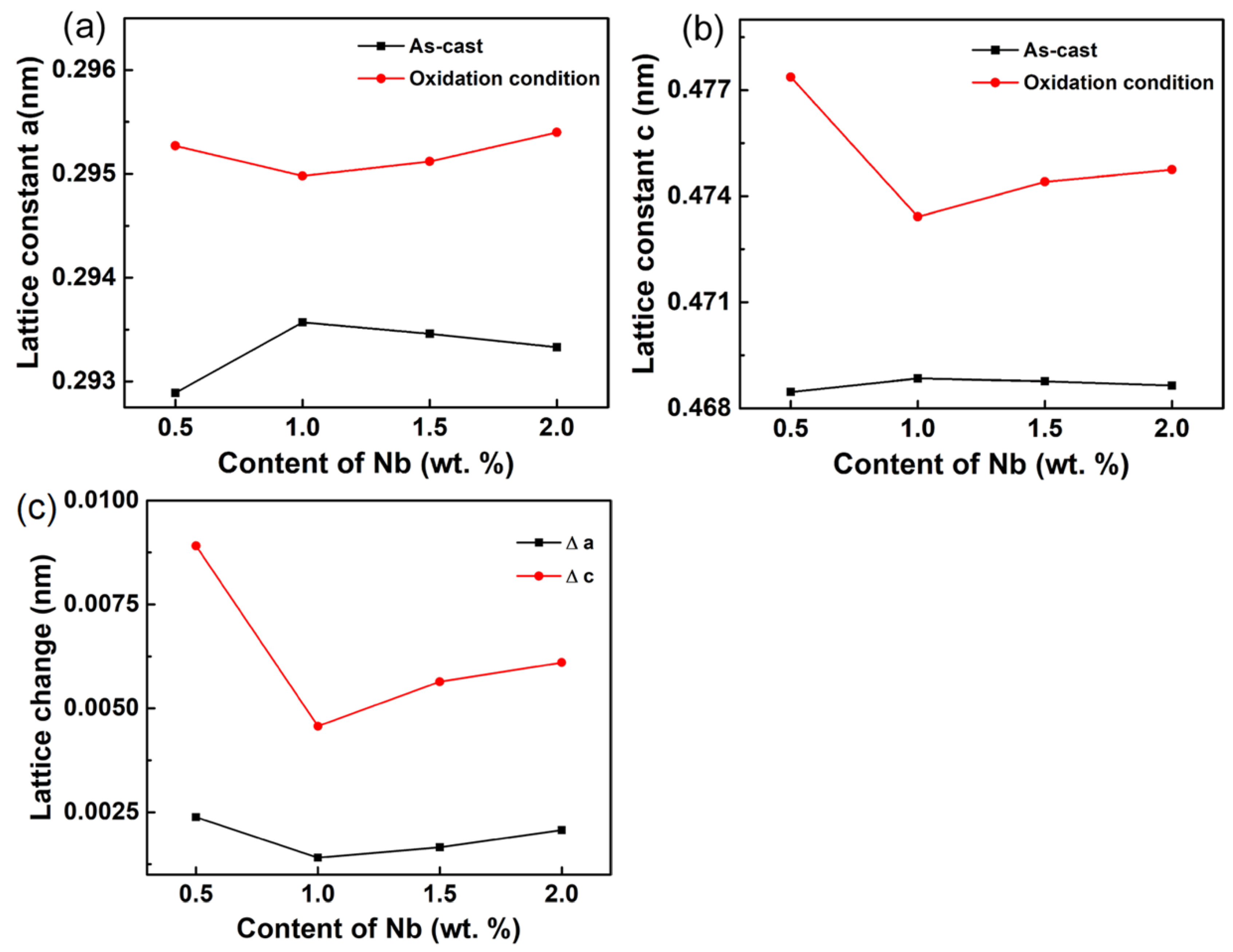
- With the increase of Nb content, both the α-Ti lattice parameters ‘a’ and ‘c’ of the as-cast alloys first rise and then decrease.
- Adding Nb to Ti-1100 alloy did not change the microstructure classification of the alloys, and the space between α-laths decreases first and then increases with the increase of Nb content.
- Nb can significantly improve the high temperature oxidation resistance. The oxidation behaviors of the alloys follow a parabolic kinetic model. The parabolic oxidation rate decreased firstly and then increased with the increase of Nb content. The Ti-1100-1.0Nb alloy has the lowest kp value, which is 5.7 × 10−13.
- Surface oxidation products are mainly composed of rutile-TiO2, TixO (x = 3, 6), NbO2 and Al2O3. The morphology of TiO2 is massive and acicular. Al2(MoO4)3 as the mainly oxide was first detected in the Ti-1100-1.5Nb alloys.
- A novel oxidation resistance was obtained in the Ti-1100-1.0Nb alloy. The interaction of Nb, O, Ti, and other elements retarded the diffusion of O atoms into the alloys, which improved the oxidation resistance property as a result.
Author Contributions
Funding
Conflicts of Interest
References
- Shams, S.A.A.; Mirdamadi, S.; Abbasi, S.M.; Lee, Y.; Lee, C.S. Coarsening kinetics of primary alpha in near alpha titanium alloy. J. Alloys. Compd. 2018, 735, 1769–1777. [Google Scholar] [CrossRef]
- Fu, B.G.; Wang, H.W.; Zou, C.M.; Wei, Z.J. The influence of Zr content on microstructure and precipitation of silicide in as-cast near α titanium alloys. Mater. Charact. 2015, 99, 17–24. [Google Scholar] [CrossRef]
- Madsen, A.; Ghonem, H. Effects of aging on the tensile and fatigue behavior of the near-α Ti-1100 at room temperature and 593 °C. Mater. Sci. Eng. A 1994, 177, 63–73. [Google Scholar] [CrossRef]
- Chandravanshi, V.; Sarkar, R.; Kamat, S.V.; Nandy, T.K. Effects of thermomechanical processing and heat treatment on the tensile and creep properties of boron-modified near alpha titanium alloy Ti-1100. Metall. Mater. Trans. A 2013, 44, 201–211. [Google Scholar] [CrossRef]
- Ouyang, P.X.; Mi, G.B.; Li, P.J.; He, L.J.; Cao, J.X.; Huang, X. Non-isothermal oxidation behavior and mechanism of a high temperature near-α titanium alloy. Materials 2018, 11, 2141. [Google Scholar] [CrossRef]
- Alam, M.Z.; Das, D.K. Effect of cracking in diffusion aluminide coatings on their cyclic oxidation performance on Ti-based IMI-834 alloy. Corros. Sci. 2009, 51, 1405–1412. [Google Scholar] [CrossRef]
- Fu, B.G.; Wang, H.W.; Zou, C.M.; Wei, Z.J. The effects of Nb content on microstructure and fracture behavior of near α titanium alloys. Mater. Des. 2015, 66, 267–273. [Google Scholar] [CrossRef]
- Dai, J.J.; Zhu, J.Y.; Chen, C.Z.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys. Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Fargas, G.; Roa, J.J.; Sefer, B.; Pederson, R.; Antti, M.L.; Mateo, A. Influence of cyclic thermal treatments on the oxidation behavior of Ti-6Al-2Sn-4Zr-2Mo alloy. Mater. Charact. 2018, 145, 218–224. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Zhou, B.; Liu, N.; Chen, L.Q. Effects of microstructure and rare-earth constituent on the oxidation behavior of Ti-5.6Al-4.8Sn-2Zr-1Mo-0.35Si-0.7Nd titanium alloy. Oxid. Met. 2014, 81, 373–382. [Google Scholar] [CrossRef]
- Jiang, H.R.; Hirohasi, M.; Lu, Y.; Imanari, H. Effect of Nb on the high temperature oxidation of Ti-(0-50 at.%) Al. Scripta. Mater. 2002, 46, 639–643. [Google Scholar] [CrossRef]
- Pflumm, R.; Donchev, A.; Mayer, S.; Clemens, H.; Schütze, M. High-temperature oxidation behavior of multi-phase Mo-containing γ-TiAl-based alloys. Intermetallics 2014, 53, 45–55. [Google Scholar] [CrossRef]
- Lin, J.P.; Zhao, L.L.; Li, G.Y.; Zhang, L.Q.; Song, X.P.; Ye, F.; Chen, G.L. Effect of Nb on oxidation behavior of high Nb containing TiAl alloys. Intermetallics 2011, 19, 131–136. [Google Scholar] [CrossRef]
- Zheng, N.; Quadakkers, W.J.; Gil, A.; Nickel, H. Studies concerning the effect of nitrogen on the oxidation behavior of TiAl-based intermetallics at 900° C. Oxid. Met. 1995, 44, 477–499. [Google Scholar] [CrossRef]
- Pérez, P.; Haanappel, V.A.C.; Stroosnijder, M.F. The effect of niobium on the oxidation behavior of titanium in N2/20% O2 atmospheres. Mater. Sci. Eng. A 2000, 284, 126–137. [Google Scholar] [CrossRef]
- Yoshihara, M.; Miura, K. Effects of Nb addition on oxidation behavior of TiAl. Intermetallics 1995, 3, 357–363. [Google Scholar] [CrossRef]
- Shida, Y.; Anada, H. The influence of ternary element addition on the oxidation behaviour of TiAl intermetallic compound in high temperature air. Corros. Sci. 1993, 35, 945–953. [Google Scholar] [CrossRef]
- Eylon, D.; Fujishiro, S.; Postans, P.J.; Froes, F.H. High temperature titanium alloys-a review. JOM 1984, 36, 55–62. [Google Scholar] [CrossRef]
- Gehring, D.; Monroe, J.A.; Karaman, I. Effects of composition on the mechanical properties and negative thermal expansion in martensitic TiNb alloys. Scripta. Mater. 2020, 178, 351–355. [Google Scholar] [CrossRef]
- Cui, W.F.; Wei, H.R.; Luo, G.Z.; Hong, Q.; Zhou, L. Oxidation behaviour of IMI 834 and Ti-1100 alloys at high temperature of 550 °C to 750 °C. Rare. Metal. Mat. Eng. 1997, 26, 31–35. [Google Scholar]
- Aniołek, K.; Kupka, M.; Łuczuk, M.; Barylski, A. Isothermal oxidation of Ti-6Al-7Nb alloy. Vacuum 2015, 114, 114–118. [Google Scholar] [CrossRef]
- Lee, H.S.; Yoon, J.H.; Yi, Y.M. Oxidation behavior of titanium alloy under diffusion bonding. Thermochim. Acta. 2007, 455, 105–108. [Google Scholar] [CrossRef]
- Baillieux, J.; Poquillon, D.; Malard, B. Observation using synchrotron X-ray diffraction of the crystallographic evolution of α-titanium after oxygen diffusion. Phil. Mag. Lett. 2015, 95, 245–252. [Google Scholar] [CrossRef]
- Jostsons, A.; Malin, A.S. The ordered structure of Ti3O. Acta. Crystallogr. B 1968, 24, 211–213. [Google Scholar] [CrossRef]
- Chase, M.W. NIST-JANAF Thermochemical Tables, 4th ed.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1998.
- Heracleous, E.; Lee, A.F.; Vasalos, I.A.; Lemonidou, A.A. Surface properties and reactivity of Al2O3-supported MoO3 catalysts in ethane oxidative dehydrogenation. Catal. Lett. 2003, 88, 47–53. [Google Scholar] [CrossRef]
- Smith, D.W. Inorganic Substances: A Prelude to the Study of Descriptive Inorganic Chemistry; Cambridge University Press: New York, NY, USA, 1990. [Google Scholar]
- Chen, L.; Cooper, A.C.; Pez, G.P.; Cheng, H.S. On the mechanisms of hydrogen spillover in MoO3. J. Phys. Chem. C 2008, 112, 1755–1758. [Google Scholar] [CrossRef]
- Nordahl, C.S.; Messing, G.L. Sintering of α-Al2O3-seeded nanocrystalline γ-Al2O3 powders. J. Eur. Ceram. Soc. 2002, 22, 415–422. [Google Scholar] [CrossRef]
- Zhong, X.; Xu, M.L.; Yang, L.L.; Qu, X.; Yang, L.H.; Zhang, M.; Liu, H.Y.; Ma, Y.M. Predicting the structure and stability of titanium oxide electrides. NPJ Comput. Mater. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Berthaud, M.; Popa, I.; Chassagnon, R.; Heintz, O.; Lavkovά, J.; Chevalier, S. Study of titanium alloy Ti6242S oxidation behaviour in air at 560 °C: Effect of oxygen dissolution on lattice parameters. Corros. Sci. 2020, 164, 108049. [Google Scholar] [CrossRef]
- Straumanis, M.E.; Ejima, T.; James, W.J. The TiO2 phase explored by the lattice constant and density method. Acta Crystallogr. 1961, 14, 493–497. [Google Scholar] [CrossRef]
- Lv, J.P.; Yang, J.Q.; Li, X.J.; Chai, Z.Y. Size dependent radiation-stability of ZnO and TiO2 particles. Dyes. Pigments. 2019, 164, 87–90. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, J.F.; Chen, H.C.; Zhong, W.L.; Zhang, P.L.; Dong, H.M.; Zhao, L.Y. Electrical properties of SnO2-ZnO-Nb2O5 varistor system. J. Phys. D Appl. Phys. 2000, 33, 96–99. [Google Scholar]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High-Temperature Oxidation of Metals; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]



| Al | Sn | Zr | Mo | Si | Nb | Ti | |
|---|---|---|---|---|---|---|---|
| 0.5 Nb | 5.71 | 2.95 | 3.81 | 0.43 | 0.41 | 0.45 | Balance |
| 1.0 Nb | 5.85 | 2.74 | 3.92 | 0.51 | 0.43 | 0.96 | Balance |
| 1.5 Nb | 5.83 | 2.81 | 3.87 | 0.46 | 0.42 | 1.47 | Balance |
| 2.0 Nb | 5.78 | 2.77 | 3.85 | 0.42 | 0.44 | 2.05 | Balance |
| Alloy | n | R2 |
|---|---|---|
| Ti-1100-0.5Nb | 1.955 | 0.996 |
| Ti-1100-1.0Nb | 1.792 | 0.989 |
| Ti-1100-1.5Nb | 1.919 | 0.995 |
| Ti-1100-2.0Nb | 2.000 | 0.995 |
| Alloy | kp (g2cm−4s−1) | R2 |
|---|---|---|
| Ti-1100-0.5Nb | 9.222 × 10−13 | 0.990 |
| Ti-1100-1.0Nb | 5.749 × 10−13 | 0.981 |
| Ti-1100-1.5Nb | 7.496 × 10−13 | 0.988 |
| Ti-1100-2.0Nb | 8.733 × 10−13 | 0.989 |
| Composition | a | c | Δa | Δc | |
|---|---|---|---|---|---|
| As-cast | 0.5Nb | 0.29289 | 0.46846 | - | - |
| 1.0Nb | 0.29357 | 0.46885 | - | - | |
| 1.5Nb | 0.29346 | 0.46877 | - | - | |
| 2.0Nb | 0.29333 | 0.46865 | - | - | |
| Oxidation condition | 0.5Nb | 0.29527 | 0.47737 | 0.00238 | 0.00891 |
| 1.0Nb | 0.29498 | 0.47342 | 0.00141 | 0.00457 | |
| 1.5Nb | 0.29512 | 0.47441 | 0.00166 | 0.00564 | |
| 2.0Nb | 0.29540 | 0.47475 | 0.00207 | 0.00610 |
| Position | O | Al | Si | Nb | Mo | Ti | Zr | Sn |
|---|---|---|---|---|---|---|---|---|
| Area1 | 34.1 | 3.9 | 0.5 | 0.6 | 1.1 | 55.5 | 2.6 | 1.7 |
| Area 2 | 34.6 | 3.2 | 0.0 | 0.0 | 0.0 | 59.9 | 0.7 | 1.6 |
| Area 3 | 51.4 | 3.3 | 0.1 | 0.2 | 9.5 | 34.4 | 0.5 | 0.6 |
| Area 4 | 44.1 | 6.9 | 0.2 | 0.7 | 19.2 | 27.2 | 0.9 | 0.8 |
| Area 5 | 38.9 | 2.7 | 0.6 | 0.3 | 0.2 | 54.8 | 1.6 | 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Fu, B.; Dong, T.; Li, G.; Wang, F.; Zhao, X.; Liu, J. Effect of Nb Content on Cyclic Oxidation Behavior of As-Cast Ti-1100 Alloys. Materials 2020, 13, 1082. https://doi.org/10.3390/ma13051082
Song Y, Fu B, Dong T, Li G, Wang F, Zhao X, Liu J. Effect of Nb Content on Cyclic Oxidation Behavior of As-Cast Ti-1100 Alloys. Materials. 2020; 13(5):1082. https://doi.org/10.3390/ma13051082
Chicago/Turabian StyleSong, Yingjun, Binguo Fu, Tianshun Dong, Guolu Li, Fei Wang, Xuebo Zhao, and Jinhai Liu. 2020. "Effect of Nb Content on Cyclic Oxidation Behavior of As-Cast Ti-1100 Alloys" Materials 13, no. 5: 1082. https://doi.org/10.3390/ma13051082
APA StyleSong, Y., Fu, B., Dong, T., Li, G., Wang, F., Zhao, X., & Liu, J. (2020). Effect of Nb Content on Cyclic Oxidation Behavior of As-Cast Ti-1100 Alloys. Materials, 13(5), 1082. https://doi.org/10.3390/ma13051082





