Electrochemical Performance of Fe40Al-X (X = Cr, Ti, Co, Ni) Alloys Exposed to Artificial Saliva
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Artificial Saliva
2.3. Electrochemical Corrosion Tests
3. Results and Discussion
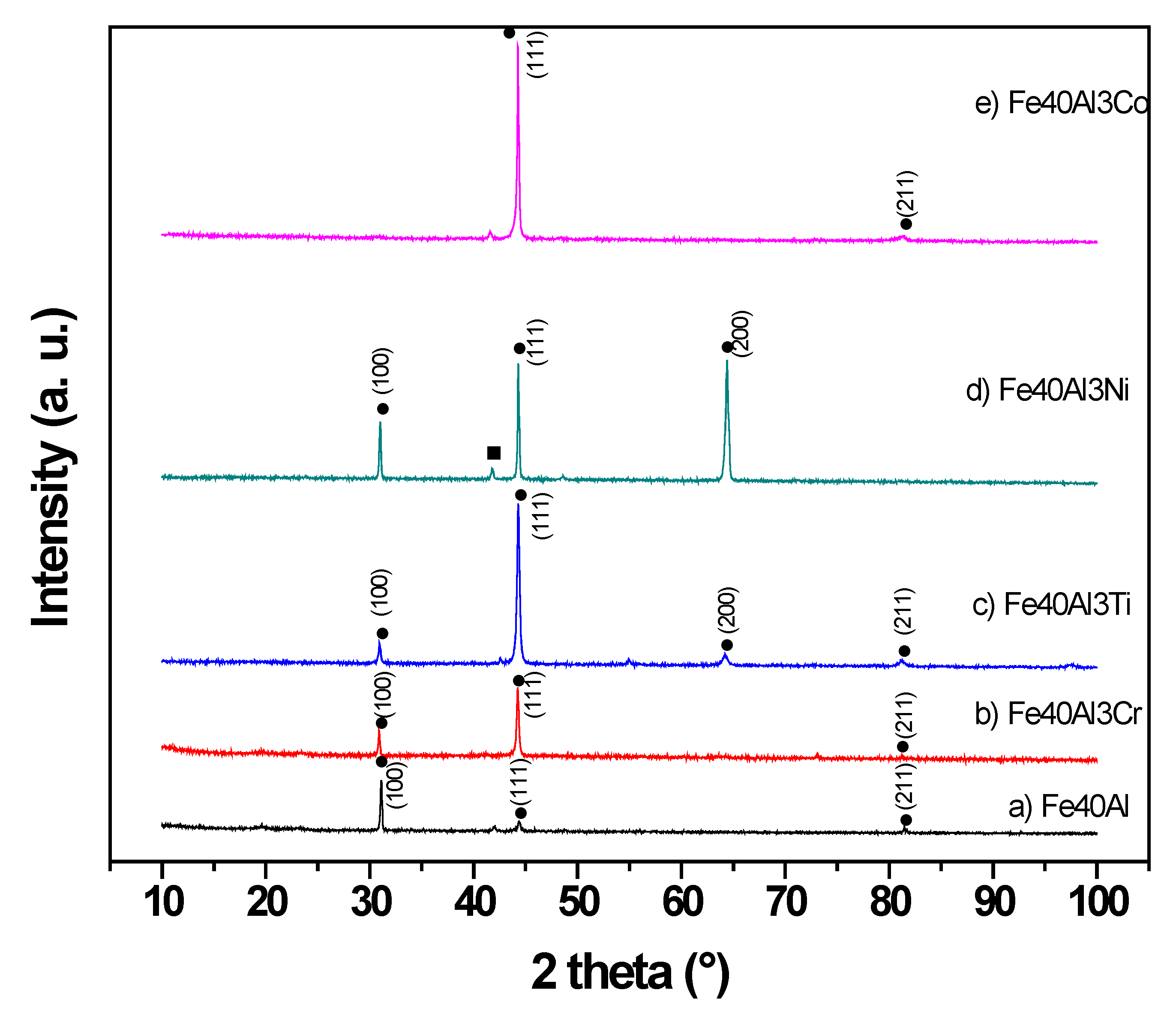
3.1. Microstructural Characterization
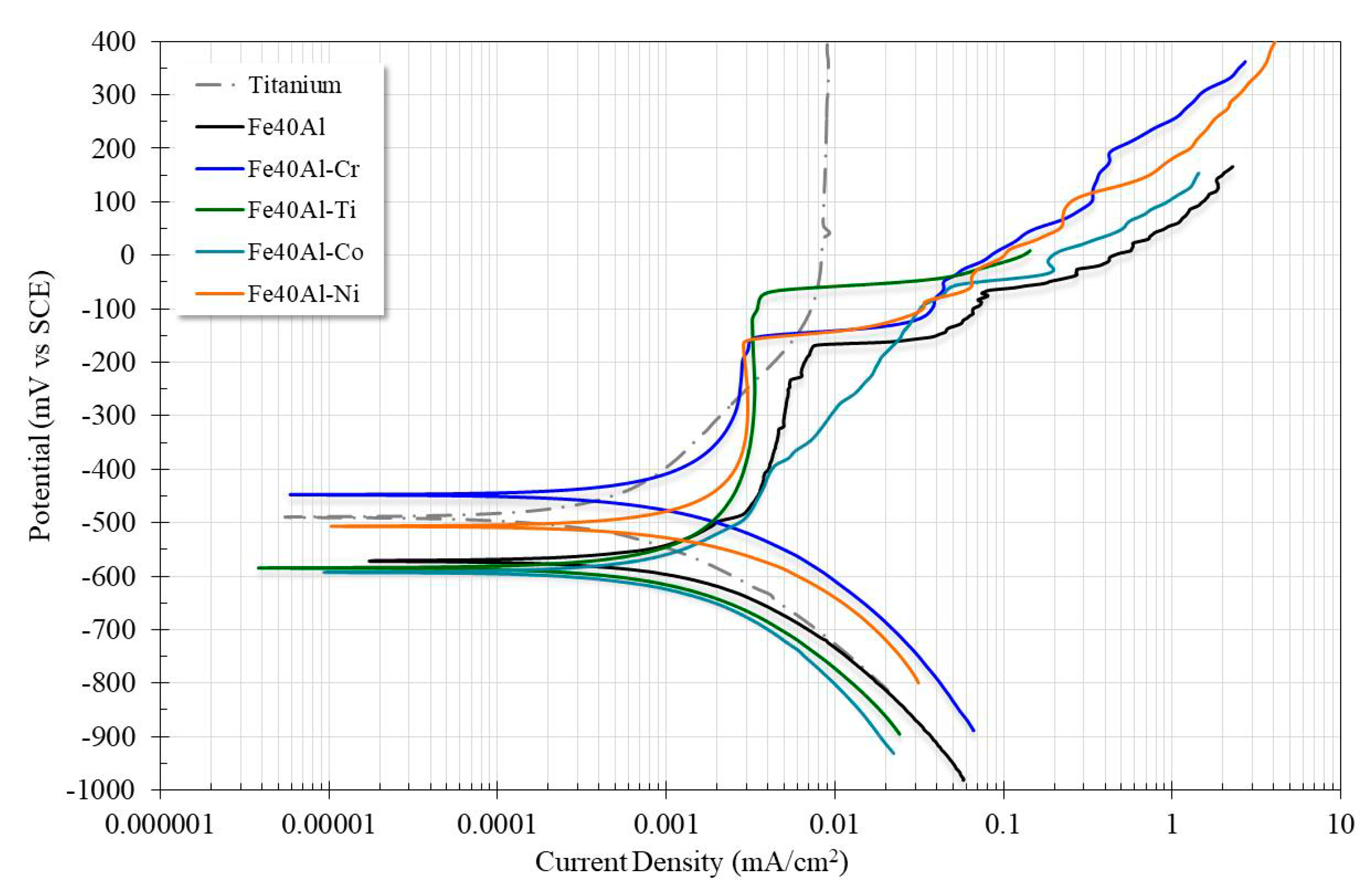
3.2. Polarization Curves
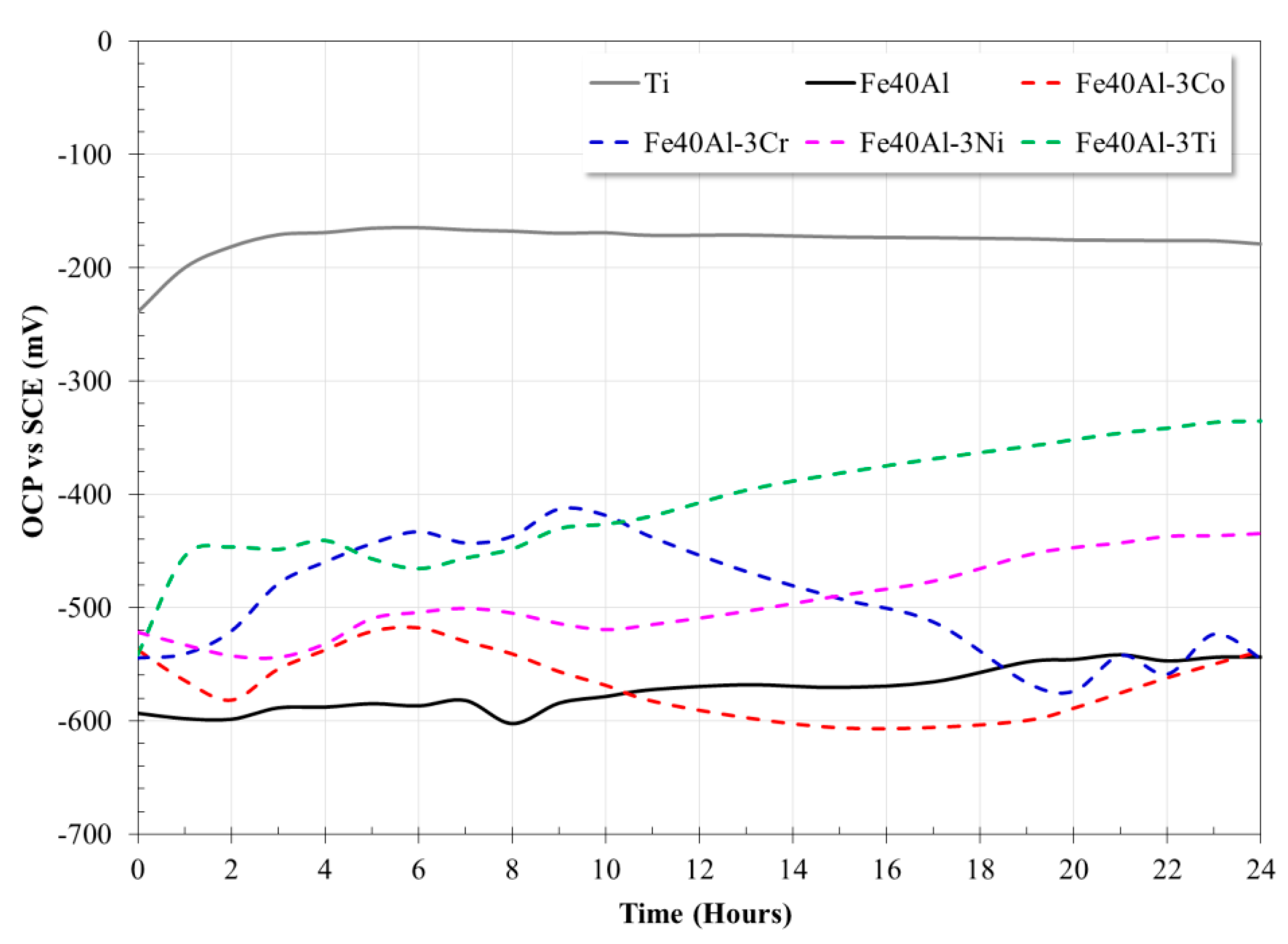
3.3. Open Circuit Potential
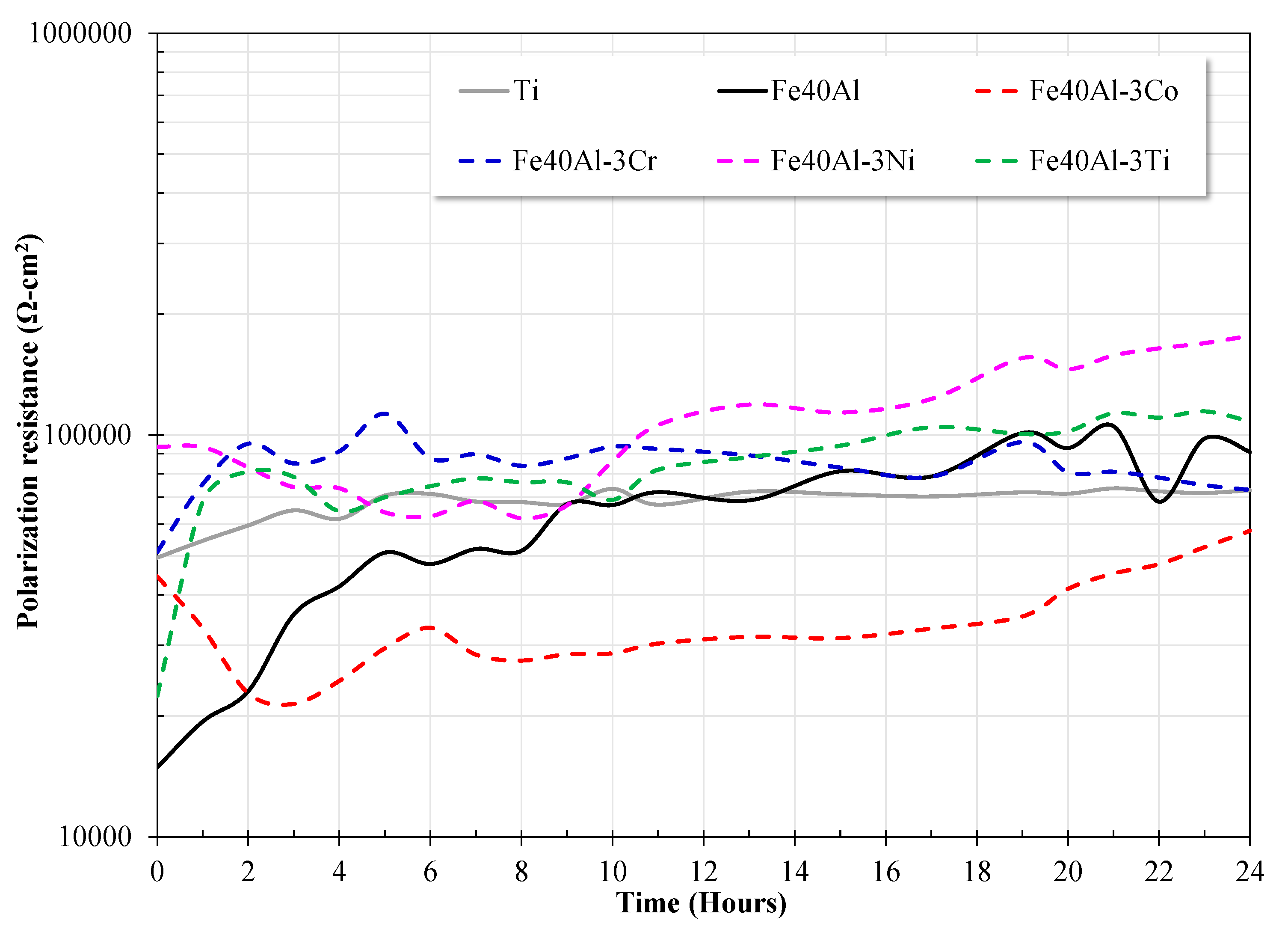
3.4. Linear Polarization Resistance (LPR) Measurements
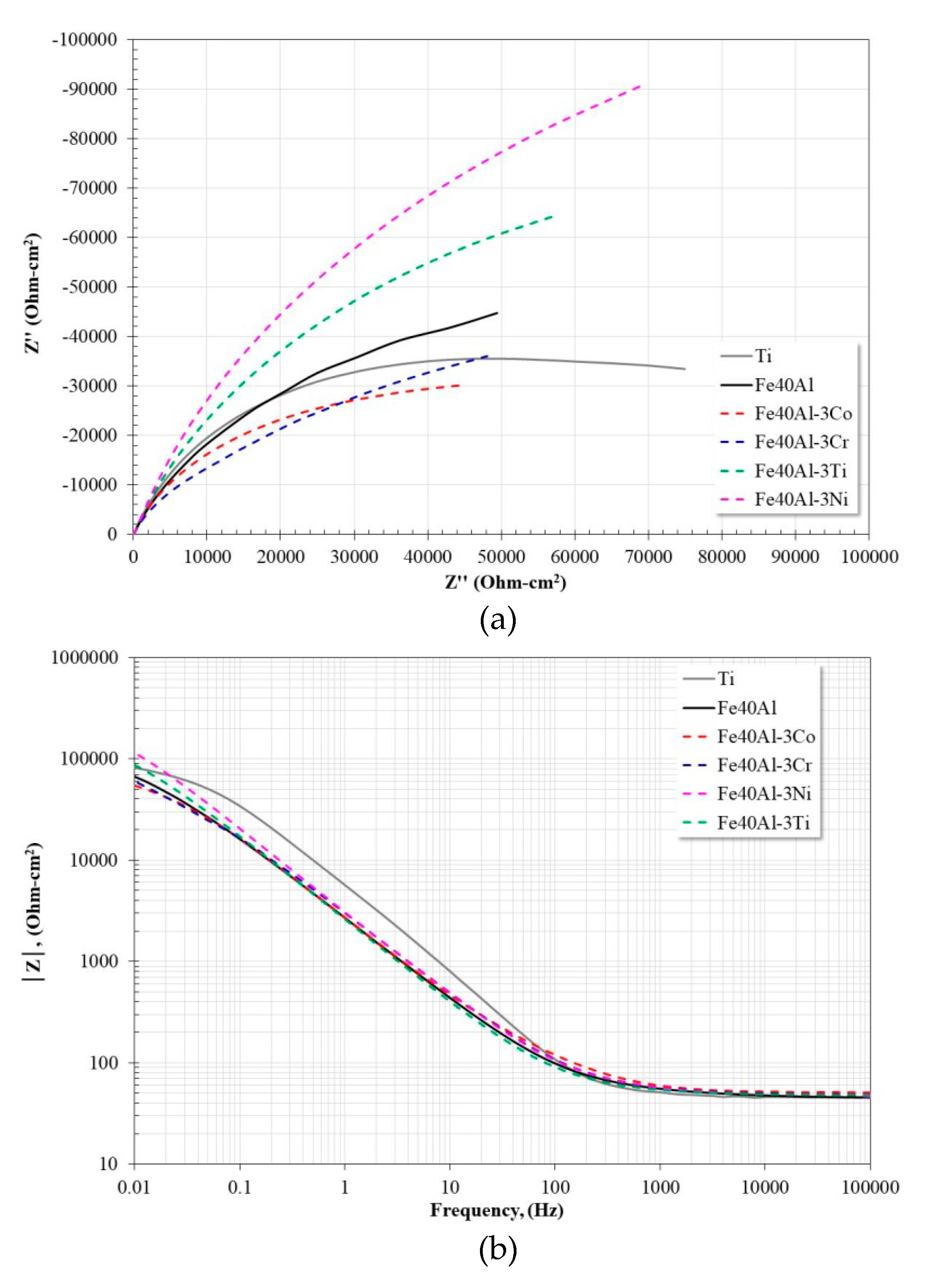
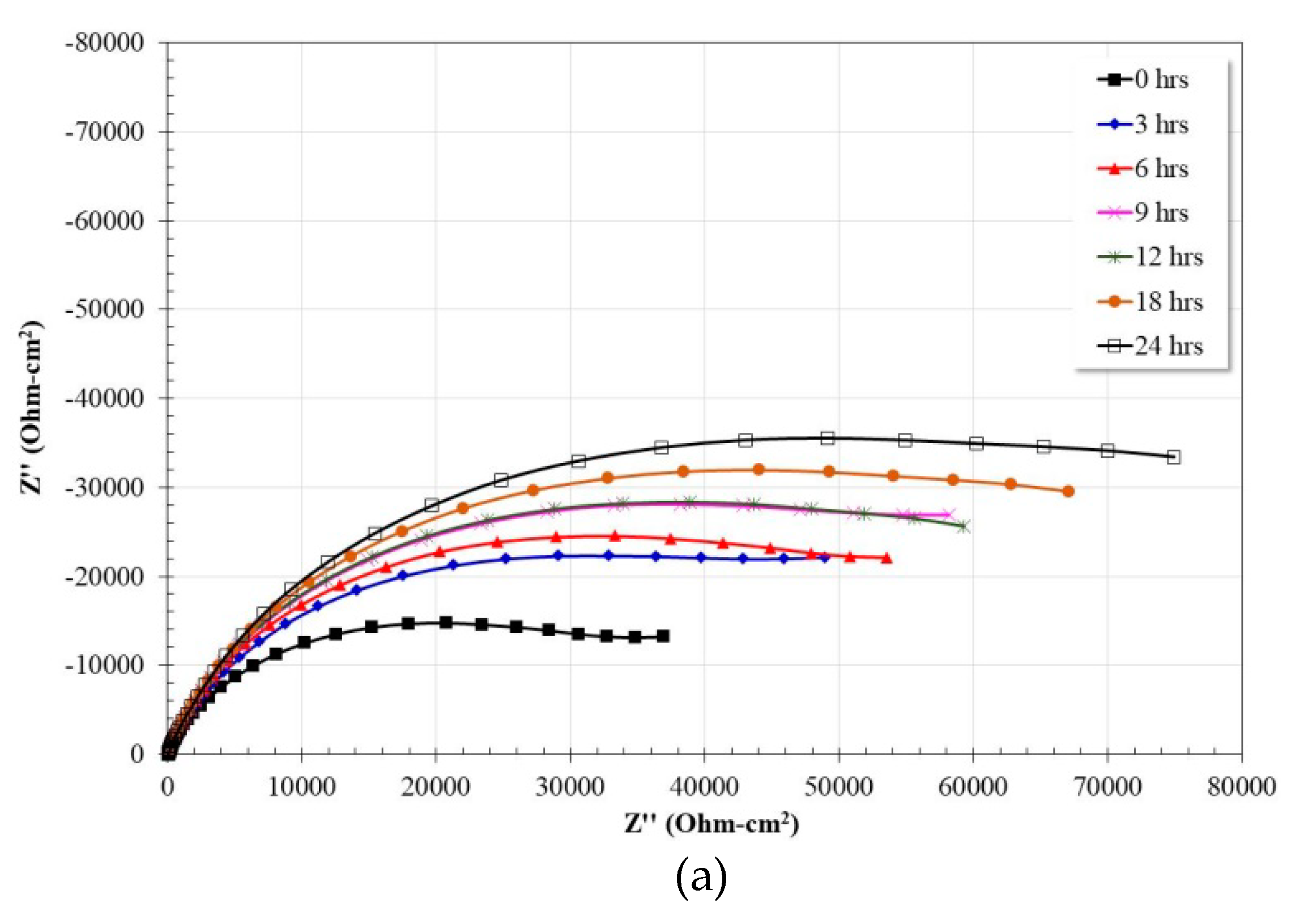
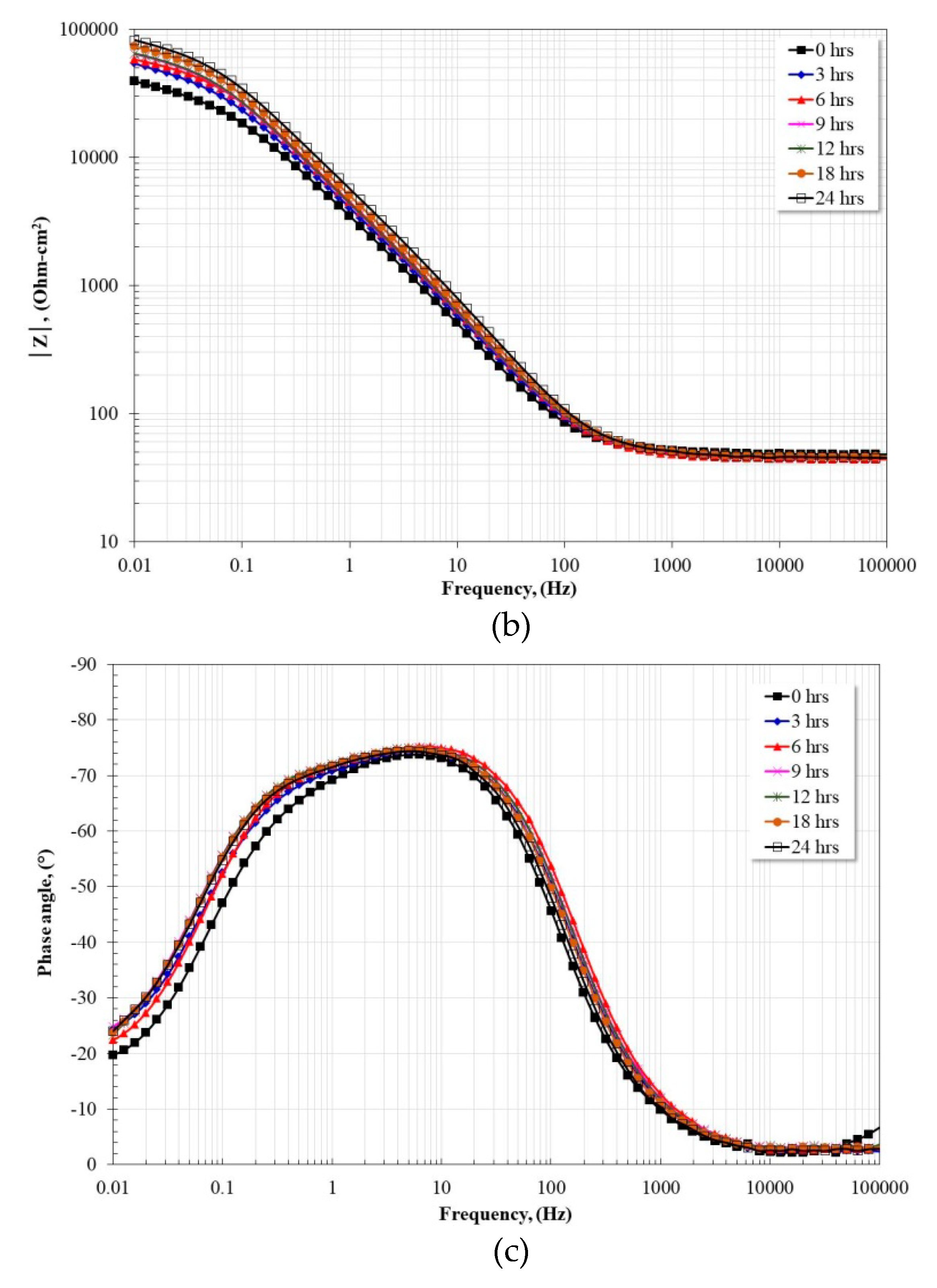
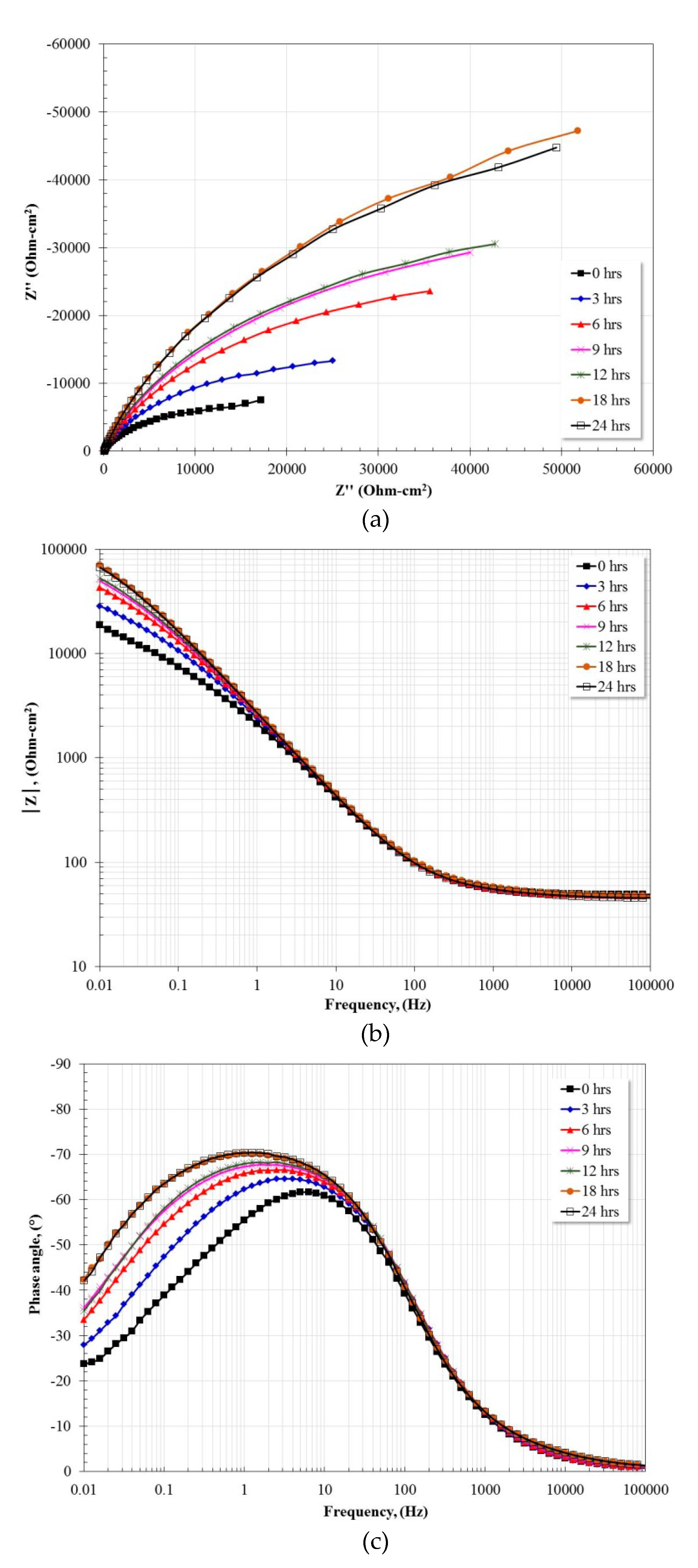
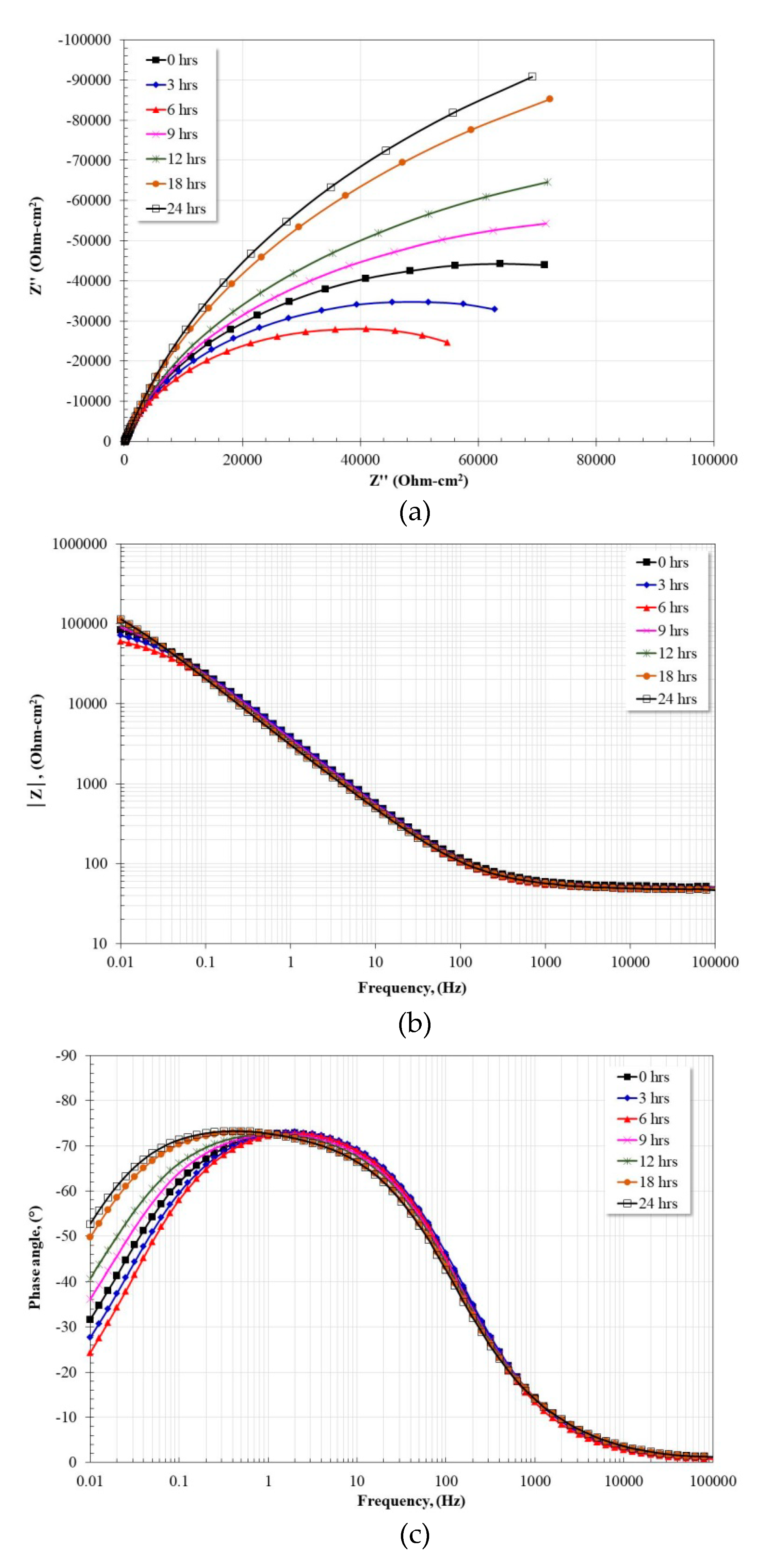
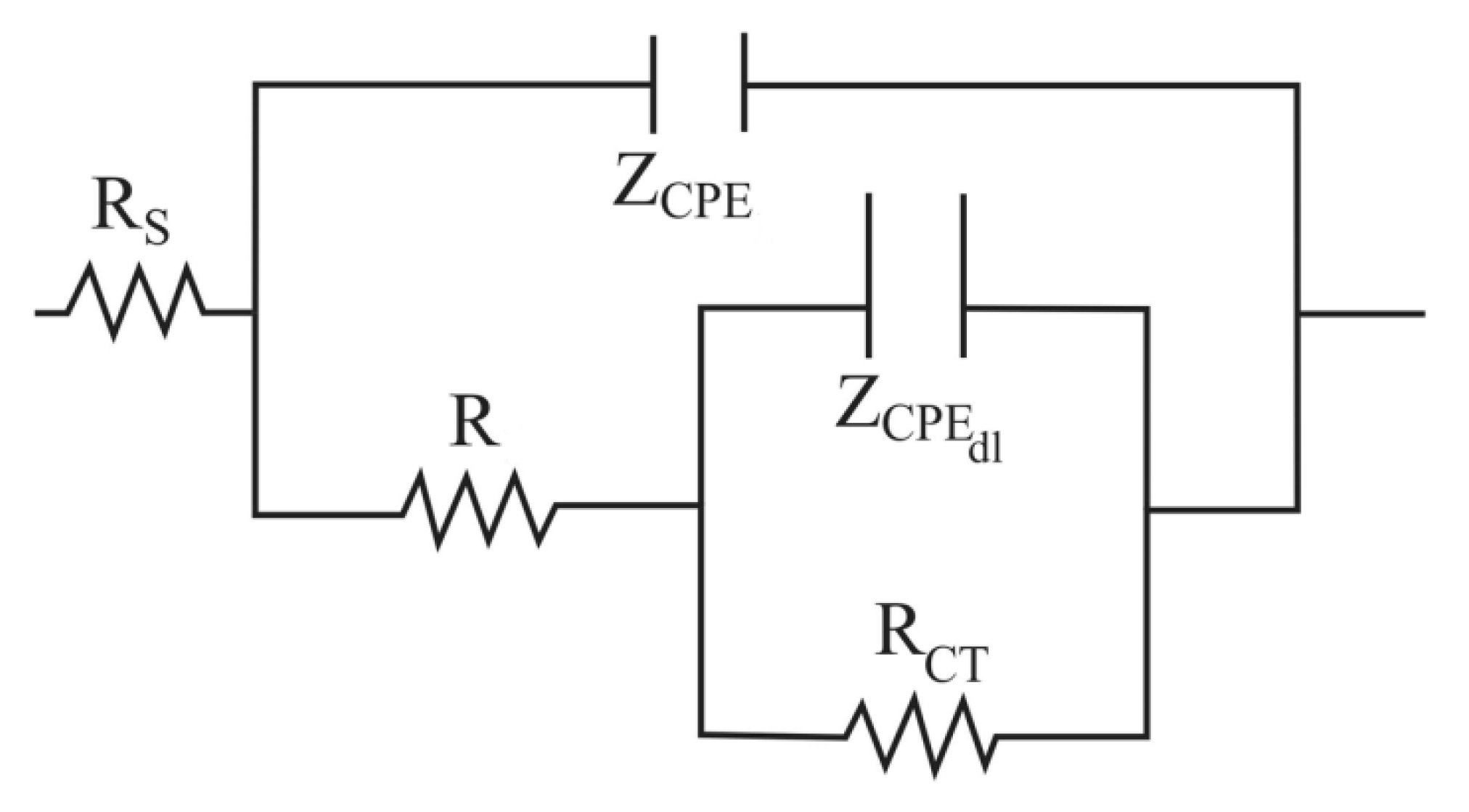
3.5. Electrochemical Impedance Spectroscopy Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloy. Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants–a review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Dahotre, N.B. Corrosion degradation and prevention by surface modification of biometallic materials. J. Mater. Sci. Mater. Med. 2007, 18, 725–751. [Google Scholar] [CrossRef] [PubMed]
- Geis-Gerstorfer, J. In vitro corrosion measurements of dental alloys. J. Dent. 1994, 22, 247–251. [Google Scholar] [CrossRef]
- Wataha, J.C. Biocompatibility of dental casting alloys: A review. J. Prosthet. Dent. 2000, 83, 223–234. [Google Scholar] [CrossRef]
- Stoloff, N.S. Iron aluminides: Present status and future prospects. Mater. Sci. Eng. A 1998, 258, 1–14. [Google Scholar] [CrossRef]
- Hotař, A.; Kejzlar, P.; Palm, M.; Mlnařík, J. The effect of Zr on high-temperature oxidation behaviour of Fe3Al-based alloys. Corrosion Sci. 2015, 100, 147–157. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Rodriguez-Diaz, R.A.; Porcayo-Palafox, E.; Colin, J.; Molina-Ocampo, A.; Martinez-Gomez, L. Effect of Cu Addition on the Electrochemical Corrosion Performance of Ni3Al in 1.0 M H2SO4. Adv. Mater. Sci. Eng. 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Busso, E.P.; McClintock, F.A. Mechanisms of cyclic deformation of NiAl single crystals at high temperatures. Acta Metall. Mater. 1994, 42, 3263–3275. [Google Scholar] [CrossRef]
- Jayaram, R.; Miller, M.K. An atom probe study of grain boundary and matrix chemistry in microalloyed NiAl. Acta Metall. Mater. 1994, 42, 1561–1572. [Google Scholar] [CrossRef]
- Huape-Padilla, E.; Sánchez-Carrillo, M.; Flores-de los Rios, J.P.; Espinosa-Medina, M.A.; Bautista-Margulis, R.G.; Ferrer-Sánchez, M.I.; Carbajal-de la Torre, G.; Bejar-Gómez, L.; Chacón-Nava, J.G.; Martínez-Villafañe, A. Corrosion study of Fe-Al Intermetallic Alloys in Simulated Acid Rain. Int. J. Electrochem. Sci. 2015, 10, 2141–2154. [Google Scholar]
- Jez, M.; Mitoraj, M.; Godlewska, E.; Jakubowska, M.; Bas, B. Evaluation of corrosion behaviour of selected metallic samples by electrochemical noise measurements. J. Solid State Electrochem. 2014, 18, 1635–1646. [Google Scholar] [CrossRef]
- Rosalbino, F.; Carlini, R.; Parodi, R.; Zanicchi, G.; Scavino, G. Investigation of passivity and its breakdown on Fe3Al–Si and Fe3Al–Ge intermetallics in chloride-containing solution. Corrosion Sci. 2014, 85, 394–400. [Google Scholar] [CrossRef]
- Senderowski, C.; Chodala, M.; Bojar, Z. Corrosion behavior of detonation gun sprayed Fe-Al type intermetallic coating. Materials 2015, 8, 1108–1123. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, R.A.; Ramirez-Ledesma, A.L.; Aguilar-Mendez, M.A.; Chavarin, J.U.; Gallegos, M.H.; Juarez-Islas, J.A. Electrochemical corrosion behavior of a Co20Cr alloy in artificial saliva. Int. J. Electrochem. Sci. 2015, 10, 7212–7226. [Google Scholar]
- Rodríguez-Diaz, R.A.; Uruchurtu-chavarín, J.; Molina-Ocampo, A.; Porcayo-Calderon, J.; Mendoza, M.E.; Valdez, S.; Juárez-Islas, J. Hot corrosion behavior of FeAl intermetallic compound modified with silver in molten salt mixture. Int. J. Electrochem. Sci. 2013, 8, 11877–11895. [Google Scholar]
- Rodríguez-Diaz, R.A.; Uruchurtu-Chavarín, J.; Porcayo-Calderon, J.; López-Oglesby, J.M.; Mendoza, M.E.; Ramos-Hernández, J.J.; Valdez, S.; Bedolla, A. Hot corrosion behavior of FeAl intermetallic compound modified with Ti, and Cr in molten salt mixture KCl-ZnCl2. Int. J. Electrochem. Sci. 2013, 8, 7257–7273. [Google Scholar]
- Sarmiento-Bustos, E.; Rodríguez-Diaz, R.A.; Colín, J.; Molina-Ocampo, A.; Gaona-Jiménez, S.; Bahena-Medina, L.A. Optimized In-vitro Corrosion Assessment of a FeAl intermetallic Compound Modified with Ag in Artificial Human Media. Int. J. Electrochem. Sci. 2016, 11, 4136–4148. [Google Scholar] [CrossRef]
- Arrieta-Gonzalez, C.D.; Porcayo-Calderon, J.; Salinas-Bravo, V.M.; Gonzalez-Rodriguez, J.G.; Chacon-Nava, J.G. Electrochemical behavior of Fe3Al modified with Ni in Hank’s solution. Int. J. Electrochem. Sci. 2011, 6, 4016–4031. [Google Scholar]
- Gonzalez-Rodriguez, J.G.; Gonzalez-Castañeda, M.; Cuellar-Hernández, M.; Domínguez-Patiño, G.; Rosas, G. Effect of Ni on the corrosion behavior of Fe–Al intermetallics in simulated human body fluid. J. Solid State Electrochem. 2008, 12, 707–713. [Google Scholar] [CrossRef]
- Garcıa-Alonso, M.C.; Lopez, M.F.; Escudero, M.L.; González-Carrasco, J.L.; Morris, D.G. Corrosion behaviour of an Fe3Al-type intermetallic in a chloride containing solution. Intermetallics 1999, 7, 185–191. [Google Scholar] [CrossRef]
- Castañeda, I.E.; Gonzalez-Rodriguez, J.G.; Colin, J.; Neri-Flores, M.A. Electrochemical behavior of Ni-Al-Fe alloys in simulated human body solution. J. Solid State Electrochem. 2010, 14, 1145–1152. [Google Scholar] [CrossRef]
- Duffó, G.S.; Quezada-Castillo, E. Development of an artificial saliva solution for studying the corrosion behavior of dental alloys. Corrosion 2004, 60, 594–602. [Google Scholar] [CrossRef]
- Ghosh, G.; Korniyenko, K.; Velikanova, T.; Sidorko, V. Aluminium–Chromium–Iron. In Iron Systems, Part 1; Springer: Berlin/Heidelberg, Germany, 2008; pp. 44–87. [Google Scholar]
- Rao, V.S. A review of the electrochemical corrosion behaviour of iron aluminides. Electrochim. Acta 2004, 49, 4533–4542. [Google Scholar] [CrossRef]
- Escudero, M.L.; Garcia-Alonso, M.C.; Gonzalez-Carrasco, J.L.; Muñoz-Morris, M.A.; Montealegre, M.A.; Garcia-Oca, C.; Morris, D.G.; Deevi, S.C. Possibilities for improving the corrosion resistance of Fe-40Al intermetallic strip by prior oxide protection. Scr. Mater. 2003, 48, 1549–1554. [Google Scholar] [CrossRef]
- Sharma, G.; Gaonkar, K.B.; Singh, P.R. Effect of Cr addition on pitting behaviour of iron aluminide intermetallic. Mater. Lett. 2007, 61, 971–973. [Google Scholar] [CrossRef]
- Negache, M.; Taibi, K.; Souami, N.; Bouchemel, H.; Belkada, R. Effect of Cr, Nb and Zr additions on the aqueous corrosion behavior of iron-aluminide. Intermetallics 2013, 36, 73–80. [Google Scholar] [CrossRef]
- Oshida, Y.; Sachdeva, R.; Miyazaki, S. Changes in contact angles as a function of time on some pre-oxidized biomaterials. J. Mater. Sci. Mater. Med. 1992, 3, 306–312. [Google Scholar] [CrossRef]
- Oshida, Y.; Sachdeva, R.C.; Miyazaki, S. Microanalytical characterization and surface modification of TiNi orthodontic archwires. Biomed. Mater. Eng. 1992, 2, 51–69. [Google Scholar] [CrossRef]
- Laub, L.W.; Stanford, J.W. Tarnish and corrosion behaviour of dental gold alloys. Gold Bull. 1981, 14, 13–18. [Google Scholar] [CrossRef]
- Kuphasuk, C.; Oshida, Y.; Andres, C.J.; Hovijitra, S.T.; Barco, M.T.; Brown, D.T. Electrochemical corrosion of titanium and titanium-based alloys. J. Prosthet. Dent. 2001, 85, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H. Effect of fluoride and albumin concentration on the corrosion behavior of Ti-6Al-4V alloy. Biomaterials 2003, 24, 275–282. [Google Scholar] [CrossRef]
- Roach, M.D.; Wolan, J.T.; Parsell, D.E.; Bumgardner, J.D. Use of x-ray photoelectron spectroscopy and cyclic polarization to evaluate the corrosion behavior of six nickel-chromium alloys before and after porcelain-fused-to-metal firing. J. Prosthet. Dent. 2000, 84, 623–634. [Google Scholar] [CrossRef]
- Ayad, M.A.; Vermilyea, S.G.; Rosenstiel, S.F. Corrosion behavior of as-received and previously cast high noble alloy. J. Prosthet. Dent. 2008, 100, 34–40. [Google Scholar] [CrossRef]
- Mareci, D.; Bolat, G.; Chelariu, R.; Sutiman, D.; Munteanu, C. The estimation of corrosion behaviour of ZrTi binary alloys for dental applications using electrochemical techniques. Mater. Chem. Phys. 2013, 141, 362–369. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y.; Lin, D. Oxidation behavior of FeAl alloys with and without titanium. J. Mater. Sci. 2001, 36, 979–983. [Google Scholar] [CrossRef]
- Aziz-Kerrzo, M.; Conroy, K.G.; Fenelon, A.M.; Farrell, S.T.; Breslin, C.B. Electrochemical studies on the stability and corrosion resistance of titanium-based implant materials. Biomaterials 2001, 22, 1531–1539. [Google Scholar] [CrossRef]
- Ibris, N.; Mirza-Rosca, J.C. EIS study of Ti and its alloys in biological media. J. Electroanal. Chem. 2002, 526, 53–62. [Google Scholar] [CrossRef]
- Marino, C.E.B.; Helena-Mascaro, L. EIS characterization of a Ti-dental implant in artificial saliva media: Dissolution process of the oxide barrier. J. Electroanal. Chem. 2004, 568, 115–120. [Google Scholar] [CrossRef]
- Wang, B.L.; Zheng, Y.F.; Zhao, L.C. Electrochemical corrosion behavior of biomedical Ti-22Nb and Ti-22Nb-6Zr alloys in saline medium. Mater. Corros. 2009, 60, 780–794. [Google Scholar] [CrossRef]
- Al-Mobarak, N.A.; Al-Swayih, A.A.; Al-Rashoud, F.A. Corrosion Behavior of Ti-6Al-7Nb Alloy in Biological Solution for Dentistry Applications. Int. J. Electrochem. Sci. 2011, 6, 2031–2042. [Google Scholar]
- Porcayo-Palafox, E.; Carrera-Chavez, S.I.; Casolco, S.R.; Porcayo-Calderon, J.; Salinas-Bravo, V.M. Electrochemical Performance of Ti-based Commercial Biomaterials. Adv. Mater. Sci. Eng. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Badawy, W.A.; Al-Kharafi, F.M.; Al-Ajmi, J.R. Electrochemical behaviour of cobalt in aqueous solutions of different pH. J. Appl. Electrochem. 2000, 30, 693–704. [Google Scholar] [CrossRef]
- Brug, G.J.; Van Den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Trompette, J.-L.; Massot, L.; Fontorbes, S. Influence of the anion specificity on the anodic polarization of titanium. Corrosion Sci. 2011, 53, 1262–1268. [Google Scholar] [CrossRef]
- Jung, H.; Alfantazi, A. Corrosion properties of electrodeposited cobalt in sulfate solutions containing chloride ions. Electrochim. Acta 2010, 55, 865–869. [Google Scholar] [CrossRef]
- Williams, D.F.; Clark, G.C.F. The corrosion of pure cobalt in physiological media. J. Mater. Sci. 1982, 17, 1675–1682. [Google Scholar] [CrossRef]


| Compound | Concentration (g/L) |
|---|---|
| NaCl | 0.600 |
| KCl | 0.720 |
| KH2PO4 | 0.680 |
| Na2HPO4·12H2O | 0.856 |
| KSCN | 0.060 |
| CaCl2·2H2O | 0.220 |
| KHCO3 | 1.500 |
| Citric acid | 0.030 |
| Alloy | Ecorr (mV) | Ba (mV/Dec) | Bc (mV/Dec) | icorr (mA/cm2) | Corrosion Rate (mm/yr) | Epit (mV) | Ipit (mV) |
|---|---|---|---|---|---|---|---|
| Fe40Al | −571 | 600 | 244 | 0.0021 | 0.0236 | −166.5 | 0.008 |
| Fe40Al-3Cr | −445 | 696 | 197 | 0.0015 | 0.0171 | −155 | 0.0022 |
| Fe40Al-3Ti | −582 | 162 | 110 | 0.00058 | 0.0064 | −68.2 | 0.004 |
| Fe40Al-3Co | −592 | 382 | 262 | 0.0016 | 0.01846 | - | . |
| Fe40Al-3Ni | −507 | 625 | 173 | 0.0017 | 0.019 | −162.6 | 0.0028 |
| Titanium | −488 | 320 | 168 | 0.00054 | 0.0047 | - | - |
| Alloy (wt.%) | Corrosion Rate (mm/yr) | Reference |
|---|---|---|
| Ti-6Al-4V | 0.011 | [34] |
| 67Ni-14Cr-8Mo-7.5Ga | 0.00024 | [32] |
| Ti-6Al-7Nb | 0.00031 | [35] |
| Ti-13Nb-13Zr | 0.00033 | [35] |
| TiNi | 0.00033 | [33] |
| Ti-5Al-2.5Fe | 0.00047 | [33] |
| Ti-4.5Al-3V-2Mo-2Fe | 0.00084 | [33] |
| 63Ni-22Cr-9Mo | 0.00086 | [32] |
| Ti-5Al-3Mo-4Zr | 0.00108 | [33] |
| 74.5Ni-11.5Cr-3.5Mo | 0.00139 | [32] |
| 68.5Ni-15.5Cr-14Mo1Al | 0.00196 | [32] |
| Ti-6Al-4V | 0.00208 | [33] |
| 76.9Ni-12.6Cr-5Mo-2.9Al-2Be | 0.046 | [32] |
| 80.7Ni-13.3Cr-4V-1.8Be | 0.0629 | [32] |
| 75.7Au-4.7Pd–11.1Ag–8.4Cu–1.4Zn (Ney-Oro-B2) | 0.502 | [31,36] |
| Zr5Ti, Zr25Ti, Zr45Ti | 0.0078-0.0229 | [37] |
| Alloy | Rs (Ω·cm2) | R (Ω·cm2) | Yo (Ω−1·cm−2·sn) | n | C (μF·cm−2) | RCT (Ω·cm2) | Yodl (Ω−1·cm−2·sn) | ndl | Cdl (μF·cm−2) |
|---|---|---|---|---|---|---|---|---|---|
| Ti | 47.45 | 21.94 | 6.70 × 10−6 | 0.98 | 5.60 × 10−6 | 94,488 | 3.14 × 10−5 | 0.80 | 4.12 × 10−5 |
| Fe40Al | 45.69 | 42.55 | 6.04 × 10−5 | 0.76 | 9.19 × 10−6 | 120,390 | 2.63 × 10−5 | 0.86 | 3.17 × 10−5 |
| Fe40Al-3Co | 45.38 | 240.9 | 4.12 × 10−5 | 0.80 | 1.30 × 10−5 | 77,669 | 4.41 × 10−5 | 0.78 | 6.24 × 10−5 |
| Fe40Al-3Cr | 49.32 | 24.38 | 1.10 × 10−5 | 0.86 | 7.81 × 10−6 | 80,720 | 6.99 × 10−5 | 0.76 | 1.21 × 10−4 |
| Fe40Al-3Ni | 48.01 | 68.93 | 3.83 × 10−5 | 0.83 | 1.14 × 10−5 | 314,180 | 3.25 × 10−5 | 0.82 | 5.41 × 10−5 |
| Fe40Al-3Ti | 48.21 | 33.53 | 3.59 × 10−5 | 0.86 | 1.20 × 10−5 | 188,530 | 4.82 × 10−5 | 0.80 | 8.37 × 10−5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrieta-Gonzalez, C.D.; Rodriguez-Diaz, R.A.; Mayen, J.; Retes-Mantilla, R.F.; Torres-Mancera, M.T.; Oros-Méndez, L.A.; Cruz-Mejía, H.; Flores-Garcia, N.S.; Porcayo-Calderon, J. Electrochemical Performance of Fe40Al-X (X = Cr, Ti, Co, Ni) Alloys Exposed to Artificial Saliva. Materials 2020, 13, 1095. https://doi.org/10.3390/ma13051095
Arrieta-Gonzalez CD, Rodriguez-Diaz RA, Mayen J, Retes-Mantilla RF, Torres-Mancera MT, Oros-Méndez LA, Cruz-Mejía H, Flores-Garcia NS, Porcayo-Calderon J. Electrochemical Performance of Fe40Al-X (X = Cr, Ti, Co, Ni) Alloys Exposed to Artificial Saliva. Materials. 2020; 13(5):1095. https://doi.org/10.3390/ma13051095
Chicago/Turabian StyleArrieta-Gonzalez, Cinthya Dinorah, Roberto Ademar Rodriguez-Diaz, Jan Mayen, Rogel Fernando Retes-Mantilla, María Teresa Torres-Mancera, Lya Adlih Oros-Méndez, Héctor Cruz-Mejía, Nestor Starlin Flores-Garcia, and Jesús Porcayo-Calderon. 2020. "Electrochemical Performance of Fe40Al-X (X = Cr, Ti, Co, Ni) Alloys Exposed to Artificial Saliva" Materials 13, no. 5: 1095. https://doi.org/10.3390/ma13051095
APA StyleArrieta-Gonzalez, C. D., Rodriguez-Diaz, R. A., Mayen, J., Retes-Mantilla, R. F., Torres-Mancera, M. T., Oros-Méndez, L. A., Cruz-Mejía, H., Flores-Garcia, N. S., & Porcayo-Calderon, J. (2020). Electrochemical Performance of Fe40Al-X (X = Cr, Ti, Co, Ni) Alloys Exposed to Artificial Saliva. Materials, 13(5), 1095. https://doi.org/10.3390/ma13051095







