Diffusion Bonding of 1420 Al–Li Alloy Assisted by Pure Aluminum Foil as Interlayer
Abstract
:1. Introduction
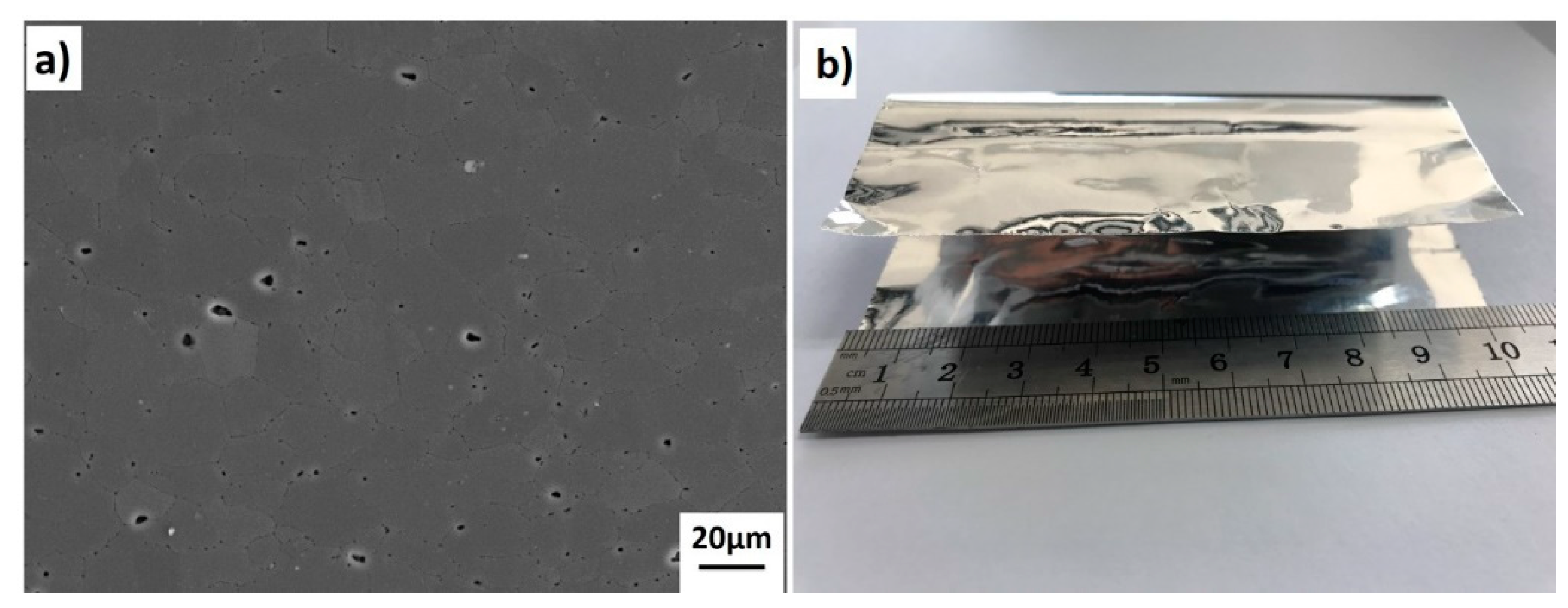
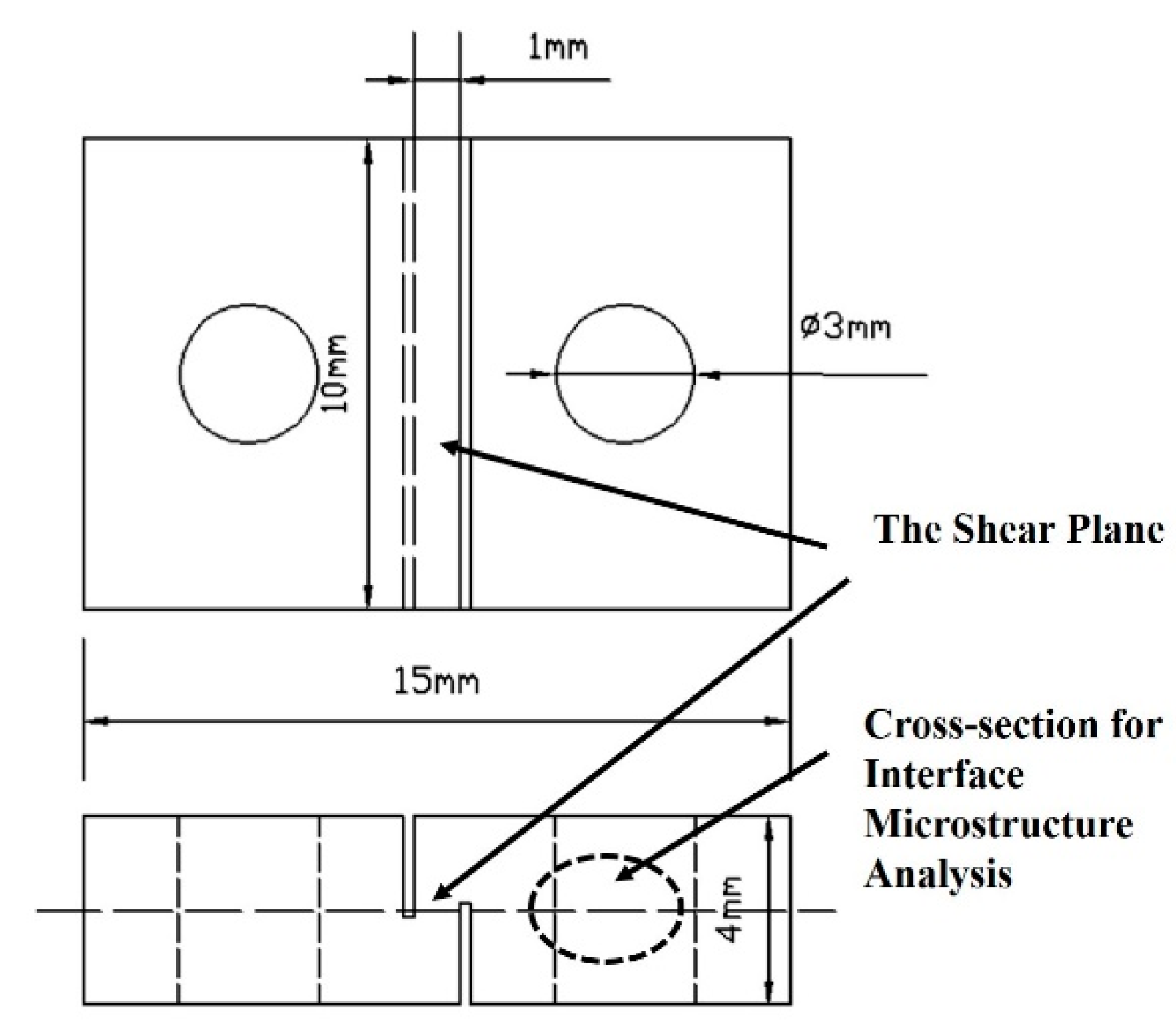
2. Materials and Methods
3. Results
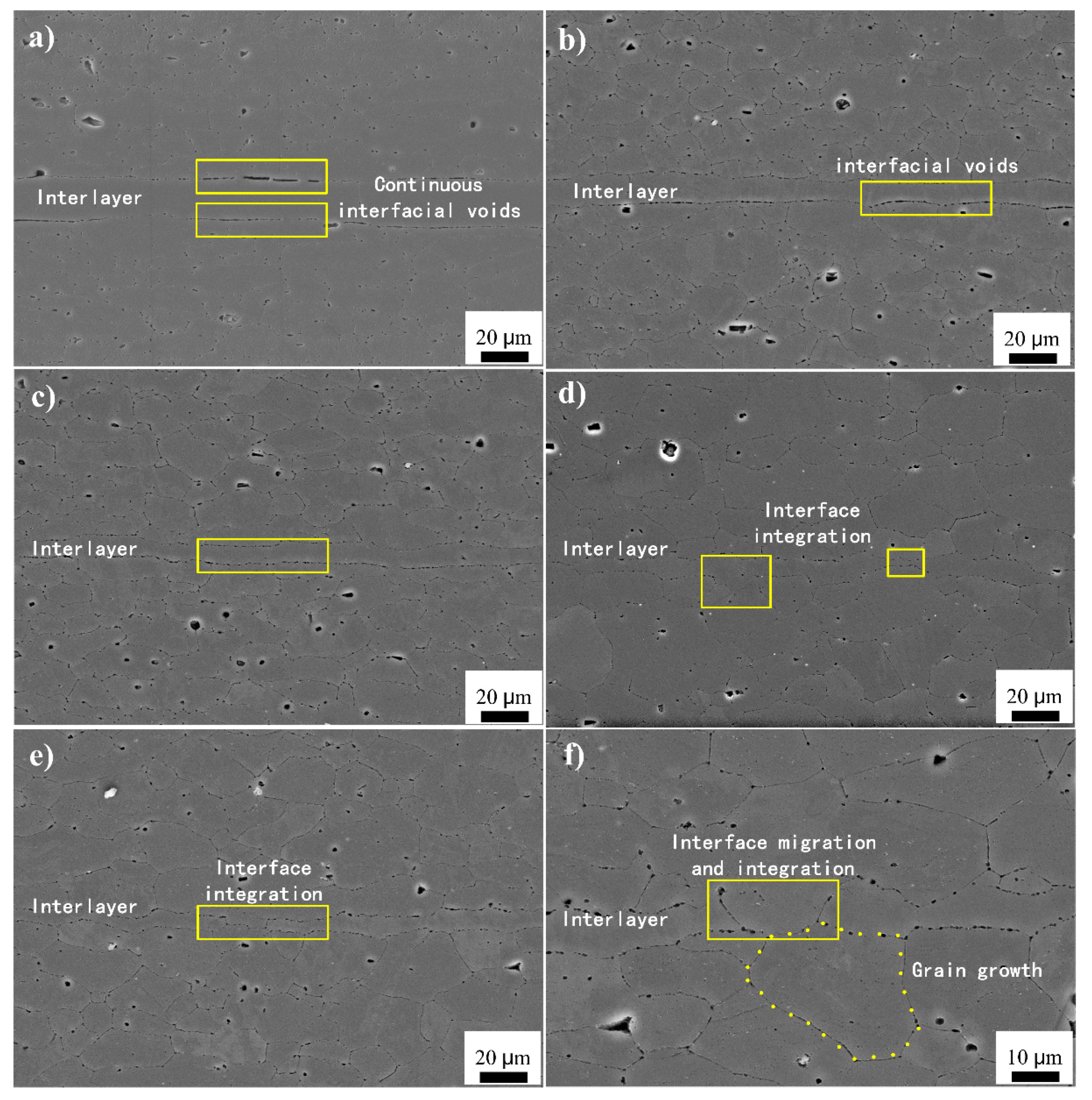
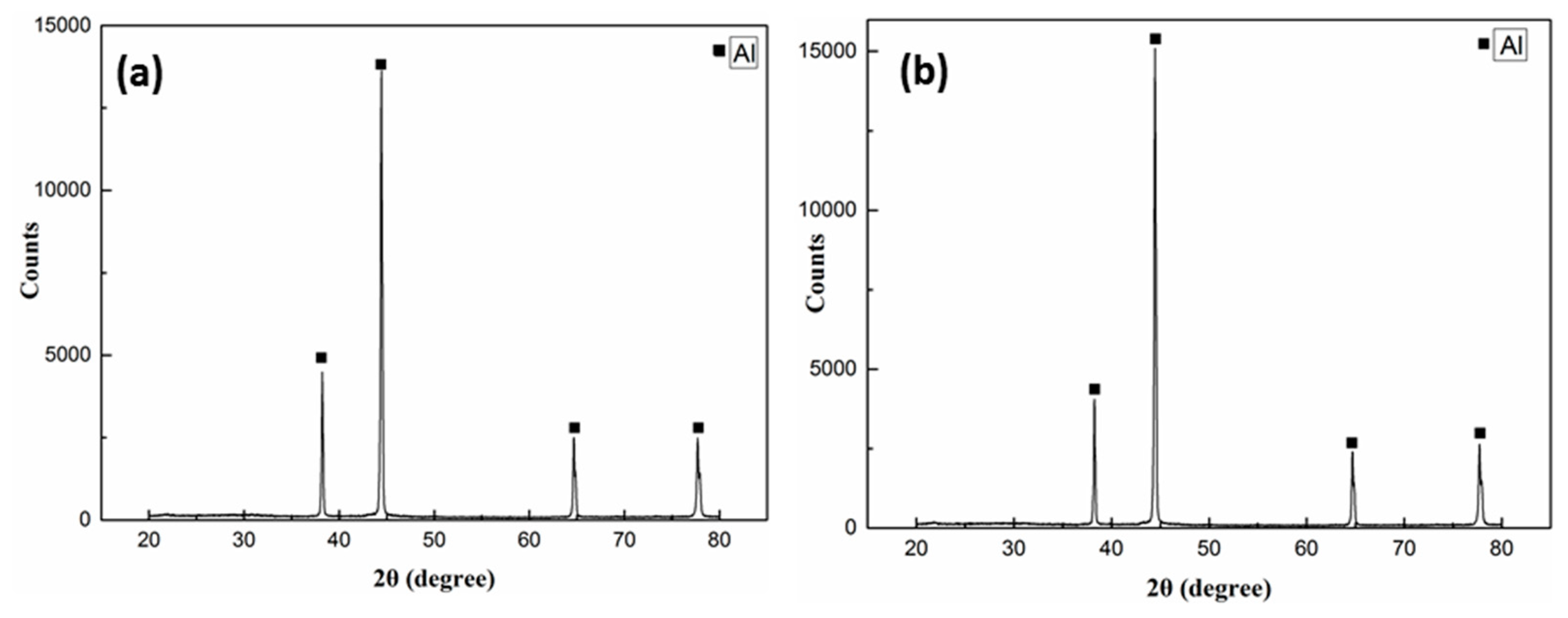
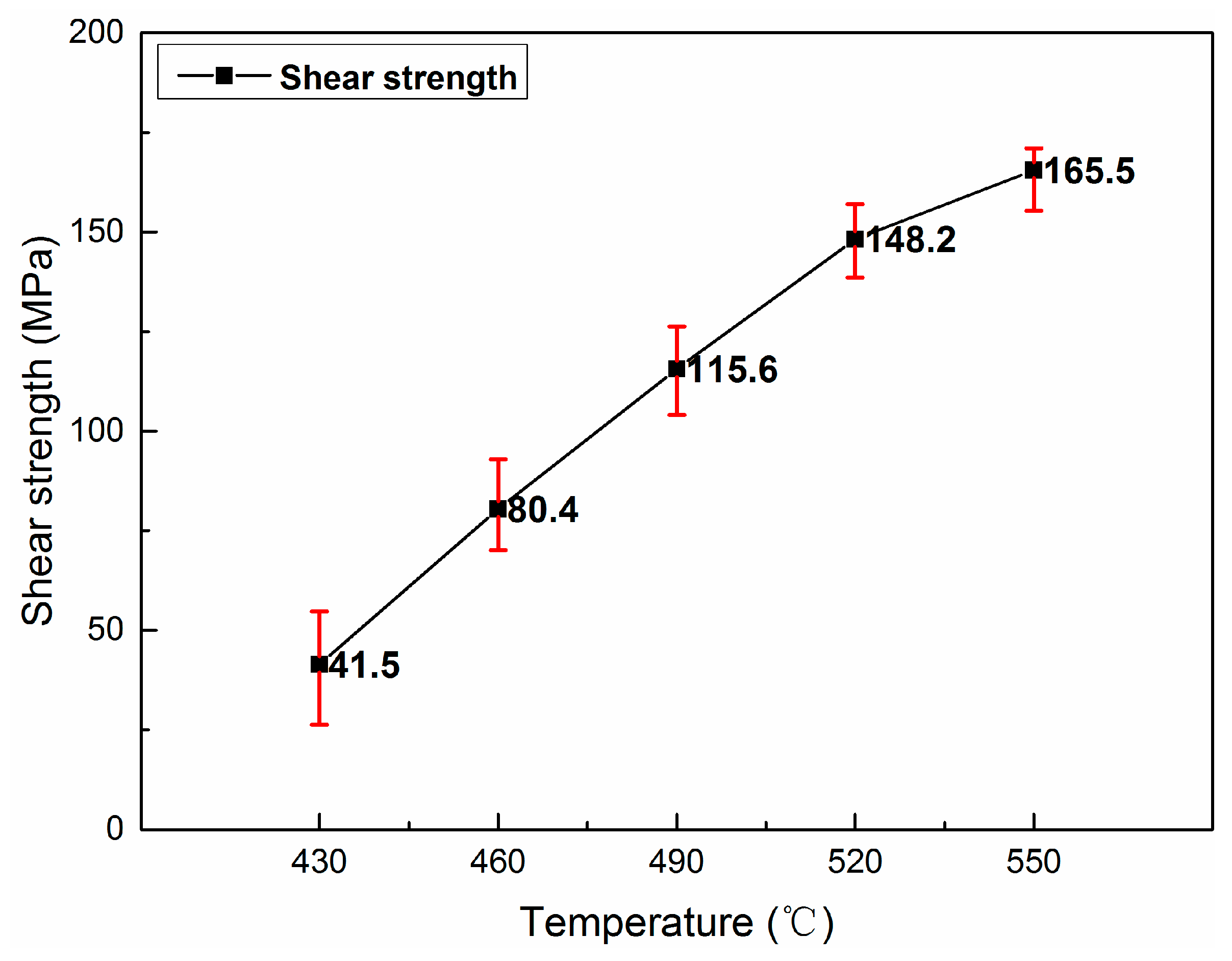
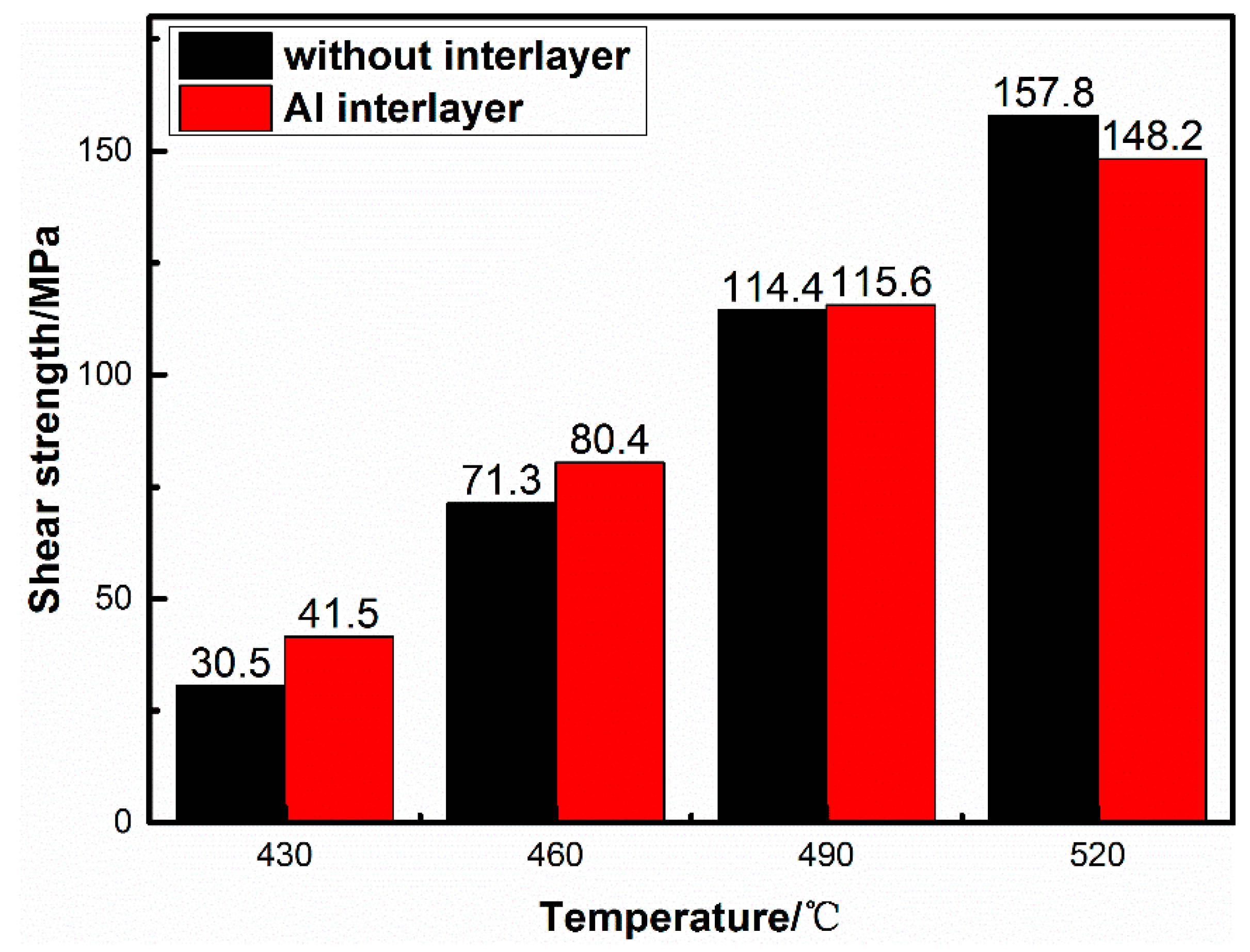
3.1. Interface Microstructure and Shear Strength
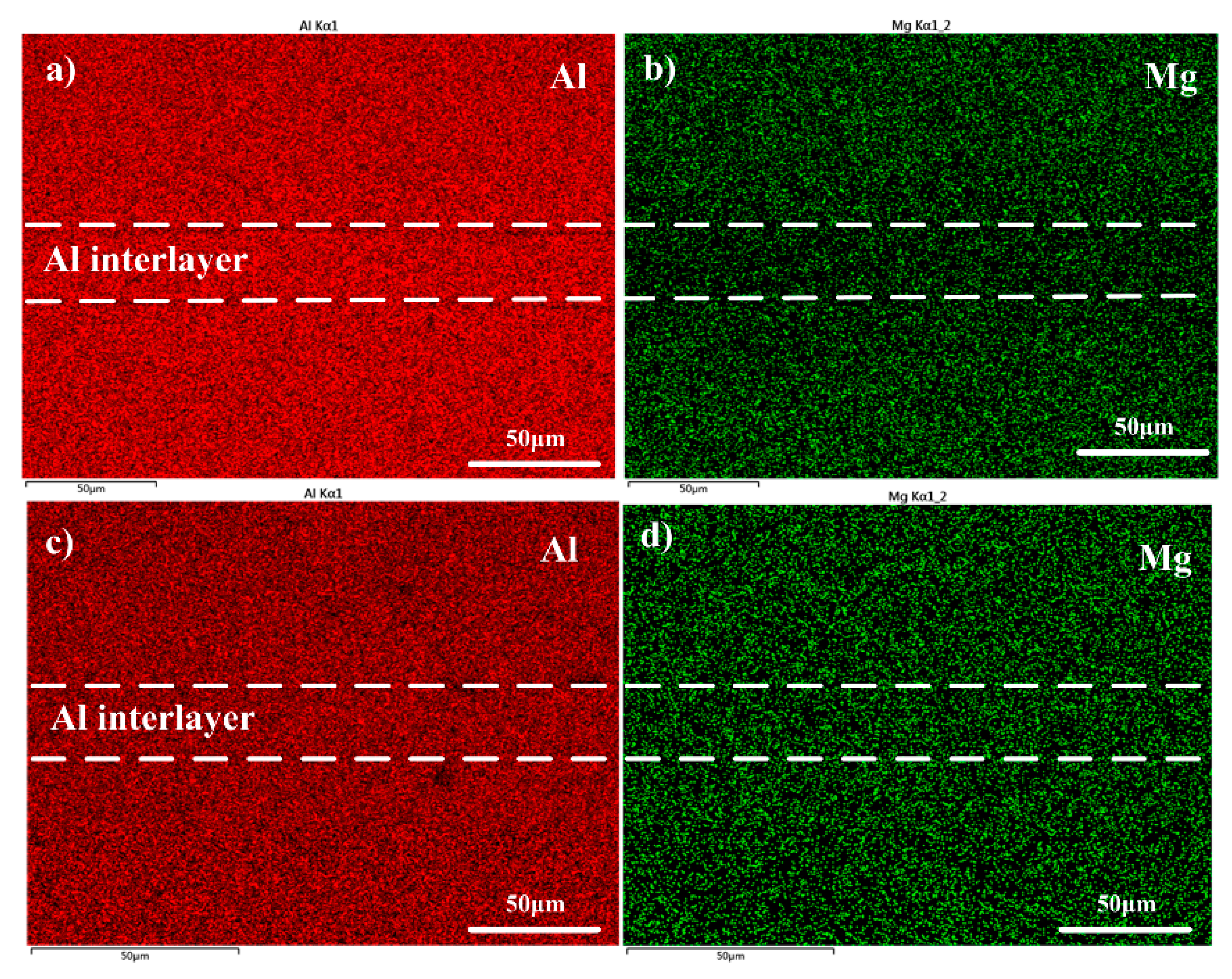
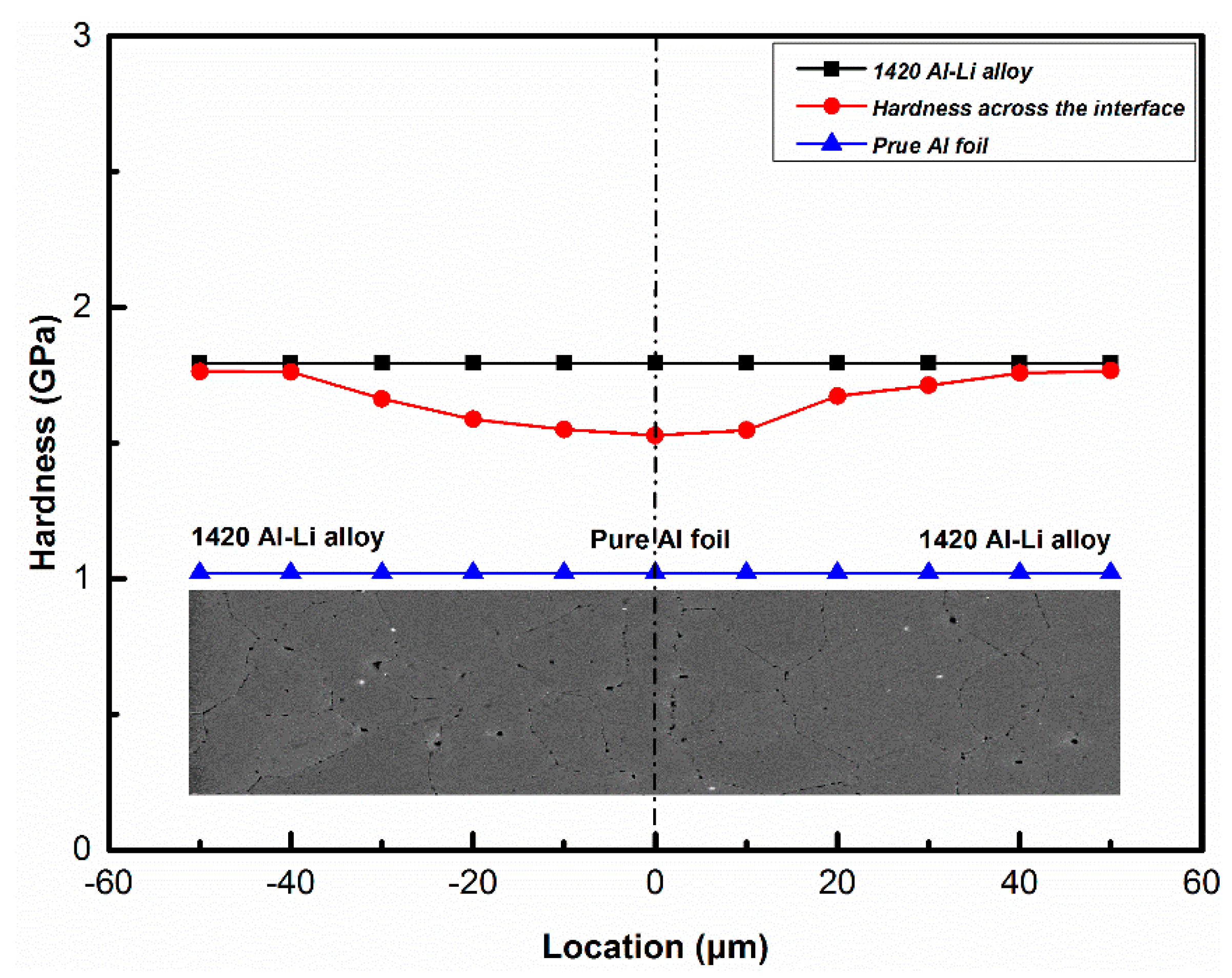
3.2. Distribution of Mg and the Nano Indentation Hardness
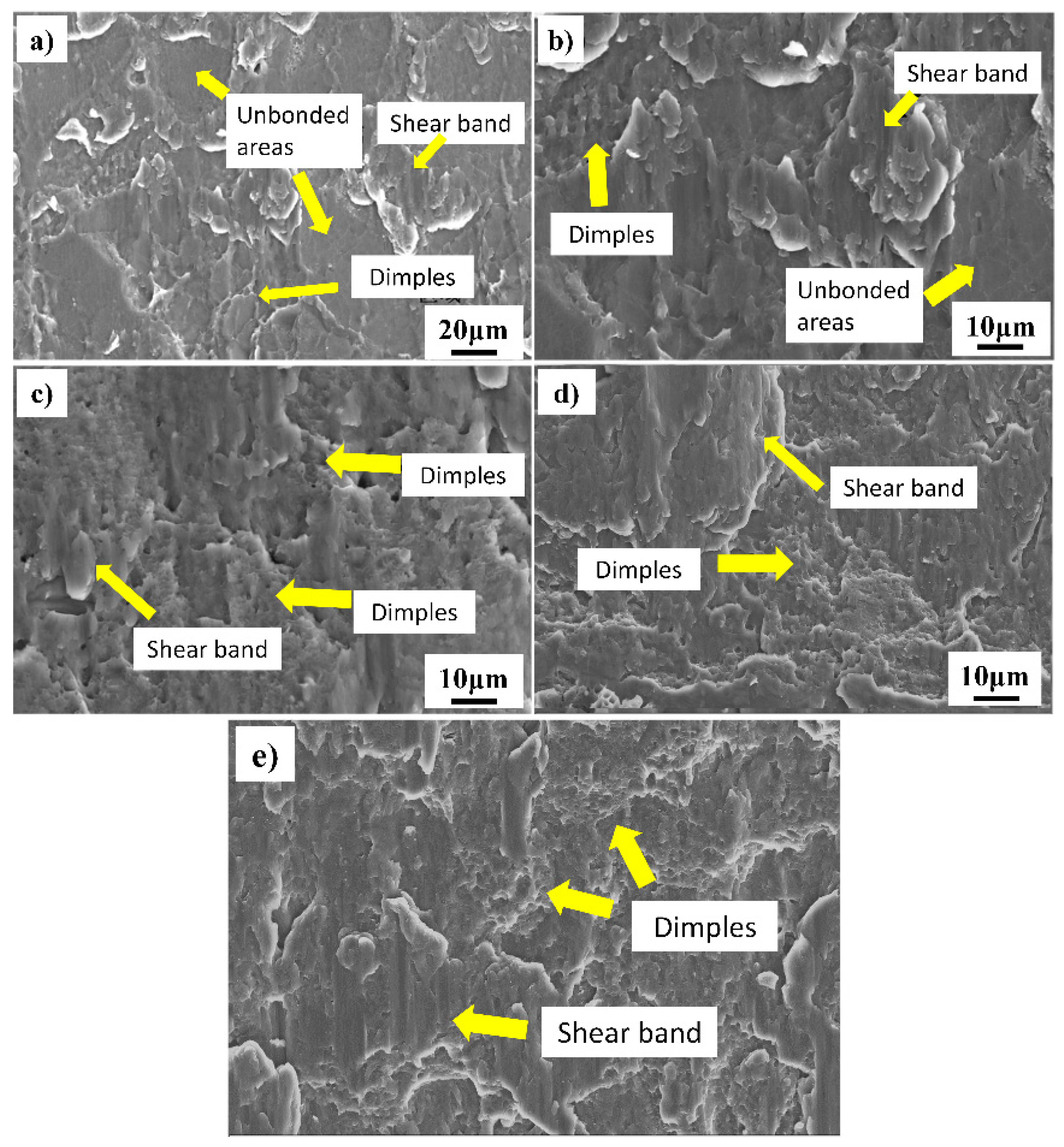
3.3. Shear Fracture Morphology
4. Discussion
4.1. Bond Formation Mechanism Assisted with Pure Al Interlayer
4.2. Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niu, J.; Liu, Z. Comparative study on electrochemical behavior of Al–Li alloy 2A97 machined surfaces in sodium chloride solution. IEEE Access 2019, 7, 134198–134205. [Google Scholar] [CrossRef]
- Jata, K.V.; Hopkins, A.K.; Rioja, R.J. The Anisotropy and Texture of Al–Li Alloys. Mater. Sci. Forum 1996, 217, 647–652. [Google Scholar] [CrossRef]
- Yi, L.; Zheng, Z.; Li, S.; Xiang, K.; Ye, H.J. Microstructures and properties of 2099 Al–Li alloy. Mater. Charact. 2013, 84, 88–99. [Google Scholar] [CrossRef]
- Song, F.; Mao, W.F.; Zhu, Y.Y. Studies on SPF/DB technology of aluminum-lithium alloys. Mater. Sci. Forum 1997, 243–245, 701–710. [Google Scholar] [CrossRef]
- Li, Z.Q.; Li, X. The applications of SPF/DB combined with welding technologies. Mater. Sci. Forum 2007, 551–552, 49–54. [Google Scholar] [CrossRef]
- Wang, N.; Chen, M. Hot flow behavior characterization for predicting the titanium alloy TC4 hollow blade surface sinkage defects in the SPF/DB process. Int. J. Mater. Form. 2019, 12, 827–844. [Google Scholar] [CrossRef]
- Orhan, N.; Khan, T.I.; Eroğlu, M. Diffusion bonding of a microduplex stainless steel to Ti–6Al–4V. Scr. Mater. 2001, 45, 441–446. [Google Scholar] [CrossRef]
- Hill, A.; Wallach, E.R. Modelling solid-state diffusion bonding. Acta Metall. 1989, 37, 2425–2437. [Google Scholar] [CrossRef]
- Derby, B.; Wallach, E.R. Diffusion bonding: Development of theoretical model. Met. Sci. 1984, 18, 427–431. [Google Scholar] [CrossRef]
- Zuruzi, A.S. Effects of surface roughness on the diffusion bonding of al alloy 6061 in air. Mater. Sci. Eng. A 1999, 270, 244–248. [Google Scholar] [CrossRef]
- Dunford, D.V.; Partridge, P.G. Critical strength criteria for DB/SPF processing of Al–Li 8090 alloy. J. Mater. Sci. 1992, 27, 3389–3394. [Google Scholar] [CrossRef]
- Nami, H.; Halvaee, A.; Adgi, H. Transient liquid phase diffusion bonding of AlMg2Si metal matrix composite. Mater. Des. 2011, 32, 3957–3965. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lim, C.H.; Lee, C.H.; Seo, K.W.; Shin, S.Y.; Lee, C.H. Diffusion bonding of al 6061 alloys using an eutectic reaction of Al-Ag-Cu. Key Eng. Mater. 2005, 297–300, 2772–2777. [Google Scholar] [CrossRef]
- Maity, J.; Pal, T.K. Transient liquid-phase diffusion bonding of aluminum metal matrix composite using a mixed Cu-Ni powder interlayer. J. Mater. Eng. Perform. 2012, 21, 1232–1242. [Google Scholar] [CrossRef]
- Nami, H.; Halvaee, A.; Adgi, H.; Hadian, A. Investigation on microstructure and mechanical properties of diffusion bonded Al/Mg2Si metal matrix composite using copper interlayer. J. Mater. Process. Technol. 2010, 210, 1282–1289. [Google Scholar] [CrossRef]
- Shirzadi, A.A.; Wallach, E.R. Novel method for diffusion bonding superalloys and aluminium alloys (USA patent 6,669,534 b2, european patent pending). Mater. Sci. Forum 2005, 502, 431–436. [Google Scholar] [CrossRef]
- Shirzadi, A.A.; Assadi, H.; Wallach, E.R. Interface evolution and bond strength when diffusion bonding materials with stable oxide films. Surf. Interface Anal. 2001, 31, 609–618. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, W.L.; Zhao, B.; Hou, H.L. Interface microstructure and bond strength of 1420/7B04 composite sheets prepared by diffusion bonding. Rare Met. 2018, 7, 613–620. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, W.; Han, Y.; Fu, X.; Xu, Y.; Hou, H. Effect of alloying elements gradient on solid-state diffusion bonding between aerospace aluminum alloys. Materials 2018, 11, 1446. [Google Scholar] [CrossRef] [Green Version]
- Ghidelli, M.; Sebastiani, M.; Collet, C.; Guillemet, R.J. Determination of the elastic moduli and residual stresses of freestanding au-tiw bilayer thin films by nanoindentation. Mater. Des. 2016, 106, 436–445. [Google Scholar] [CrossRef]
- Ast, J.; Ghidelli, M.; Durst, K.; Göken, M.; Sebastiani, M.; Korsunsky, A.M. A review of experimental approaches to fracture toughness evaluation at the micro-scale. Mater. Des. 2019, 173, 107762. [Google Scholar] [CrossRef]
- Dunford, D.V.; Partridge, P.G. Strength and fracture behaviour of diffusion-bonded joints in Al–Li (8090) alloy. J. Mater. Sci. 1991, 26, 2625–2629. [Google Scholar] [CrossRef]
- AydN, K.; Kaya, Y.; Kahraman, N. Experimental study of diffusion welding/bonding of titanium to copper. Mater. Des. 2012, 37, 356–368. [Google Scholar] [CrossRef]
- Pilling, J.; Ridley, N. Solid state bonding of superplastic AA 7475. Mater. Sci. Technol. 1987, 3, 353–359. [Google Scholar] [CrossRef]
- Kurgan, N. Investigation of the effect of diffusion bonding parameters on microstructure and mechanical properties of 7075 aluminium alloy. Int. J. Adv. Manuf. Technol. 2014, 71, 2115–2124. [Google Scholar] [CrossRef]
- Derby, B.; Wallach, E.R. Joining methods in space: A theoretical model for diffusion bonding. Acta Astronaut. 1980, 7, 685–698. [Google Scholar] [CrossRef]
- Takahashi, Y.; Inoue, K.; Nishiguchi, K. Identification of void shrinkage mechanisms. Acta Metall. Mater. 1993, 41, 3077–3084. [Google Scholar] [CrossRef]
- Fan, W.; Kashyap, B.P. Effects of strain rate and test temperature on flow behaviour and microstructural evolution in AA8090 Al–Li alloy. Metal. Sci. J. 2013, 17, 431–438. [Google Scholar] [CrossRef]
- Ureña, A.; Salazar, J.M.G.D.; Quiñones, J.; Martin, J.J. TEM characterization of diffusion bonding of superplastic 8090 Al–Li alloy. Scr. Mater. 1996, 34, 617–623. [Google Scholar] [CrossRef]
- Gilmore, C.J.; Dunford, D.V.; Partridge, P.G. Microstructure of diffusion-bonded joints in Al–Li 8090 alloy. J. Mater. Sci. 1996, 26, 3119–3124. [Google Scholar] [CrossRef]
| Sample | Mg | Li | Zr | Cu | Fe | Si | Al |
|---|---|---|---|---|---|---|---|
| 1420 Al–Li alloy | 5.2 | 2.0 | 0.12 | 0.03 | 0.09 | 0.03 | Bal. |
| Sample | Temperature (°C) | Presssure (MPa) | Time (min) |
|---|---|---|---|
| S1 | 430 | 6 | 60 |
| S2 | 460 | 6 | 60 |
| S3 | 490 | 6 | 60 |
| S4 | 520 | 6 | 60 |
| S5 | 550 | 6 | 60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Chen, W.; Zhao, B.; Hou, H.; Zhou, W.; Li, Z. Diffusion Bonding of 1420 Al–Li Alloy Assisted by Pure Aluminum Foil as Interlayer. Materials 2020, 13, 1103. https://doi.org/10.3390/ma13051103
Wu F, Chen W, Zhao B, Hou H, Zhou W, Li Z. Diffusion Bonding of 1420 Al–Li Alloy Assisted by Pure Aluminum Foil as Interlayer. Materials. 2020; 13(5):1103. https://doi.org/10.3390/ma13051103
Chicago/Turabian StyleWu, Fan, Wei Chen, Bing Zhao, Hongliang Hou, Wenlong Zhou, and Zhiqiang Li. 2020. "Diffusion Bonding of 1420 Al–Li Alloy Assisted by Pure Aluminum Foil as Interlayer" Materials 13, no. 5: 1103. https://doi.org/10.3390/ma13051103





