Acid Red 66 Dye Removal from Aqueous Solution by Fe/C-based Composites: Adsorption, Kinetics and Thermodynamic Studies
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the Fe-Based Composites
2.3. Characterizations
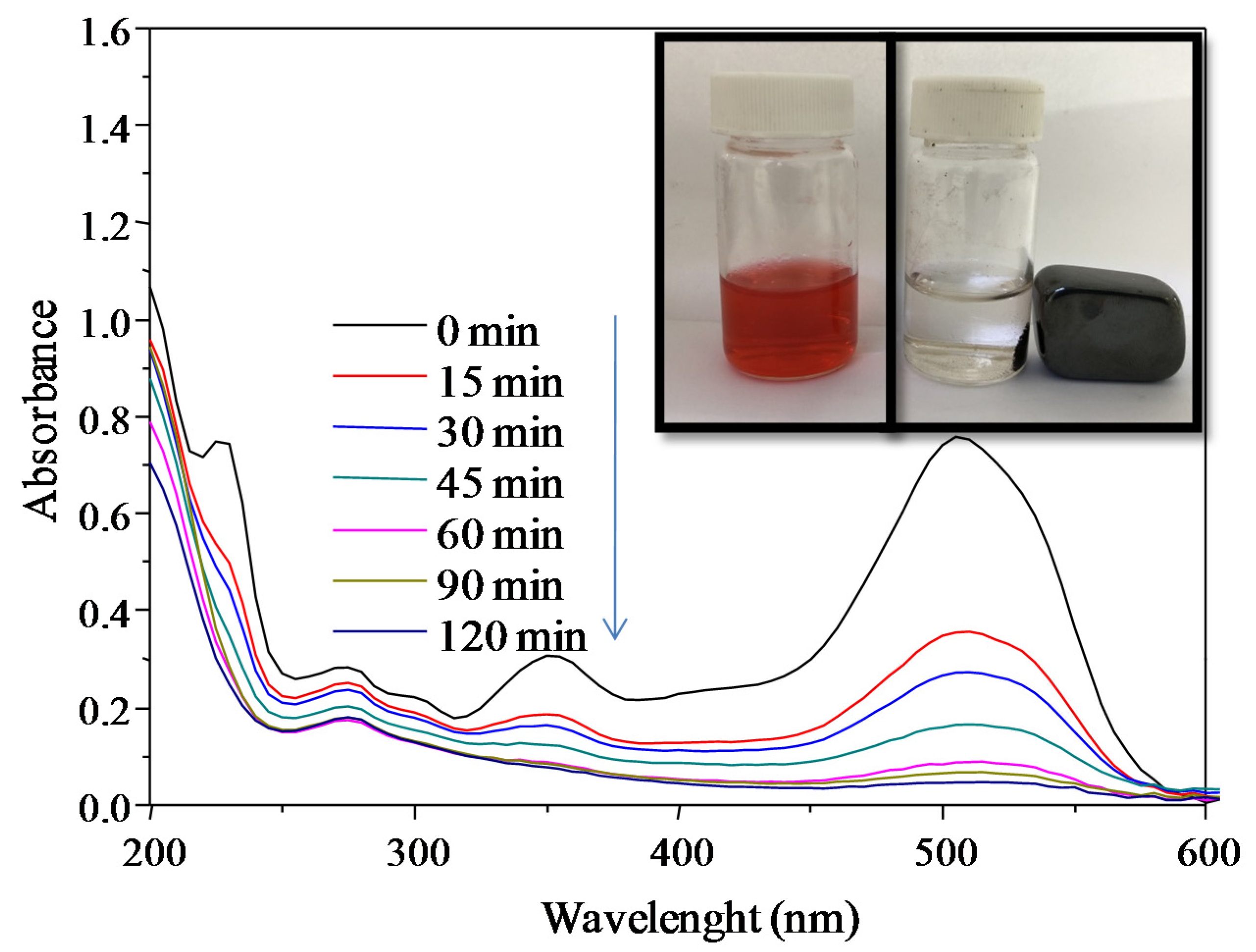
2.4. Adsorption Studies
3. Results and Discussion
3.1. Structure by XRD, Raman and FTIR
3.2. Textural Properties and Zeta Potential Determination
3.3. Morphological Aspects of the Solids by SEM-EDS
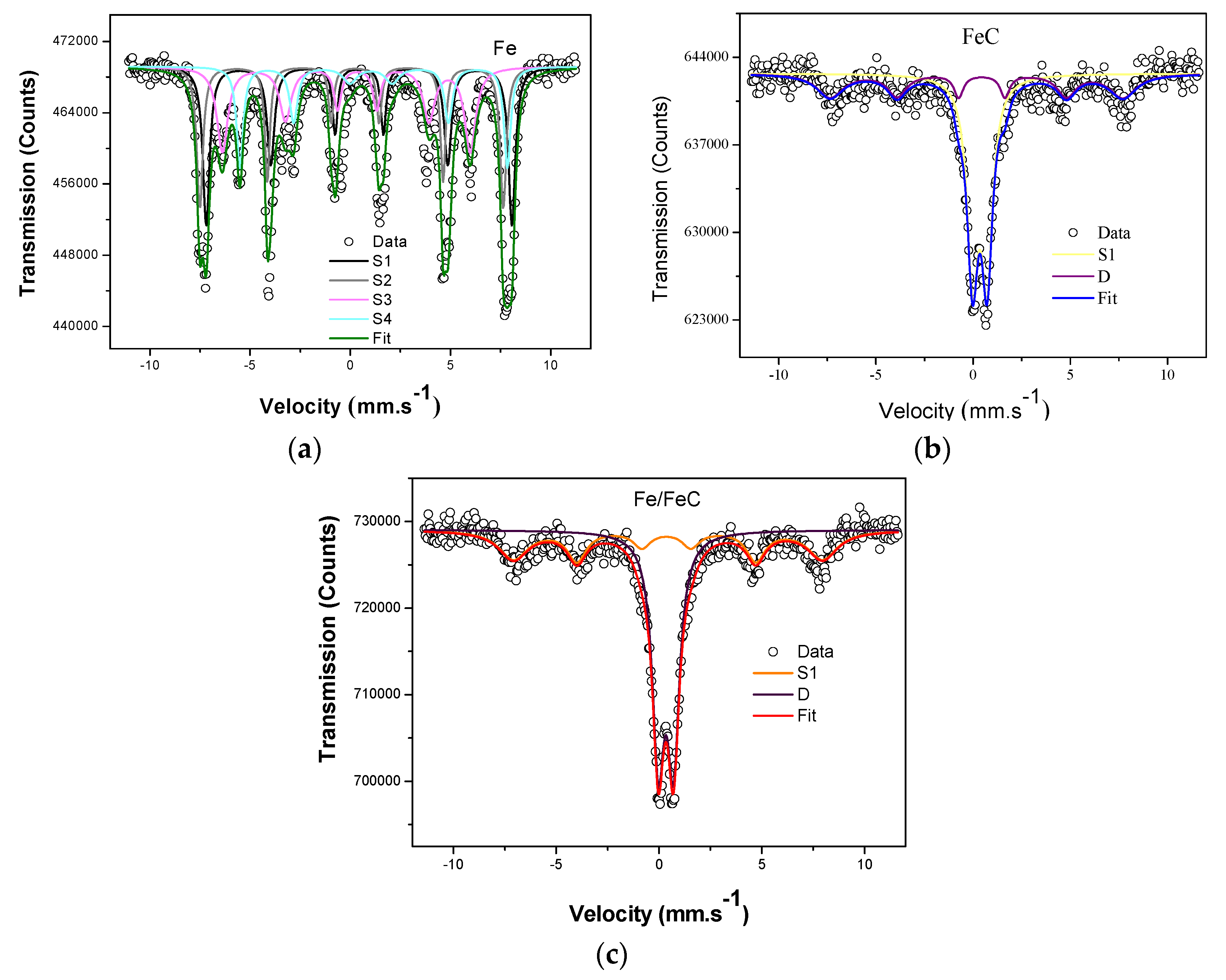
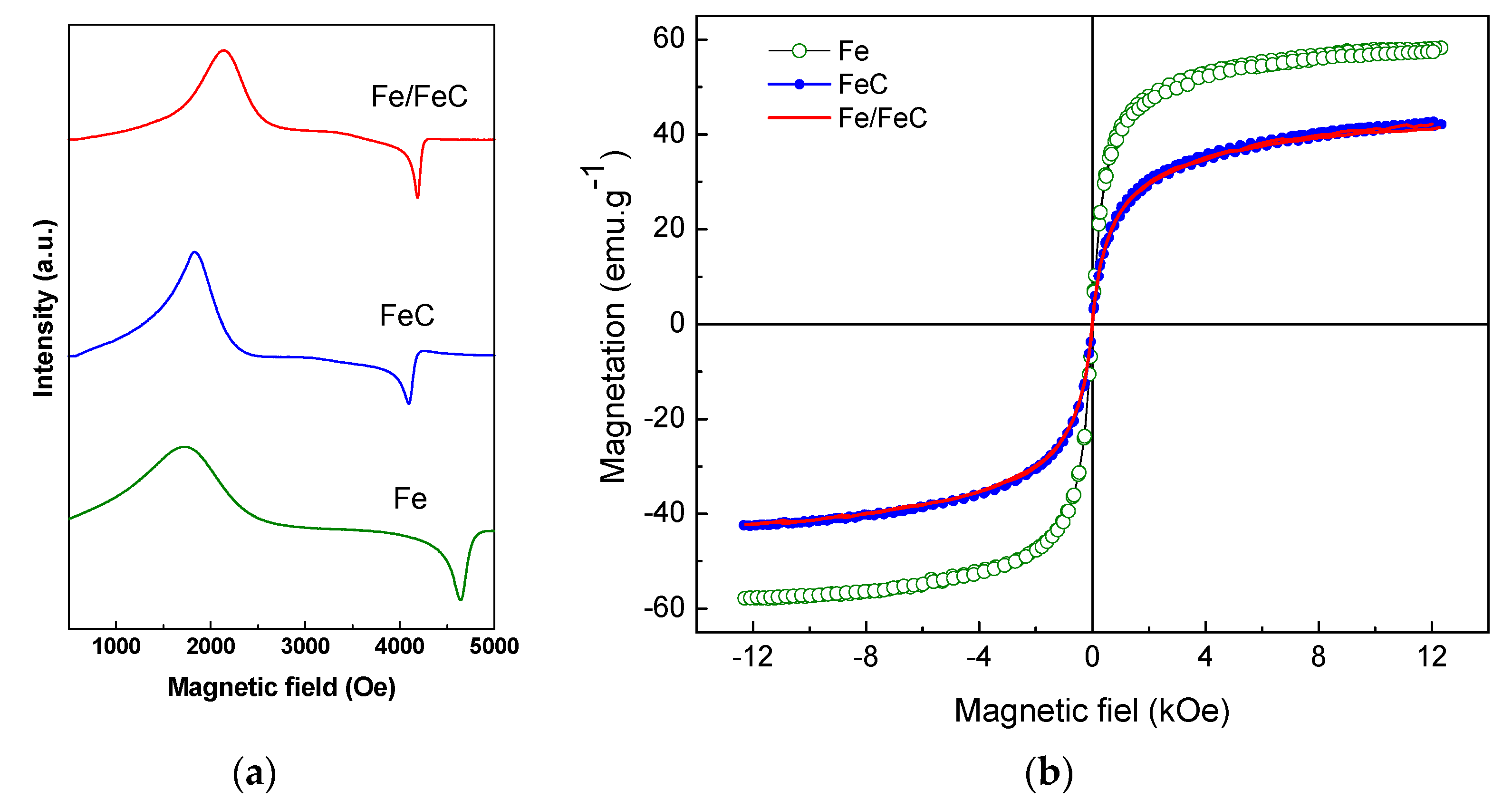
3.4. Mössbauer, EPR and Magnetization Curves
3.5. Adsorption Studies
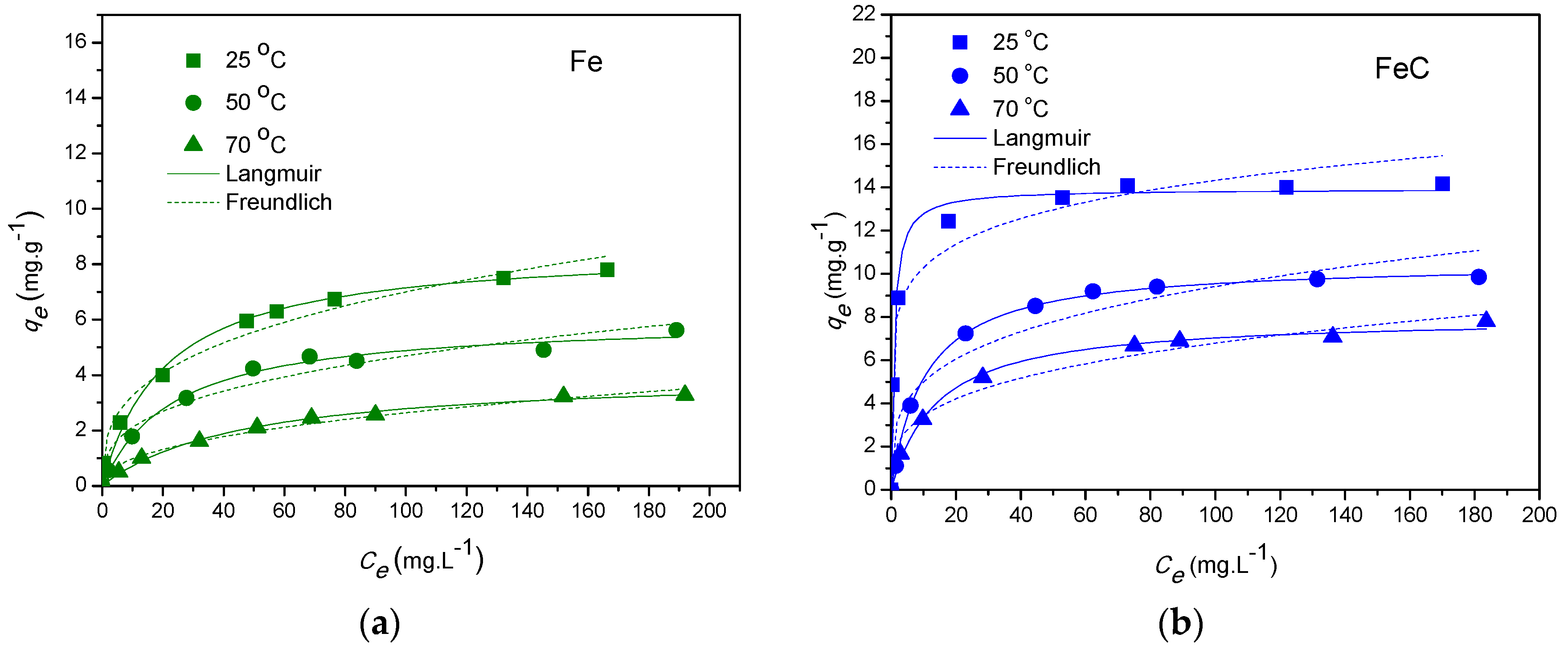
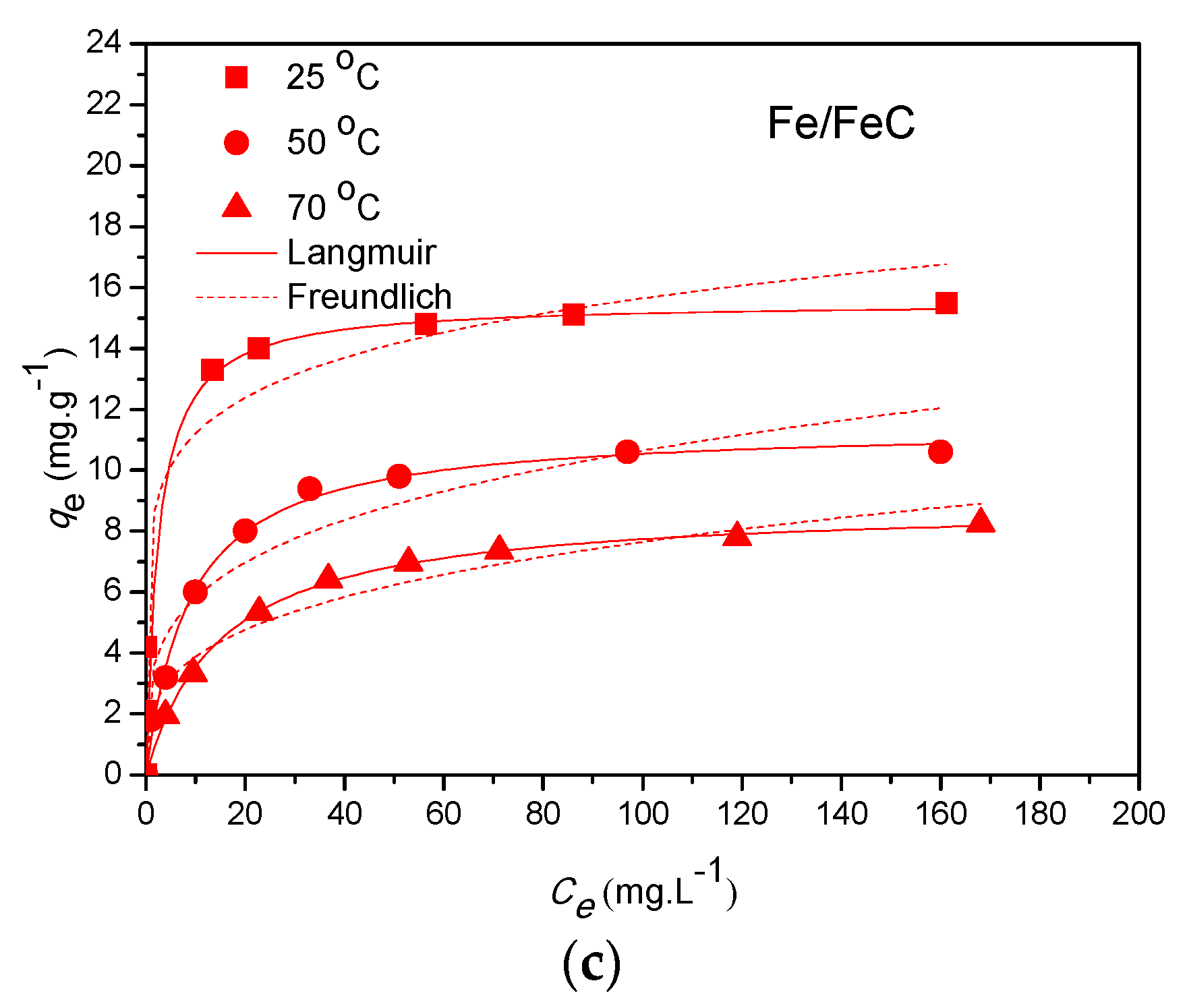
3.5.1. Equilibrium Study
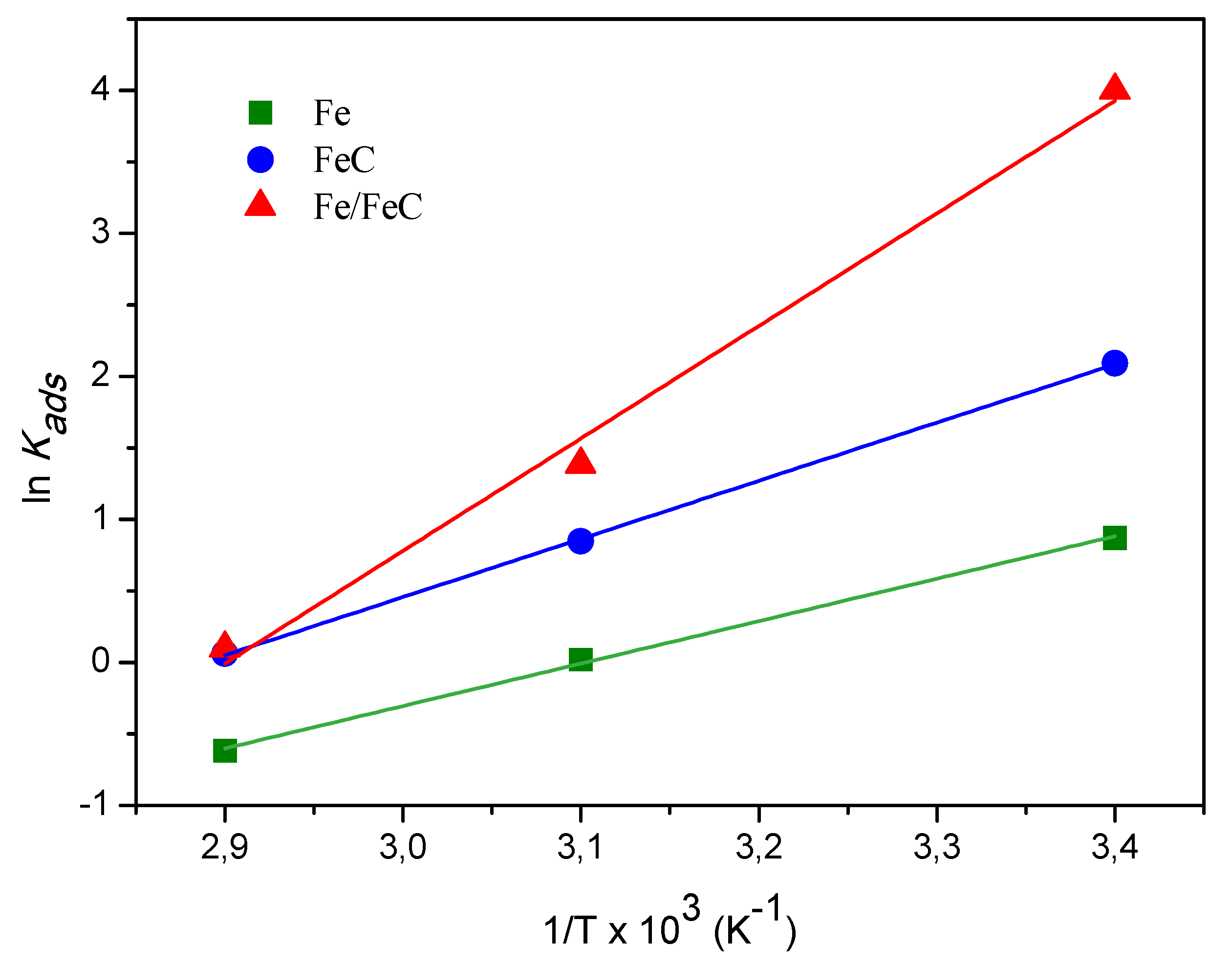
3.5.2. Thermodynamic Studies
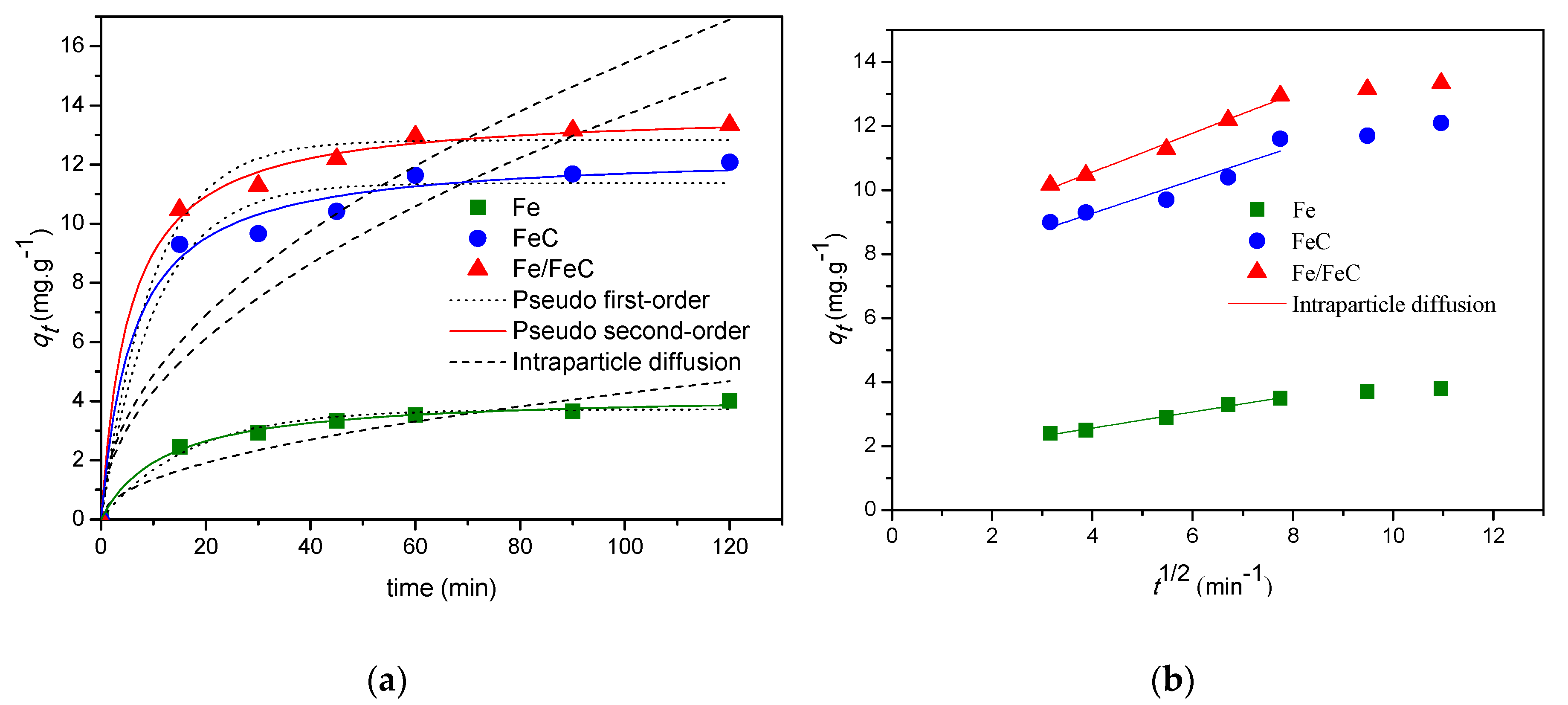
3.5.3. Adsorption Kinetics
Pseudo-First and Second Order Models
Intra-Particle Diffusion
3.6. XPS Measurements of the Spent Adsorbents
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muhammad, A.; Shah, A.-U.-H.; Bilal, S.; Rahman, G. Basic Blue Dye Adsorption from Water using Polyaniline/Magnetite(Fe3O4) Composites: Kinetic and Thermodynamic Aspects. Materials 2019, 12, 1764. [Google Scholar] [CrossRef]
- Li, C.; Liu, H.; Xiahoui, J.; Waterhouse, G.I.N. Hierarchical Fe3O4/C with a flower-like morphology: A highly efficient and reusable dye adsorbent. Synth. Met. 2018, 248, 24645–24656. [Google Scholar] [CrossRef]
- Hu, X.-S.; Liang, R.; Sun, G. Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution. J. Mater. Chem. A 2018, 6, 17612–17624. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Zillohu, A.U.; Abdelaziz, R.; Hedayati, M.K.; Elbahri, M. A Novel Nanohybrid Nanofibrous Adsorbent for Water Purification from Dye Pollutants. Materials 2016, 9, 848. [Google Scholar] [CrossRef]
- Patil, S.M.; Deshmukh, S.P.; More, K.V.; Shevale, V.B.; Delekar, S.D. Sulfated TiO2/WO3 nanocomposite: An efficient photocatalyst for degradation of Congo red and methyl red dyes under visible light irradiation. Mater. Chem. Phys. 2019, 225, 247–255. [Google Scholar] [CrossRef]
- Yadav, A.; Mukherji, S.; Garg, A. Removal of Chemical Oxygen Demand and Color from Simulated Textile Wastewater Using a Combination of Chemical/Physicochemical Processes. Ind. Eng. Chem. Res. 2013, 52, 10063–10071. [Google Scholar] [CrossRef]
- Buscio, V.; García-Jiménez, M.; Vilaseca, M.; Lopez-Grimau, V.; Crespi, M.; Gutiérrez-Bouzán, C. Reuse of Textile Dyeing Effluents Treated with Coupled Nanofiltration and Electrochemical Processes. Materials 2016, 9, 490. [Google Scholar] [CrossRef]
- Zhao, B.; Shao, Q.; Hao, L.; Zhang, L.; Liu, Z.; Zhang, B.; Ge, S.; Guo, Z. Yeast-template synthesized Fe-doped cerium oxide hollow microspheres for visible photodegradation of acid orange 7. J. Colloid Interface Sci. 2018, 511, 39–47. [Google Scholar] [CrossRef]
- Chu, T.P.M.; Nguyen, N.T.; Vu, T.L.; Dao, T.H.; Dinh, L.C.; Nguyen, H.L.; Hoang, T.H.; Le, T.S.; Pham, T.D. Synthesis, Characterization, and Modification of Alumina Nanoparticles for Cationic Dye Removal. Materials 2019, 12, 450. [Google Scholar] [CrossRef]
- Dastgerdi, Z.H.; Abkhiz, V.; Meshkat, S.S.; Ghorbani, N. Preparation of novel magnetic grafted raft agent nanocomposite: Application in carmine dye adsorptive removal from waste water. J. Environ. Chem. Eng. 2019, 7, 103109. [Google Scholar] [CrossRef]
- Ren, S.; Deng, L.; Zhang, B.; Lei, Y.; Ren, H.; Lu, J.X.; Zhao, R.; Chen, X. Effect of Air Oxidation on Texture, Surface Properties and Dye Adsorption of Wood-Derived Porous Carbon Materials. Materials 2019, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Choi, P.-P. Enhanced Congo red dye removal from aqueous solutions using iron nanoparticles: Adsorption, kinetics, and equilibrium studies. Dalton Trans. 2017, 46, 15470–15479. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.; Travlou, N.A.; Kalogirou, O.; Deliyanni, E. Magnetic Graphene Oxide: Effect of Preparation Route on Reactive Black 5 Adsorption. Materials 2013, 6, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Cojocariu, B.; Mocanu, A.M.; Nacu, G.; Bulgariu, L. Possible utilization of PET waste as adsorbent for Orange G dye removal from aqueous media. Desalin. Water Treat. 2018, 104, 338–345. [Google Scholar] [CrossRef]
- Yu, B.; He, L.; Wang, Y.; Cong, H. Multifunctional PMMA@Fe3O4@DR Magnetic Materials for Efficient Adsorption of Dyes. Materials 2017, 10, 1239. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, T.; Zhang, Z.; Xie, M. Hydrothermal synthesis of a graphene/magnetite/montmorillonite nanocomposite and its ultrasonically assisted methylene blue adsorption. J. Mater. Sci. 2019, 54, 11037–11055. [Google Scholar] [CrossRef]
- González-Alfaro, Y.; Aranda, P.; Fernandes, F.; Wicklein, B.; Darder, M.; Ruiz-Hitzky, E. Multifunctional Porous Materials Through Ferrofluids. Adv. Mater. 2011, 23, 5224–5228. [Google Scholar] [CrossRef]
- Darder, M.; González-Alfaro, Y.; Aranda, P.; Ruiz-Hitzky, E. Silicate-based multifunctional nanostructured materials wi.th magnetite and Prussian blue: Application to cesium uptake. RSC Adv. 2014, 4, 35415–35421. [Google Scholar] [CrossRef]
- Aboutaleb, W.A.; El-Salamony, R.A. Effect of Fe2O3-CeO2 nanocomposite synthesis method on the Congo red dye photodegradation under visible light irradiation. Mater. Chem. Phys. 2019, 236, 121724. [Google Scholar] [CrossRef]
- Sukumar, M.; Sivasamy, A.; Swaminathan, G. In situ biodecolorization kinetics of Acid Red 66 in aqueous solutions by Trametes versicolor. J. Hazard. Mater. 2009, 167, 660–663. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, T.; Liu, P.; Cui, P.; Hu, P. Manufacture of nano graphite oxides derived from aqueous glucose solutions and in-situ synthesis of magnetite–graphite oxide composites. Mater. Chem. Phys. 2015, 153, 202–208. [Google Scholar] [CrossRef]
- Cruz, M.G.; Bastos-Neto, M.; Oliveira, A.C.; Filho, J.M.; Soares, J.S.; Rodriguez-Castellon, E.; Fernandes, F.A.N. On the structural, textural and morphological features of Fe-based catalysts supported on polystyrene mesoporous carbon for Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2015, 495, 72–83. [Google Scholar] [CrossRef]
- Cornell, R.M. Effect of simple sugars on the alkaline transformation of ferrihydride into goethite and hematite. Clays Clay Min. 1985, 33, 219–227. [Google Scholar] [CrossRef]
- Lian, S.; Wang, E.; Kang, Z.; Bai, Y.; Gao, L.; Jiang, M.; Hu, C.; Xu, L. Synthesis of magnetite nanorods and porous hematite nanorods. Solid State Commun. 2004, 129, 485–490. [Google Scholar] [CrossRef]
- De Castro, A.J.R.; Marques, S.P.; Soares, J.S.; Filho, J.M.; Saraiva, G.D.; Oliveira, A.C. Nanosized aluminum derived oxides catalysts prepared with different methods for styrene production. Chem. Eng. J. 2012, 209, 345–355. [Google Scholar] [CrossRef]
- Rout, K.; Mohapatra, M.; Layek, S.; Dash, A.; Verma, H.; Anand, S. The influence of precursors on phase evolution of nano iron oxides/oxyhydroxides: Optical and magnetic properties. New J. Chem. 2014, 38, 3492–3506. [Google Scholar] [CrossRef]
- Vinothkannana, M.; Karthikeyana, C.; Kumara, G.G.; Kim, A.R.; Yoo, D.J. One-pot green synthesis of reduced graphene oxide (RGO)/Fe3O4 nanocomposites and its catalytic activity toward methylene blue dyedegradation. Spectochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 256–264. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman spectroscopic study of magnetite (FeFe2O4): A new assignment for the vibrational spectrum. J. Solid State Chem. 2003, 174, 424–430. [Google Scholar] [CrossRef]
- Wang, L.; Lin, K.; Ren, J.; Du, K.; Chang, Y.; Han, L.; Yao, P.; Tian, F. Direct synthesis of ultrasmall and stable magnetite nanoparticles coated with one single carbon layer for sensitive surface-enhanced Raman scattering. Appl. Surf. Sci. 2019, 478, 601–606. [Google Scholar] [CrossRef]
- Iyengar, S.J.; Joy, M.; Ghosh, C.K.; Dey, S.; Kotnala, R.K.; Ghosh, S. Magnetic, X-ray and Mössbauer studies on magnetite/maghemite core–shell nanostructures fabricated through an aqueous route. RSC Adv. 2014, 4, 64919–64929. [Google Scholar] [CrossRef]
- Stoia, M.; Istratie, R.; Păcurariu, C. Investigation of magnetite nanoparticles stability in air by thermal analysis and FTIR spectroscopy. J. Therm. Anal. Calorim. 2016, 125, 1185–1198. [Google Scholar] [CrossRef]
- Luo, X.; Liu, S.; Zhou, J.; Zhang, L. In situ synthesis of Fe3O4/cellulose microspheres with magnetic-induced protein delivery. J. Mater. Chem. 2009, 19, 3538–3545. [Google Scholar] [CrossRef]
- De Castro, A.J.R.; Soares, J.S.; Filho, J.M.; Oliveira, A.C.; Campos, A.; Milet, E.R. Oxidative dehydrogenation of ethylbenzene with CO2 for styrene production over porous iron-based catalysts. Fuel 2013, 108, 740–748. [Google Scholar] [CrossRef]
- Aslam, S.; Zeng, J.; Subhan, F.; Li, M.; Lyu, F.; Li, Y.; Yan, Z. In situ one-step synthesis of Fe3O4@MIL-100(Fe) core-shells for adsorption of methylene blue from water. J. Colloid Interface Sci. 2017, 505, 186–195. [Google Scholar] [CrossRef]
- Ding, Y.; Morber, J.R.; Snyder, R.L.; Wang, Z.L. Nanowire Structural Evolution from Fe3O4 to ϵ-Fe2O3. Adv. Funct. Mater. 2007, 17, 1172–1178. [Google Scholar] [CrossRef]
- Roychowdhury, A.; Pati, S.P.; Mishra, A.K.; Kumar, S.; Das, D. Magnetically addressable fluorescent Fe3O4/ZnO nanocomposites: Structural, optical and magnetization studies. J. Phys. Chem. Solids 2013, 74, 811–818. [Google Scholar] [CrossRef]
- He, Y.; Xu, J.; Yang, F.; Lin, J.; Yang, H.; He, Y. Magnetic and Mössbauer Spectroscopy Studies of Zinc-Substituted Cobalt Ferrites Prepared by the Sol-Gel Method. Materials 2018, 11, 1799. [Google Scholar]
- Salama, W.; Aref, M.E.; Gaupp, R. Spectroscopic characterization of iron ores formed in different geological environments using FTIR, XPS, Mössbauer spectroscopy and thermoanalyses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1816–1826. [Google Scholar] [CrossRef]
- Singh, L.H.; Govindaraj, R.; Mythili, R.; Amarendra, G. Stability and magnetic interactions between magnetite nanoparticles dispersed in zeolite as studied using Mössbauer spectroscopy. J. Magn. Magn. Mater. 2016, 418, 248–252. [Google Scholar] [CrossRef]
- Astrath, N.; Baesso, M.L.; Bento, A.; Colucci, C.C.; Medina, A.; Evangelista, L.R. Phonon–roton-like elementary excitations and low-temperature behaviour of non-crystalline solids. Philos. Mag. 2006, 86, 227–235. [Google Scholar] [CrossRef]
- Oliveira, A.P.S.; Gomes, I.S.; Neto, A.S.B.; Oliveira, A.C.; Filho, J.M.; Saraiva, G.D.; Soares, J.M.; Tehuacanero-Cuapa, S. Catalytic performance of MnFeSi composite in selective oxidation of styrene, ethylbenzene and benzyl alcohol. Mol. Catal. 2017, 436, 29–42. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Kubiak, T.; Dobosz, B.; Schroeder, G.; Kurczewska, J. EPR spectroscopy and imaging of TEMPO-labeled magnetite nanoparticles. Curr. Appl. Phys. 2014, 14, 798–804. [Google Scholar] [CrossRef]
- Klencsár, Z.; Ábrahám, A.; Szabó, L.; Szabó, E.G.; Stichleutner, S.; Kuzmann, E.; Homonnay, Z.; Tolnai, G. The effect of preparation conditions on magnetite nanoparticles obtained via chemical co-precipitation. Mater. Chem. Phys. 2019, 223, 122–132. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Sinnwell, M.A.; Banerjee, D.; Devaraj, A.; Kukkadapu, R.K.; Droubay, T.C.; Nie, Z.; Kovarik, L.; Vijayakumar, M.; Manandhar, S.; et al. Reduced Magnetism in Core–Shell Magnetite@MOF Composites. Nano Lett. 2017, 17, 6968–6973. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, Z.; Bawazir, W.A.; Al-Bukhari, S.M.A.; Basaleh, A.S.; Bawazier, W.A. Adsorption, equilibrium isotherm, and thermodynamic studies to the removal of acid orange 7. Mater. Chem. Phys. 2019, 232, 109–120. [Google Scholar] [CrossRef]
- De Lima, A.C.A.; Nascimento, R.; De Sousa, F.F.; Filho, J.M.; Oliveira, A.C. Modified coconut shell fibers: A green and economical sorbent for the removal of anions from aqueous solutions. Chem. Eng. J. 2012, 185, 274–284. [Google Scholar] [CrossRef]
- Li, Y.; Lu, H.; Wang, Y.; Zhao, Y.; Li, X. Efficient removal of methyl blue from aqueous solution by using poly(4-vinylpyridine)–graphene oxide–Fe3O4 magnetic nanocomposites. J. Mater. Sci. 2019, 54, 7603–7616. [Google Scholar] [CrossRef]
- Konicki, W.; Hełminiak, A.; Arabczyk, W.; Mijowska, E. Adsorption of cationic dyes onto Fe@graphite core–shell magnetic nanocomposite: Equilibrium, kinetics and thermodynamics. Chem. Eng. Res. Des. 2018, 129, 259–270. [Google Scholar] [CrossRef]
- Bulut, E.; Özacar, M.; Şengil, I.A. Equilibrium and kinetic data and process design for adsorption of Congo Red onto bentonite. J. Hazard. Mater. 2008, 154, 613–622. [Google Scholar] [CrossRef]
- Allen, S.J.; McKay, G.; Porter, J. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interface Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef]
- Luo, Z.; Gao, M.; Yang, S.; Yang, Q. Adsorption of phenols on reduced-charge montmorillonites modified by bispyridinium dibromides: Mechanism, kinetics and thermodynamics studies. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 222–230. [Google Scholar] [CrossRef]
- Cojocaru, C.; Samoila, P.; Pascariu, P. Chitosan-based magnetic adsorbent for removal ofwater-soluble anionic dye: Artificial neural network modeling and molecular docking insights. Int. J. Biol. Macromol. 2019, 123, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.M.D.G.; Ferreira, M.D.; Hespanhol, M.C.; Rezende, J.P.; Pires, A.C.S.; Gurgel, L.V.A.; Silva, L.H.M. Adsorption of red azo dyes on mullti-walled carbon nanotubes and activated carbon: A thermodynamic study. Colloid Surf. A Physicochem. Eng. Asp. 2017, 529, 531–540. [Google Scholar] [CrossRef]
- Zhu, H.-Y.; Jiang, R.; Xiao, L. Adsorption of an anionic azo dye by chitosan/kaolin/γ-Fe2O3 composites. Appl. Clay Sci. 2010, 48, 522–526. [Google Scholar] [CrossRef]
- Pirillo, S.; Cornaglia, L.; Ferreira, M.L.; Rueda, E.H. Removal of Fluorescein using different iron oxides as adsorbents: Effect of pH. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 636–643. [Google Scholar] [CrossRef]
- Reddy, Y.S.; Magdalane, C.M.; Kaviyarasu, K.; Mola, G.T.; Kennedy, J.; Maaza, M. Equilibrium and kinetic studies of the adsorption of acid blue 9 and Safranin O from aqueous solutions by MgO decked FLG coated Fuller’s Earth. J. Phys. Chem. Solids. 2018, 123, 43–51. [Google Scholar] [CrossRef]
- Wu, R.; Liu, J.-H.; Zhao, L.; Zhang, X.; Xie, J.; Yu, B.; Ma, X.; Yang, S.-T.; Wang, H.; Liu, Y. Hydrothermal preparation of magnetic Fe3O4@C nanoparticles for dye adsorption. J. Environ. Chem. Eng. 2014, 2, 907–913. [Google Scholar] [CrossRef]
- Konicki, W.; Aleksandrzak, M.; Moszyński, D.; Mijowska, E. Adsorption of anionic azo-dyes from aqueous solutions onto graphene oxide: Equilibrium, kinetic and thermodynamic studies. J. Colloid Interface Sci. 2017, 496, 188–200. [Google Scholar] [CrossRef]
- Nasef, M.; Saidi, H. Surface studies of radiation grafted sulfonic acid membranes: XPS and SEM analysis. Appl. Surf. Sci. 2006, 252, 3073–3084. [Google Scholar] [CrossRef]
- Di Lecce, D.; Marangon, V.; Benítez, A.; Caballero, Á.; Morales, J.; Rodríguez-Castellón, E.; Hassoun, J. High capacity semi-liquid lithium sulfur cells with enhanced reversibility for application in new-generation energy storage systems. J. Power Sources 2019, 412, 575–585. [Google Scholar] [CrossRef]






| Sample | BET Surface Areas (m2·g−1) | Pore Volumes (cm3·g−1) | t-Plot Surface Areas (m2·g−1) | Pore Sizes (Å) |
|---|---|---|---|---|
| Fe | 163 | 0.23 | 170 | 27 |
| FeC | 122 | 0.17 | 126 | 27 |
| Fe/FeC | 202 | 0.28 | 205 | 28 |
| Sample | Zeta Potential (mV) | Composition (wt%) | |||
|---|---|---|---|---|---|
| Fe | C | O | S | ||
| Fe | −5.56 | 73.1 | – | 23.9 | 0.1 |
| FeC | 10.8 | 63.9 | 6.7 | 29.1 | – |
| Fe/FeC | 24.9 | 62.6 | 6.7 | 30.0 | – |
| Samples | Electric Quadrupole and Isomer Shift | |||
|---|---|---|---|---|
| BHF (T) | Δ (mm/s) | δ (mm/s) | R.A. (%) | |
| Fe (S1) | 47.42 | −0.01 | 0.44 | 30.2 |
| Fe (S2) | 47.09 | −0.18 | 0.15 | 24.5 |
| Fe (S3) | 38.46 | −0.54 | 0.07 | 26.4 |
| Fe (S4) | 41.60 | 0.13 | 1.02 | 18.8 |
| Fe/FeC (S1) | 46.83 | 0.08 | 0.44 | 39.8 |
| Fe/FeC (D) | – | 0.73 | 0.33 | 60.2 |
| FeC (S1) | 46.74 | −0.27 | 0.31 | 37.4 |
| FeC (D) | – | 0.74 | 0.34 | 62.6 |
| Adsorbent | Temperature | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (°C) | qo (mg·g−1) | b (L·mg−1) | R2 | SD | KF (mg1−1/n·L1/n·g−1) | 1/n | R2 | SD | |
| Fe | 25 | 8.616 | 0.049 | 0.989 | 0.065 | 1.512 | 0.333 | 0.981 | 0.086 |
| 50 | 6.030 | 0.043 | 0.991 | 0.034 | 0.954 | 0.345 | 0.951 | 0.091 | |
| 70 | 4.080 | 0.022 | 0.995 | 0.054 | 0.361 | 0.435 | 0.981 | 0.061 | |
| FeC | 25 | 13.92 | 1.125 | 0.968 | 0.009 | 7.394 | 0.011 | 0.975 | 0.011 |
| 50 | 10.54 | 0.096 | 0.999 | 0.009 | 2.661 | 0.270 | 0.923 | 0.083 | |
| 70 | 8.007 | 0.072 | 0.994 | 0.022 | 1.732 | 0.294 | 0.973 | 0.051 | |
| Fe/FeC | 25 | 15.53 | 0.404 | 0.919 | 0.011 | 8.025 | 0.145 | 0.972 | 0.066 |
| 50 | 11.46 | 0.114 | 0.993 | 0.014 | 3.174 | 0.263 | 0.914 | 0.091 | |
| 70 | 8.901 | 0.067 | 0.999 | 0.009 | 1.972 | 0.294 | 0.949 | 0.067 | |
| Adsorbent | ΔHo (kJ·mol−1) | ΔSo (J·mol−1·K−1) | R2 | ΔGo (kJ·mol−1) | ||
|---|---|---|---|---|---|---|
| 25 °C | 50 °C | 70 °C | ||||
| Fe | −28.1 | −86.9 | 0.998 | −2.16 | −0.05 | 1.77 |
| FeC | −38.5 | −111.7 | 0.999 | −5.18 | −2.27 | −0.17 |
| Fe/FeC | −74.3 | −216.9 | 0.988 | −9.91 | −3.72 | −0.29 |
| Adsorbent | Dye Removal (%) | qe (mg·g−1) | Pseudo 1st Order | Pseudo 2nd Order | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe1 (mg·g−1) | R2 | SD | k2 (g·mg−1·min−1) | qe2 (mg·g−1) | R2 | SD | |||
| Fe | 52.6 | 4.001 | 0.014 | 3.720 | 0.976 | 0.055 | 0.0094 | 4.249 | 0.994 | 0.024 |
| FeC | 73.3 | 12.13 | 0.022 | 11.37 | 0.968 | 0.067 | 0.0132 | 12.40 | 0.987 | 0.043 |
| Fe/FeC | 98.4 | 13. 31 | 0.024 | 12.82 | 0.985 | 0.045 | 0.0134 | 13.85 | 0.997 | 0.022 |
| Adsorbent | Intraparticle Diffusion | ||
|---|---|---|---|
| kint (mg·g−1·min−1/2) | R2 | SD | |
| Fe | 0.427 | 0.831 | 0.183 |
| FeC | 1.367 | 0.687 | 0.234 |
| Fe/FeC | 1.542 | 0.647 | 0.244 |
| Sample | Binding energy (eV) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe 2p3/2 | Fe 2p1/2 | S 2p3/2 | Na 1s | Cl 2p3/2 | N 1s | C 1s | O 1s | |
| Fe | 710.8 | 724.2 | 399.6 | 284.9(82) | 530.0(68) | |||
| 286.4 (8) | 531.5(32) | |||||||
| 288.6 (4) | ||||||||
| FeC | 710.9 | 724.3 | 168.1 | 399.7 | 285.0(45) | 529.9(44) | ||
| 286.1(43) | 531.4(36) | |||||||
| 288.0(12) | 532.8(20) | |||||||
| Fe(FeC) | 710.6 | 724.3 | 168.1 | 399.7 | 285.0(50) | 530.4(48) | ||
| 286.2(38) | 531.5(35) | |||||||
| 288.1(12) | 532.9(17) | |||||||
| Acid Red dye | 168.1 | 1071.8 | 198.2 | 399.9 | 284.8(70) | |||
| 285.9 (9) | ||||||||
| 286.7(17) | 531.8(47) | |||||||
| 288.3 (4) | 533.2(53) | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paz, C.B.; Araújo, R.S.; Oton, L.F.; Oliveira, A.C.; Soares, J.M.; Medeiros, S.N.; Rodríguez-Castellón, E.; Rodríguez-Aguado, E. Acid Red 66 Dye Removal from Aqueous Solution by Fe/C-based Composites: Adsorption, Kinetics and Thermodynamic Studies. Materials 2020, 13, 1107. https://doi.org/10.3390/ma13051107
Paz CB, Araújo RS, Oton LF, Oliveira AC, Soares JM, Medeiros SN, Rodríguez-Castellón E, Rodríguez-Aguado E. Acid Red 66 Dye Removal from Aqueous Solution by Fe/C-based Composites: Adsorption, Kinetics and Thermodynamic Studies. Materials. 2020; 13(5):1107. https://doi.org/10.3390/ma13051107
Chicago/Turabian StylePaz, Camila B., Rinaldo S. Araújo, Lais F. Oton, Alcineia C. Oliveira, João M. Soares, Susana N. Medeiros, Enrique Rodríguez-Castellón, and Elena Rodríguez-Aguado. 2020. "Acid Red 66 Dye Removal from Aqueous Solution by Fe/C-based Composites: Adsorption, Kinetics and Thermodynamic Studies" Materials 13, no. 5: 1107. https://doi.org/10.3390/ma13051107
APA StylePaz, C. B., Araújo, R. S., Oton, L. F., Oliveira, A. C., Soares, J. M., Medeiros, S. N., Rodríguez-Castellón, E., & Rodríguez-Aguado, E. (2020). Acid Red 66 Dye Removal from Aqueous Solution by Fe/C-based Composites: Adsorption, Kinetics and Thermodynamic Studies. Materials, 13(5), 1107. https://doi.org/10.3390/ma13051107







