PVC-Based Copper Electric Wires under Various Fire Conditions: Toxicity of Fire Effluents
Abstract
:1. Background
2. Methodology
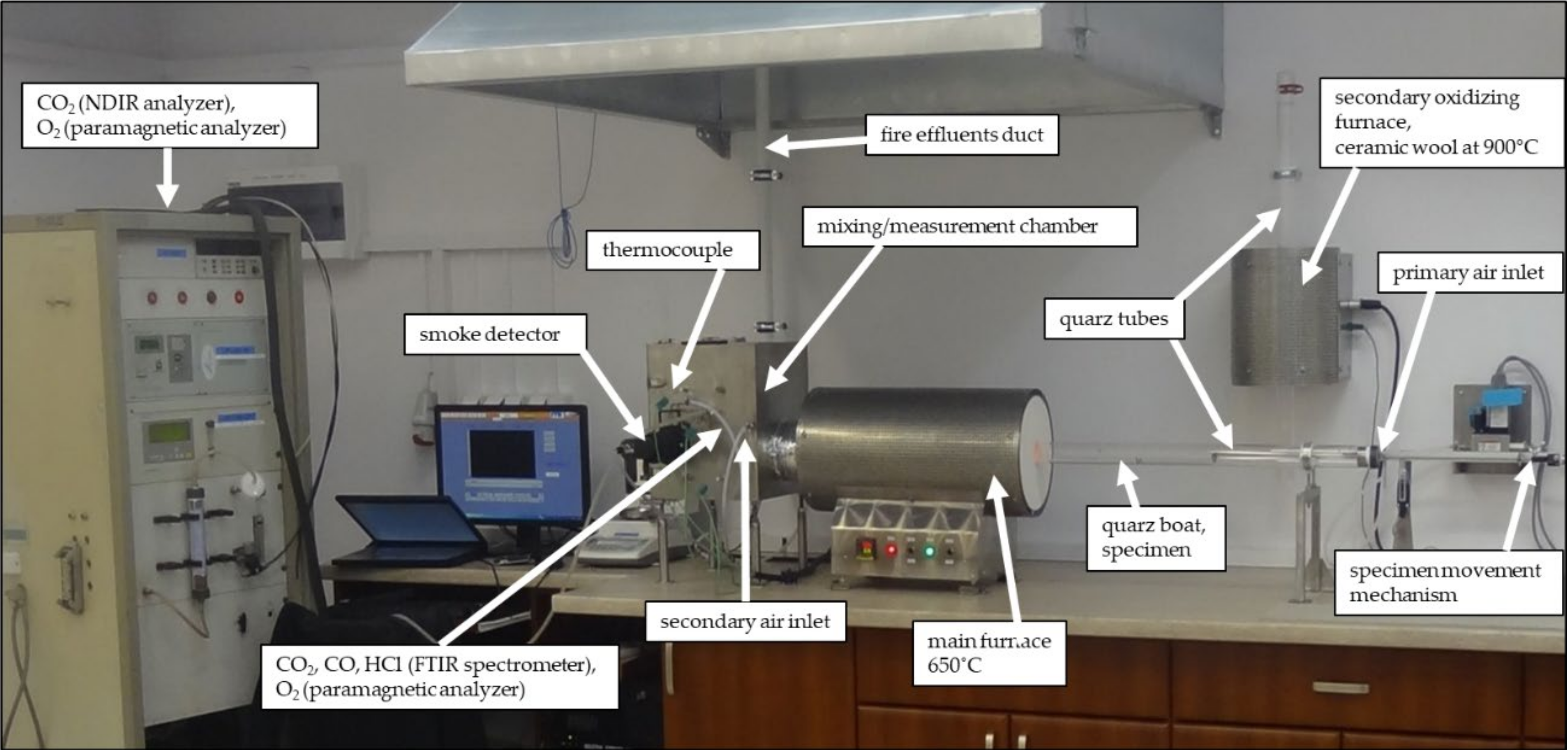
2.1. Experiments
2.2. Statistical Analysis
3. Results and Discussion
4. Summary and Conclusions
- 1.
- Fire gases yields generated from PVC-based electric copper wire were approximately four times higher than from pure polymers (pure rigid PVC and pure LDPE) tested under the same ventilated conditions (10 L/min).
- 2.
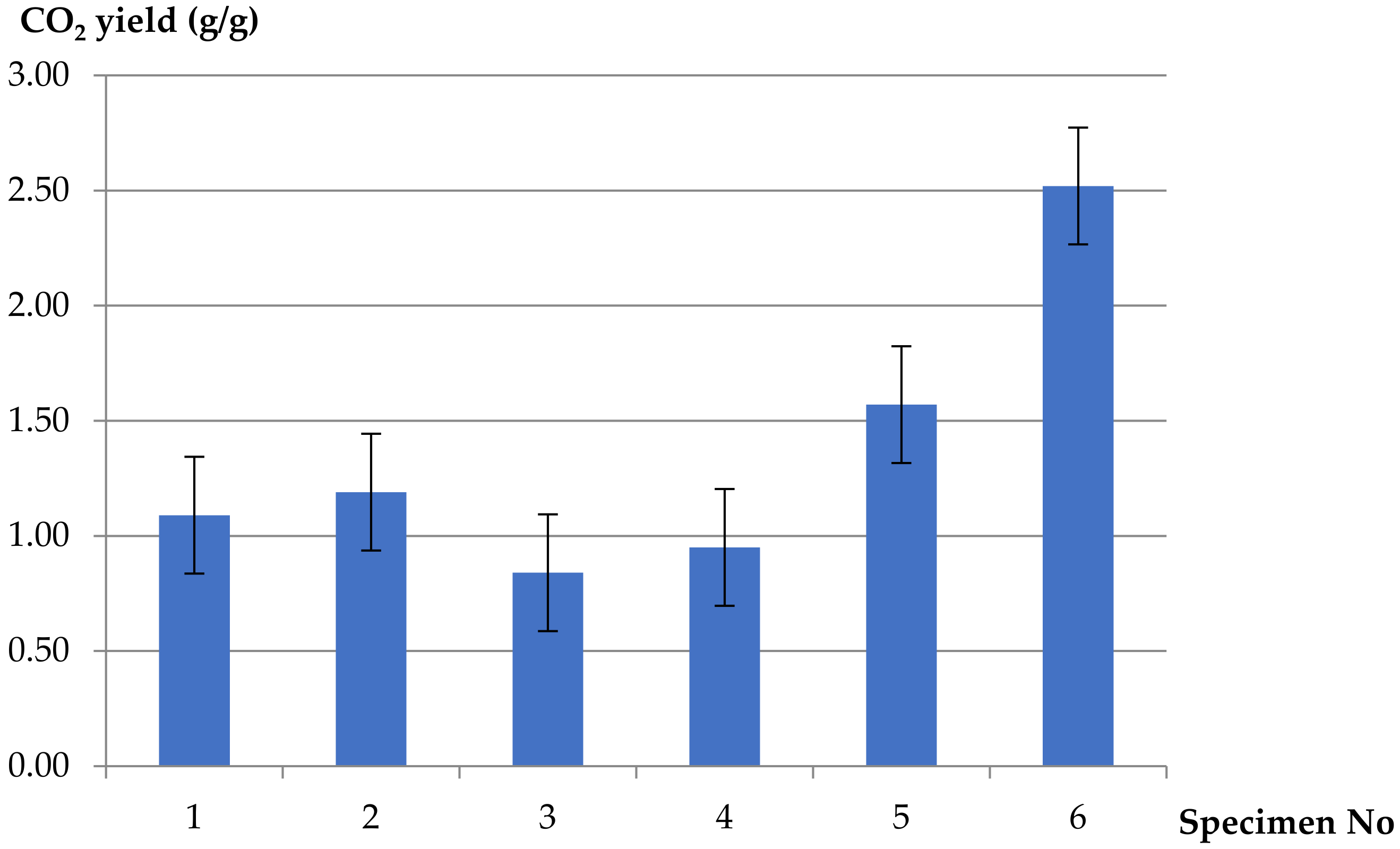
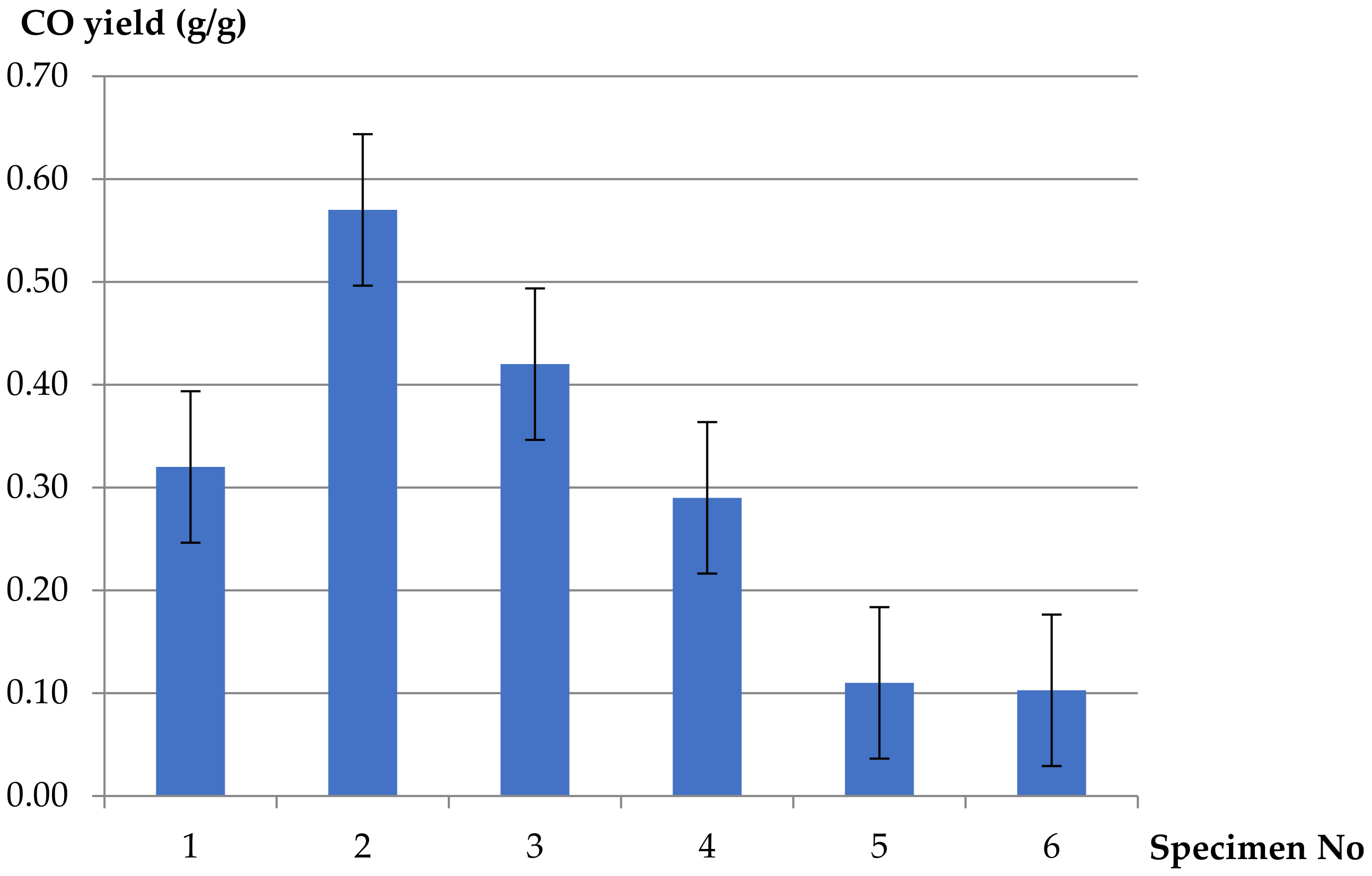
- Decreasing values of CO2 yields at different ventilation conditions were obtained for the PVC-insulated wire, compared to the reference sample of pure unplasticized PVC and additionally for pure LDPE. The values of the yields increase in well-ventilated conditions: threefoldin the case of pure LDPE and twofold for pure PVC. A different tendency was observed in the case of carbon monoxide. Increasing values of CO yields were obtained for the PVC-based electric copper wire in comparison to pure polymers. The maximum value of CO yield (0.57 g/g) was determined in the case of 5 L/min of primary airflow (ϕ = 0.42) and decreased with increasing ventilation. The minimum value of CO yield, equal to 0.29 g/g, was observed at higher ventilation conditions (ϕ = 0.27). This phenomenon confirms the significant contribution of the hyperventilation effect caused by CO2 inhalation during a cable fire.
- 3.
- In the case of light hydrocarbons (products of incomplete combustion), which are highly irritating to the skin and respiratory track, there was no clear tendency observed; in essence, the measured yields were similar to the reference values except for ϕ = 0.27, where the obtained hydrocarbon yield was equal to 0.45 g/g. The large amount of observed hydrocarbons in comparison with carbon monoxide in the case of ϕ = 0.27 might be caused by lots of smal-size volatile hydrocarbon species, while large-size hydrocarbon species create soot in the combustion zone.
- 4.
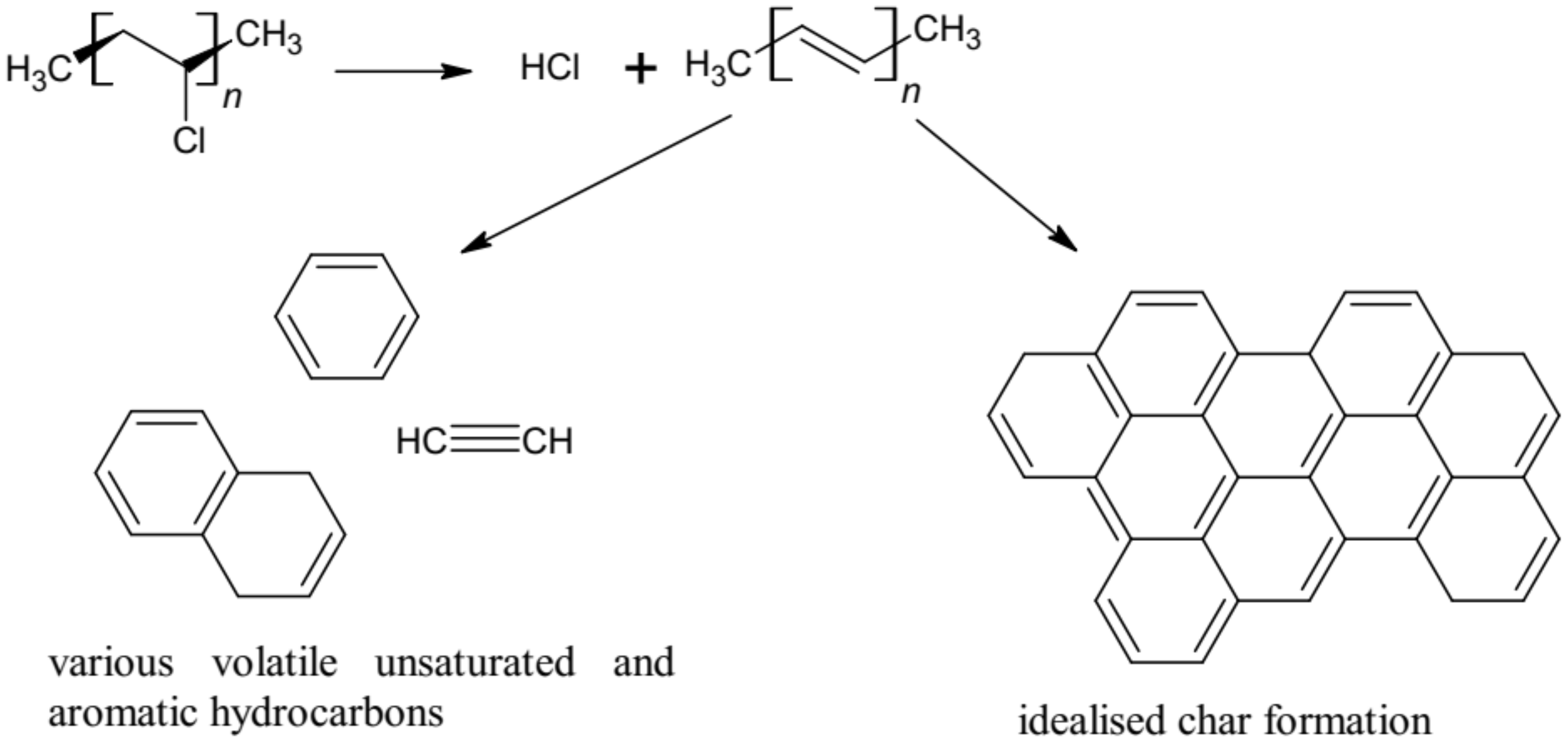
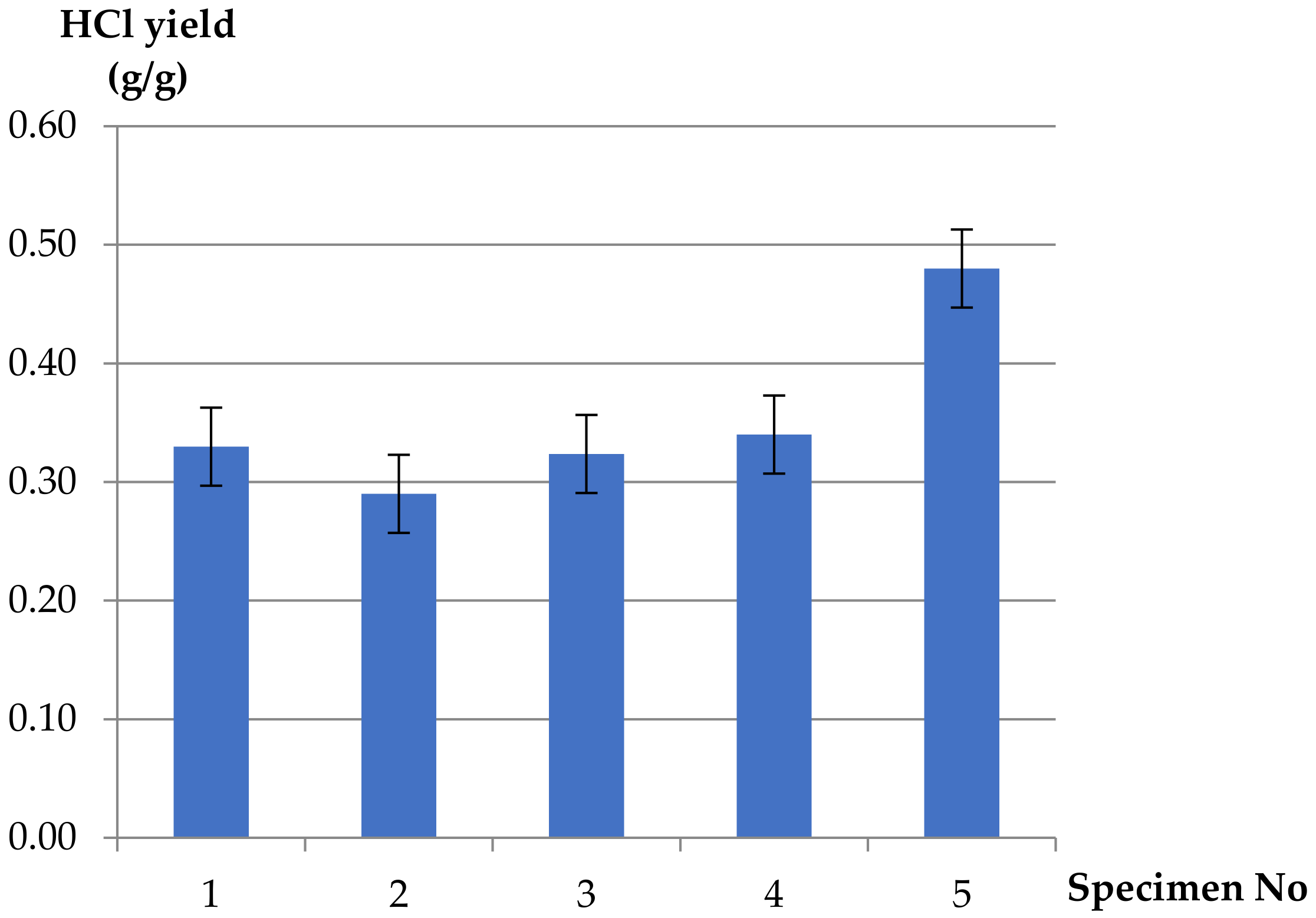
- The corrosive and toxic HCl occurring in fire effluents from the plasticized PVC-based electric copper wire was found to be independent of ventilation conditions. This is due to the composition of the cable, which contains copper wire and inorganic fillers acting as flame retardants. The reaction between copper and the HCl compound, as well as the flame-retardant mechanisms of the additives, caused lower values of HCl in fire effluents from the PVC-based electric copper wire as compared to pure unplasticized rigid PVC (about 1.5 times lower). High yields of HCl, resulting from the chain stripping of PVC, and of CO as an effect of the inhibition of the oxidation of CO by HCl demonstrate the increased toxicological significance of HCl and CO in PVC-based materials under fire conditions. The strong effect of HCl is particularly evident when incapacitation prevents escape during fires.
Author Contributions
Funding
Conflicts of Interest
References
- Fire Safety, Smoke Toxicity and Acidity. Available online: https://pdfs.semanticscholar.org/8a3c/0b2110638d8bb351105ca451efaf22f10031.pdf (accessed on 17 January 2020).
- Murakami, K.; Traber, D.L. Pathophysiological Basis of Smoke Inhalation Injury. News Physiol. Sci. 2003, 18, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Gann, R.G.; Babrauskas, V.; Grayson, S.J.; Marsh, N.D. Hazards of combustion products: Toxicity, opacity, corrosivity, and heat release: The experts’ views on capability and issues. Fire Mater. 2011, 35, 115–127. [Google Scholar] [CrossRef]
- Matthew, E.; Warden, G.; Dedman, J. A Murine Model of Smoke Inhalation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, 716–723. [Google Scholar] [CrossRef]
- Hull, T.R.; Stec, A.A.; Lebek, K.; Price, D. Factors Affecting the Combustion Toxicity of Polymeric Materials. Polym. Degrad. Stab. 2007, 92, 2239–2246. [Google Scholar] [CrossRef]
- Purser, D.A. Toxicity Assessment of Combustion Products. In SFPE Handbook of Fire Protection Engineering, 3rd ed.; National Fire Protection Association: Quincy, MA, USA, 2002; pp. 2–83. [Google Scholar]
- Tilley, S.K.; Fry, R.C. Priority Environmental Contaminants. In Systems Biology in Toxicology and Environmental Health; Academic Press: Boston, MA, USA, 2015; pp. 117–169. [Google Scholar]
- Hull, T.R.; Lebek, K.; Stec, A.A.; Paul, K.T.; Price, D. Bench-Scale Assessment of Fire Toxicity. In Advances in the Flame Retardancy of Polymeric Materials: Current Perspectives Presented at FRPM’05; Schartel, B., Ed.; Books on Demand GmbH: Norderstedt, Germany, 2007. [Google Scholar]
- Babrauskas, V. The Generation of CO in Bench-scale Fire Tests and the Prediction for Real-scale Fires. Fire Mater. 1995, 19, 205–213. [Google Scholar] [CrossRef]
- Stec, A.A.; Hull, T.R.; Lebek, K. Characterisation of Steady State Tube Furnace (ISO 19700) for Fire Toxicity Assessment. Polym. Degrad. Stab. 2008, 93, 2058–2065. [Google Scholar] [CrossRef] [Green Version]
- ISO. Guidelines for Assessing the Fire Threat to People; ISO/TS 19706; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Toxic Yield. Available online: https://tsapps.nist.gov/publication/get_pdf.cfm?pub_id=911816 (accessed on 17 January 2020).
- Kaplan, H.L.; Grand, A.F.; Switzer, W.G.; Mitchell, D.S.; Rogers, W.R.; Harzell, G.E. Effects of Combustion Gases on Escape Performance of the Baboon and the Rat. J. Fire Sci. 1985, 3, 228–244. [Google Scholar] [CrossRef]
- Kaczorek, K. Bench-Scale Fire Toxicity Measurements of Polymers and Cables. Master’s Thesis, University of Central Lancashire, Preston, UK, February 2009. [Google Scholar]
- Wang, Z.; Wang, J.; Richter, H.; Howard, J.B.; Carlson, J.; Levendis, Y.A. Comparative Study on Polycyclic Aromatic Hydrocarbons, Light Hydrocarbons, Carbon Monoxide, and Particulate Emissions from the Combustion of Polyethylene, Polystyrene, and Poly(vinyl chloride). Energy Fuels 2003, 17, 999–1013. [Google Scholar] [CrossRef]
- Kaczorek, K.; Stec, A.A.; Hull, T.R. Effect of Temperature and Ventilation Condition on the Combustion Efficiency of Halogenated and Aromatic Fuels. In Proceedings of the 4th FireSeat Symposium on Fire Safety Engineering, Edinburgh, UK, 10 November 2010; pp. 27–35. [Google Scholar]
- Hirschler, M.M. Poly(vinyl chloride) and its fire properties. Fire Mater. 2017, 41, 993–1006. [Google Scholar] [CrossRef]
- Di Blasi, C. The Burning of Plastics. In Plastics Flammability Handbook, 3rd ed.; Carl Hanser Verlag GmbH & Co. KG: Munich, Germany, 2004; pp. 47–132. [Google Scholar]
- Yasuhara, A.; Hiroyasu, I. Combustion Products of Poly(vinyl chloride). J. Environ. Chem. 1991, 1, 525–528. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.M.; Jiang, X.G.; Yan, J.H.; Chi, Y.; Cen, K.F. TG-FTIR analysis of PVC thermal degradation and HCl removal. J. Anal. Appl. Pyrolysis 2007, 82, 1–9. [Google Scholar] [CrossRef]
- NcNeill, I.C.; Memetea, L. Pyrolysis products of poly(vinyl chloride), dioctyl phthalate and their mixture. Polym. Degrad. Stab. 1994, 43, 9–25. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.C.; Wang, X.H.; He, J.J.; Wang, J. Pyrolysis and Combustion of Polyvinyl Chloride (PVC) Sheath for New and Aged Cables via Thermogravimetric Analysis-Fourier Transform Infrared (TG-FTIR) and Calorimeter. Materials 1997, 11. [Google Scholar] [CrossRef] [Green Version]
- Chong, N.S.; Abdulramoni, S.; Patterson, D.; Brown, H. Releases of Fire-Derived Contaminants from Polymer Pipes Made of Polyvinyl Chloride. Toxics 2019, 11, 57. [Google Scholar] [CrossRef] [Green Version]
- Kaczorek-Chrobak, K.; Fangrat, J. Influence of constructional-material parameters on the fire properties of electric cables. Energies 2019, 12, 4569. [Google Scholar] [CrossRef] [Green Version]
- Szlezyngier, W.; Brzozowski, Z.K. Tworzywa Sztuczne. Środki Pomocnicze i Specjalne Zastosowanie Polimerów. (Plastics. Additives and Special Applications of Polymers); Wydawnictwo Oświatowe FOSZE: Rzeszów, Poland, 2012; Volume 1, pp. 213–222. ISBN 978-83-7586-096-6. [Google Scholar]
- Hounsham, I.D.; Titow, W.V. Fillers in PVC. In PVC Technology; Springer: Dordrecht, The Netherlands, 1984; pp. 215–254. [Google Scholar] [CrossRef]
- Hirschler, M.M. Fire performance of poly(Vinyl Chloride)—Update and Recent Developments. In Proceedings of the Flame Retardants ’98, London, UK, 3–4 February 1998; Interscience Communications: London, UK, 1988; pp. 103–123. [Google Scholar]
- Yang, L.; Wang, Y.Y. Smoke suppressant and flame retardant properties of PVC/Zinc Hydroxystannate composites. Adv. Mat. Res. 2012, 512–515, 2804–2807. [Google Scholar] [CrossRef]
- Ning, Y.; Guo, S.Y. Flame-retardant and smoke-suppressant properties of zinc borate and aluminium trihydrate-filled rigid PVC. J. Appl. Polym. Sci. 2000, 77. [Google Scholar] [CrossRef]
- Purser, D.A.; Fardell, P.J.; Rowley, J.; Vollam, S.; Bridgeman, B. An improved tube furnace method for the generation and measurement of toxic combustion products under a wide range of fire conditions. In Proceedings of Flame Retardants ’94 Conference: London, UK; 27 January 1994; Interscience Communications Ltd.: London, UK, 1994; pp. 263–274. [Google Scholar]
- ISO. Controlled equivalence ratio method for the determination of hazardous components of fire effluents; ISO/TS 19700; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- Purser, J.A.; Purser, D.; Stec, A.A.; Moffat, C.; Hull, T.R.; Su, J.Z.; Bijloos, M.; Blomqvist, P. Repeatability and reproducibility of the ISO/TS 19700 steady state tube furnace. Fire Saf. J. 2013, 55, 22–34. [Google Scholar] [CrossRef]
- Stec, A.A.; Hull, T.R.; Lebek, K.; Purser, J.A.; Purser, D.A. The effect of temperature and ventilation condition on the toxic product yields from burning polymers. Fire Mater. 2007. [Google Scholar] [CrossRef] [Green Version]
- Kaczorek, K.; Stec, A.A.; Hull, T.R. Carbon Monoxide Generation in Fires: Effect of Temperature on Halogenated and Aromatic Fuels. Fire Saf. J. 2011, 10, 253–263. [Google Scholar] [CrossRef]
- Smoke Toxicity from Combustion Products Based on Polymers Containing Flame Retardant Additives. Available online: https://hal-ineris.archives-ouvertes.fr/ineris-00976169/document (accessed on 17 January 2020).
- Gann, R.G.; Bryner, N.P. Combustion products and their effects on life safety. In Fire Protection Handbook, 20th ed.; National Fire Protection Association: Quincy, MA, USA, 2008; pp. 6–27. [Google Scholar]
- Cullis, C.F.; Hirschler, M.M. The Combustion of Organic Polymers; Oxford University Press: Oxford, UK, 1981. [Google Scholar]
- Schnipper, A.; Smith-Hansen, L.; Thomsen, S.E. Reduced Combustion Efficiency of Chlorinated Compounds, Resulting in Higher Yields of Carbon Monoxide. Fire Mater. 1995, 19, 61–64. [Google Scholar] [CrossRef]
- Grimes, S.M.; Lateef, H.; Jafari, A.J.; Mehta, L. Studies of the effects of copper, copper(II) oxide and copper(II) chloride on the thermal degradation of poly(vinyl chloride). Polym. Degrad. Stab. 2006, 91, 3274–3280. [Google Scholar] [CrossRef]




| Specimen No | Specimen Description | ϕ, - | Primary Airflow, L/min |
|---|---|---|---|
| 1 | PVC wire | 0.82 | 2 |
| 2 | PVC wire | 0.42 | 5 |
| 3 | PVC wire | 0.37 | 10 |
| 4 | PVC wire | 0.27 | 15 |
| 5 | Pure PVC polymer | 0.04 | 10 |
| 6 | Pure LDPE polymer | 0.10 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczorek-Chrobak, K.; Fangrat, J. PVC-Based Copper Electric Wires under Various Fire Conditions: Toxicity of Fire Effluents. Materials 2020, 13, 1111. https://doi.org/10.3390/ma13051111
Kaczorek-Chrobak K, Fangrat J. PVC-Based Copper Electric Wires under Various Fire Conditions: Toxicity of Fire Effluents. Materials. 2020; 13(5):1111. https://doi.org/10.3390/ma13051111
Chicago/Turabian StyleKaczorek-Chrobak, Katarzyna, and Jadwiga Fangrat. 2020. "PVC-Based Copper Electric Wires under Various Fire Conditions: Toxicity of Fire Effluents" Materials 13, no. 5: 1111. https://doi.org/10.3390/ma13051111






