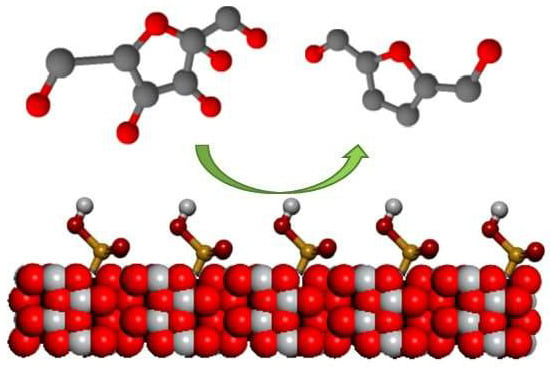
Dehydration of Fructose to 5-HMF over Acidic TiO2 Catalysts
Abstract
Share and Cite
Testa, M.L.; Miroddi, G.; Russo, M.; La Parola, V.; Marcì, G. Dehydration of Fructose to 5-HMF over Acidic TiO2 Catalysts. Materials 2020, 13, 1178. https://doi.org/10.3390/ma13051178
Testa ML, Miroddi G, Russo M, La Parola V, Marcì G. Dehydration of Fructose to 5-HMF over Acidic TiO2 Catalysts. Materials. 2020; 13(5):1178. https://doi.org/10.3390/ma13051178
Chicago/Turabian StyleTesta, Maria Luisa, Gianmarco Miroddi, Marco Russo, Valeria La Parola, and Giuseppe Marcì. 2020. "Dehydration of Fructose to 5-HMF over Acidic TiO2 Catalysts" Materials 13, no. 5: 1178. https://doi.org/10.3390/ma13051178
APA StyleTesta, M. L., Miroddi, G., Russo, M., La Parola, V., & Marcì, G. (2020). Dehydration of Fructose to 5-HMF over Acidic TiO2 Catalysts. Materials, 13(5), 1178. https://doi.org/10.3390/ma13051178