Biobased Materials from Microbial Biomass and Its Derivatives
Abstract
:1. Introduction
2. Fungal Biomass as Source of Materials
2.1. Yeast Biomass as Source of Biobased Materials
2.2. Yeast Cell Structure
2.3. Use of Saccharomyces Cerevisiae Cells as Film Forming Material
2.4. Use of Fungal β-Glucans as Film Forming Materials
2.5. Yeast Cells as an Ideal Carrier for Encapsulated Compounds
2.6. Mycelium Biobased Materials
2.7. Applications of Fungal Biomass
3. Kefir Grains as Source of Materials
3.1. Milk Kefir Grains and Water Kefir Grains
3.2. Milk Kefir Grains as Source of Biobased Materials: Kefiran-Based Materials
3.3. Composite Materials Made with Kefiran
3.4. Water Kefir Grains as Source of Biobased Materials: Dextran Based Materials
3.5. Applications of Kefiran and Dextran-Based Materials
4. Cellulose from Microbial Sources: Bacterial Cellulose
4.1. Acetic Acid Bacteria as Cellulose Cell Factory
4.2. Kombucha Tea Fermentation Produces Cellulose
4.3. Technological Applications of Microbial Cellulose
4.4. Agro-Industrial Residues as Sustainable Substrate for Cellulose Production
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakajima, H.; Dijkstra, P.; Loos, K. The recent developments in biobased polymers toward general and engineering applications: Polymers that are upgraded from biodegradable polymers, analogous to petroleum-derived polymers, and newly developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Domb, A.J.; Kost, J.; Wiseman, D.M. Handbook of Biodegradable Polymers; Harwood Academic Publishers: London, UK, 1997; ISBN 90-5702-153-6. [Google Scholar]
- Mohsenzadeh, A.; Zamani, A.; Taherzadeh, M.J. Bioethylene Production from Ethanol: A review and Techno-economical Evaluation. Chem. Bio Eng. Rev. 2017, 4, 75–91. [Google Scholar] [CrossRef] [Green Version]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sust. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Kobayashi, S.; Müllen, K. Encyclopedia of Polymeric Nanomaterials; Springer-Verlag: Berlin/Heidelberg, Germany, 2015; p. 2658. [Google Scholar]
- Leceta, I.; Etxabide, A.; Cabezudo, S.; De La Caba, K.; Guerrero, P. Bio-based films prepared with by-products and wastes: Environmental assessment. J. Clean. Prod. 2014, 64, 218–227. [Google Scholar] [CrossRef]
- Vijayendra, S.V.N.; Shamala, T.R. Film forming microbial biopolymers for commercial applications. A review. Crit. Rev. Biotechnol. 2014, 34, 338–357. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Burrola-Núñez, H.; Martínez-Encinas, E.G. Composite Materials Based on PLA and its Applications in Food Packaging. In Composites Materials for Food Packaging; Scrivener Publishing: Beverly, MA, USA, 2018; pp. 355–399. [Google Scholar]
- Lagarón, J.M. Polylactic acid (PLA) nanocomposites for food packaging applications. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Woodhead Publishing: Cambridge, UK, 2011; pp. 485–497. [Google Scholar]
- Arjmandi, R.; Hassan, A.; Zakaria, Z. Polylactic Acid Green Nanocomposites for Automotive Applications. In Green Biocomposites; Springer: Cham, Switzerland, 2017; pp. 193–208. [Google Scholar]
- Pawar, R.P.; Tekale, S.U.; Shisodia, S.U.; Totre, J.U.; Domb, A.J. Biomedical applications of poly (lactic acid). Recent Pat. Regen. Med. 2014, 4, 40–51. [Google Scholar] [CrossRef]
- Ranjbar, M.; Dehghan Noudeh, G.; Hashemipour, M.A.; Mohamadzadeh, I. A systematic study and effect of PLA/Al2O3 nanoscaffolds as dental resins: Mechanochemical properties. Artif. Cells Nanomed. Biotechnol. 2019, 47, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Getachew, A.; Woldesenbet, F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res. Notes 2016, 9, 509. [Google Scholar] [CrossRef] [Green Version]
- Hottel, T.; Bilec, M.; Landis, A. Sustainability assessments of bio-based polymers. Polym. Degrad. Stabil. 2013, 98, 1898–1907. [Google Scholar] [CrossRef]
- Bos, H.L.; Meesters, K.P.; Conijn, S.G.; Corré, W.J.; Patel, M.K. Accounting for the constrained availability of land: A comparison of bio-based ethanol, polyethylene, and PLA with regard to non-renewable energy use and land use. Biofuels Bioprod. Biorefin. 2012, 6, 146–158. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Shoda, M.; Sugano, Y. Recent advances in bacterial cellulose production. Biotechnol. Bioproc. E 2005, 10, 1. [Google Scholar] [CrossRef]
- Rani, M.U.; Appaiah, A. Optimization of culture conditions for bacterial cellulose production from Gluconacetobacter hansenii UAC09. Ann. Microbiol. 2011, 61, 781–787. [Google Scholar] [CrossRef]
- Coma, M.E.; Peltzer, M.A.; Delgado, J.F.; Salvay, A.G. Water kefir grains as an innovative source of materials: Study of plasticiser content on film properties. Eur. Polym. 2019, 120, 109234. [Google Scholar] [CrossRef]
- Zajšek, K.; Kolar, M.; Goršek, A. Characterisation of the exopolysaccharide kefiran produced by lactic acid bacteria entrapped within natural kefir grains. Int. J. Dairy Technol. 2011, 64, 544–548. [Google Scholar] [CrossRef]
- Göksungur, Y.; Uzunoğulları, P.; Dağbağlı, S. Optimization of pullulan production from hydrolysed potato starch waste by response surface methodology. Carbohydr. Polym. 2011, 83, 1330–1337. [Google Scholar] [CrossRef]
- Han, Y.W.; Watson, M.A. Production of microbial levan from sucrose, sugarcane juice and beet molasses. J. Ind. Microbiol. 1992, 9, 257–260. [Google Scholar] [CrossRef]
- Patel, S.; Majumder, A.; Goyal, A. Potentials of exopolysaccharides from lactic acid bacteria. Indian J. Microbiol. 2012, 52, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Zhu, S.; Li, C.; Zhang, C.; Ji, Y. Cost-effective optimization of gellan gum production by Sphingomonas paucimobilis using corn steep liquor. Prep. Biochem. Biotech. 2020, 50, 1–7. [Google Scholar] [CrossRef]
- Peltzer, M.; Delgado, J.F.; Salvay, A.G.; Wagner, J.R. β-glucan, a promising polysaccharide for bio-based films developments for food contact materials and medical applications. Curr. Org. Chem. 2018, 22, 1249–1254. [Google Scholar] [CrossRef]
- Kyanko, M.V.; Canel, R.S.; Ludemann, V.; Pose, G.; Wagner, J.R. β-Glucan content and hydration properties of filamentous fungi. Appl. Biochem. Microbiol. 2013, 49, 41–45. [Google Scholar] [CrossRef]
- Cerimi, K.; Akkaya, K.C.; Pohl, C.; Schmidt, B.; Neubauer, P. Fungi as source for new bio-based materials: A patent review. Fungal Biol. Biotechnol. 2019, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Nelson, G.; Duckham, S.C.; Crothers, M.E.D. Microencapsulation in yeast cells and applications in drug delivery. In Polymeric Drug Delivery I: Particulate Drug Carriers; American Chemical Society: Washington, DC, USA, 2006; pp. 268–281. ISBN 978-0841239180. [Google Scholar]
- Delgado, J.F.; Peltzer, M.A.; Salvay, A.G.; de la Osa, O.; Wagner, J.R. Characterization of thermal, mechanical and hydration properties of novel films based on Saccharomyces cerevisiae biomass. Innov. Food Sci. Emerg. Technol. 2018, 48, 240–247. [Google Scholar] [CrossRef]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; de la Osa, O.; Wagner, J.R. Use of Residual Yeast Cell Wall for New Biobased Materials Production: Effect of Plasticization on Film Properties. Food Bioproc. Tech. 2018, 11, 1995–2007. [Google Scholar] [CrossRef]
- Meyer, V.; Andersen, M.R.; Brakhage, A.A.; Braus, G.H.; Caddick, M.X.; Cairns, T.C.; Krappmann, S. Current challenges of research on filamentous fungi in relation to human welfare and a sustainable bio-economy: A white paper. Fungal Biol. Biotechnol. 2016, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Choque, E.; El Rayess, Y.; Raynal, J.; Mathieu, F. Fungal naphtho-γ-pyrones secondary metabolites of industrial interest. Appl. Microbiol. Biotechnol. 2015, 99, 1081–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López de León, L.; Caceres, I.; Bornot, J.; Choque, E.; Raynal, J.; Taillandier, P.; Mathieu, F. Influence of Culture Conditions on Production of NGPs by Aspergillus tubingensis. J. Microbiol. Biotechnol. 2019, 29, 1412–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santhosh, B.S.; Bhavana, D.R.; Rakesh, M.G. Mycelium composites: An emerging green building material. Int. Res. J. Eng. Technol. 2018, 5, 3066–3068. [Google Scholar]
- Sun, W.; Tajvidi, M.; Hunt, C.G.; McIntyre, G.; Gardner, D.J. Fully Bio-Based Hybrid Composites Made of Wood, Fungal Mycelium and Cellulose Nanofibrils. Sci. Rep. 2019, 9, 3766. [Google Scholar] [CrossRef]
- Kadimaliev, D.; Kezina, E.; Telyatnik, V.; Revin, V.; Parchaykina, O.; Syusin, I. Residual Brewer’s Yeast Biomass and Bacterial Cellulose as an Alternative to Toxic Phenol-Formaldehyde Binders in Production of Pressed Materials from Waste Wood. BioResources 2015, 10, 1644–1656. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, L.H.S.; Peixoto-Nogueira, S.C.; Michelin, M.; Rizzatti, A.C.S.; Sandrim, V.C.; Zanoelo, F.F.; Polizeli, M.D.L. Screening of filamentous fungi for production of enzymes of biotechnological interest. Braz. J. Microbiol. 2006, 37, 474–480. [Google Scholar] [CrossRef]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant Sci. 2015, 5, 47–56. [Google Scholar]
- Türker, M. Yeast Biotechnology: Diversity and Applications. Advances in Science and Industrial Productions Of Baker’s Yeast. In Proceedings of the 27th VH Yeast Conference, Istanbul, Turkey, 14–15 April 2014. [Google Scholar]
- Żymańczyk-Duda, E.; Brzezińska-Rodak, M.; Klimek-Ochab, M.; Duda, M.; Zerka, A. Yeast as a Versatile Tool in Biotechnology. Yeast Ind. Appl. 2017, 1, 3–40. [Google Scholar]
- Pérez-Torrado, R.; Gamero, E.; Gómez-Pastor, R.; Garre, E.; Aranda, A.; Matallana, E. Yeast biomass, an optimised product with myriad applications in the food industry. Trends Food Sci. Technol. 2015, 46, 167–175. [Google Scholar] [CrossRef]
- Peris, D.; Pérez-Torrado, R.; Hittinger, C.T.; Barrio, E.; Querol, A. On The Origins and Industrial Applications of Saccharomyces cerevisiae and Saccharomyces kudriavzevii Hybrids. Yeast 2017, 35, 51–69. [Google Scholar] [CrossRef] [Green Version]
- Wagner, J.R.; Rambla, O.; Miguel, A.; Legarreta, G. Las Levaduras y sus Productos Derivados como Ingredientes en la Industria de Alimentos, 1st ed.; Editorial de la Universidad Nacional de Quilmes: Bernal, Argentina, 2008. [Google Scholar]
- Santipanichwong, R.; Suphantharika, M. Influence of different β-glucans on the physical and rheological properties of egg yolk stabilized oil-in-water emulsions. Food Hydrocoll. 2009, 23, 1279–1287. [Google Scholar] [CrossRef]
- Caridi, A. Enological functions of parietal yeast mannoproteins. Antonie Van Leeuwenhoek 2006, 89, 417–422. [Google Scholar] [CrossRef]
- Klis, F.M.; Boorsma, A.; De Groot, P.W. Cell wall construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef]
- Delgado, J.; Sceni, P.; Peltzer, M.A.; Salvay, A.G.; de la Osa, O.; Wagner, J.R. Development of Innovative Biodegradable Films Based on Biomass Of Saccharomyces cerevisiae. Innov. Food Sci. Emerg. Technol. 2016, 36, 83–91. [Google Scholar] [CrossRef]
- Van der Rest, M.E.; Kamminga, A.H.; Nakano, A.; Anraku, Y.; Poolman, B.; Konings, W.N. The plasma membrane of Saccharomyces cerevisiae: Structure, function, and biogenesis. Microbiol. Mol. Biol. Rev. 1995, 59, 304–322. [Google Scholar] [CrossRef]
- Kozlowski, L.P. Proteome-pI: Proteome isoelectric point database. Nucleic Acids Res. 2016, 45, D1112–D1116. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, P.; Retegi, A.; Gabilondo, N.; De la Caba, K. Mechanical and thermal properties of soy protein films processed by casting and compression. J. Food Eng. 2010, 100, 145–151. [Google Scholar] [CrossRef]
- Novák, M.; Synytsya, A.; Gedeon, O.; Slepička, P.; Procházka, V.; Synytsya, A.; Čopíková, J. Yeast β(1-3),(1-6)-d-glucan films: Preparation and characterization of some structural and physical properties. Carbohydr. Polym. 2012, 87, 2496–2504. [Google Scholar] [CrossRef]
- Roca, C.; Chagas, B.; Farinha, I.; Freitas, F.; Mafra, L.; Aguiar, F.; Oliveira, R.; Reis, M.A.M. Production of yeast chitin–glucan complex from biodiesel industry byproduct. Process Biochem. 2012, 47, 1670–1675. [Google Scholar] [CrossRef]
- Rudnik, E.; Matuschek, G.; Milanov, N.; Kettrup, A. Thermal stability and degradation of starch derivatives. J. Therm. Anal. Calorim. 2006, 85, 267–270. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Wagner, J.R.; Salvay, A.G. Hydration and Water Vapour Transport Properties in Yeast Biomass Based Films: A Study of Plasticizer content and thickness effects. Eur. Polym. J. 2018, 99, 9–17. [Google Scholar] [CrossRef]
- Kogan, G.; Pajtinka, M.; Babincova, M.; Rauko, P.; Slamenova, D.; Korolenko, T.A. Yeast Cell Wall Polysaccha.; rides as Antioxidants and Antimutagens: Can They Fight Cancer? Neoplasma 2008, 55, 387–393. [Google Scholar]
- Pizarro, S.; Ronco, A.M.; Gotteland, M. β-glucanos: ¿Qué Tipos Existen y Cuáles Son Sus Beneficios en la Salud? Rev. Chil. Nutr. 2014, 3, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Paramera, E.I.; Karathanos, V.T.; Konteles, S.J. Yeast cells and yeast-based materials for microencapsulation. In Microencapsulation in the Food Industry; Gaonkar, A., Vasisht, N., Khare, A., Sobel, R., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 267–281. [Google Scholar]
- Mokhtari, S.; Jafari, S.M.; Khomeiri, M. The Cell Wall Compound of Saccharomyces cerevisiae as a Novel Wall Material for Encapsulation of Probiotics. Food Res. Int. 2017, 96, 19–26. [Google Scholar] [CrossRef]
- Salari, R.; Bazzaz, B.S.; Rajabi, O.; Khashyarmanesh, Z. New Aspects of Saccharomyces cerevisiae as a Novel Carrier for Berberine. DARU. J. Pharm. Sci. 2013, 21, 73. [Google Scholar]
- Normand, V.; Dardelle, G.; Bouquerand, P.-E.; Nicolas, L.; Johnston, D.J. Flavour Encapsulation in Yeast: Limonene Used as a Model System for Characterization of the Release Mechanism. J. Agr. Food Chem. 2005, 53, 7532–7543. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L. The magnitude of fungal diversity: The 1.5 million species estimate revisited. Mycol. Res. 2001, 105, 1422–1432. [Google Scholar] [CrossRef] [Green Version]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1. [Google Scholar] [CrossRef] [Green Version]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced materials from fungal mycelium: Fabrication and tuning of physical properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef]
- Martinez, E.; Salvay, A.G.; Ludemann, V.; Peltzer, M.A. In Proceedings of the XVI Congress CYTAL 2017. Argentinean Congress in Food Science and Technology, Mar del Plata, Argentina, 18–20 September 2017; ISBN 978-987-22165-8-0.
- Sabu, C.; Mufeedha, P.; Pramod, K. Yeast-inspired drug delivery: Biotechnology meets bioengineering and synthetic biology. Expert Op. Drug Del. 2019, 16, 27–41. [Google Scholar] [CrossRef]
- Kofuji, K.; Huang, Y.; Tsubaki, K.; Kokido, F.; Nishikawa, K.; Isobe, T.; Murata, Y. Preparation and evaluation of a novel wound dressing sheet comprised of β-glucan–chitosan complex. React. Funct. Polym. 2010, 70, 784–789. [Google Scholar] [CrossRef]
- Zeller, P.; Zocher, D. Ecovative’s breakthrough biomaterials. Fungi Mag. 2012, 5, 51–56. [Google Scholar]
- Girometta, C.; Picco, A.M.; Baiguera, R.M.; Dondi, D.; Babbini, S.; Cartabia, M.; Savino, E. Physico-mechanical and thermodynamic properties of mycelium-based biocomposites: A review. Sustainability 2019, 11, 281. [Google Scholar] [CrossRef] [Green Version]
- Radhouani, H.; Gonçalves, C.; Maia, F.R.; Oliveira, J.M.; Reis, R.L. Biological performance of a promising kefiran-biopolymer with potential in regenerative medicine applications: A comparative study with hyaluronic acid. J. Mater. Sci. Mater. Med. 2018, 29, 124. [Google Scholar] [CrossRef]
- Pidoux, M. The microbial flora of sugary kefir grain (the gingerbeer plant): Bio-synthesis of the grain from Lactobacillus hilgardii producing a polysaccharide gel. MIRCEN Appl. Microbiol. Biotechol. 1989, 5, 223–238. [Google Scholar] [CrossRef]
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Chemical and microbiological characterisation of kefir grains. J. Dairy Res. 2001, 68, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.S.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk kefir: Composition, microbial cultures, biological activities, and related products. Front. Microbiol. 2015, 6, 1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado Prado, M.R.; Boller, C.; Guetter Mello Zibetti, R.; de Souza, D.; Lopes Pedroso, L.; Soccol, C.R. Anti-inflammatory and angiogenic activity of polysaccharide extract obtained from Tibetan kefir. Microvasc. Res. 2016, 108, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Laureys, D.; De Vuyst, L. Microbial species diversity, community dynamics, and metabolite kinetics of water kefir fermentation. Appl. Environ. Microbiol. 2014, 80, 2564–2572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moinas, M.; Horisberger, M.; Bauer, H. The structural organization of the Tibi grain as revealed by light, scanning and transmission microscopy. Arch. Microb. 1980, 128, 157–161. [Google Scholar] [CrossRef]
- Fels, L.; Jakob, F.; Vogel, R.F.; Wefers, D. Structural characterization of the exopolysaccharides from water kefir. Carbohydr. Polym. 2018, 189, 296–303. [Google Scholar] [CrossRef]
- Galli, A.; Fiori, E.; Franzetti, L.; Pagani, M.A.; Ottogalli, G. Microbiological and chemical composition of sugar kefir grains. Ann. Microbiol. Enzimol. 1995, 45, 85–95. [Google Scholar]
- Gulitz, A.; Stadie, J.; Wenning, M.; Ehrmann, M.A.; Vogel, R.F. The microbial diversity of water kefir. Int. J. Food Microbiol. 2011, 151, 284–288. [Google Scholar] [CrossRef]
- Mali, S.; Sakanaka, L.S.; Yamashita, F.; Grossmann, M.V.E. Water Sorption and Mechanical Properties of Cassava Starch Films and Their Relation to Plasticizing Effect. Carbohydr. Polym. 2005, 60, 283–289. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Rheological and structural characterisation of film-forming solutions and biodegradable edible film made from kefiran as affected by various plasticizer types. Int. J. Biol. Macromol. 2011, 49, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Piermaria, J.; Pinotti, A.; García, M.A.; Abraham, A.G. Films based on kefiran, an exopolysaccharide obtained from kefir grains: Development and characterization. Food Hydrocoll. 2009, 23, 684–690. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A.; Yarmand, M.S. Development and characterisation of a new biodegradable edible film made from kefiran, an exopolysaccharide obtained from kefir grains. Food Chem. 2011, 127, 1496–1502. [Google Scholar] [CrossRef]
- Rimada, P.S.; Abraham, A.G. Polysaccharide production by kefir grains during whey fermentation. J. Dairy Res. 2001, 68, 653–661. [Google Scholar] [CrossRef]
- Piermaria, J.; Bosch, A.; Pinotti, A.; Yantorno, O.; Garcia, M.A.; Abraham, A.G. Kefiran films plasticized with sugars and polyols: Water vapor barrier and mechanical properties in relation to their microstructure analyzed by ATR/FT-IR spectroscopy. Food Hydrocoll. 2011, 25, 1261–1269. [Google Scholar] [CrossRef]
- Couchman, P.R.; Karaz, F.E. A classical thermodynamic discussion of the effect of composition on glass-transition temperature. Macromolecules 1978, 11, 177–188. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Physical, mechanical, barrier, and thermal properties of polyol-plasticized biodegradable edible film made from kefiran. Carbohydr. Polym. 2011, 84, 477–483. [Google Scholar] [CrossRef]
- Jangchud, A.; Chinnan, M.S. Properties of peanut protein film: Sorption isotherm and plasticizer effect. LWT Food Sci. Technol. 1999, 32, 89–94. [Google Scholar] [CrossRef]
- Piermaria, J.; Diosma, G.; Aquino, C.; Garrote, G.; Abraham, A. Edible kefiran films as vehicle for probiotic microorganisms. Innov. Food Sci. Emerg. Technol. 2015, 32, 193–199. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A.; Yarmand, M.S. Characterization of edible emulsified films with low affinity to water based on kefiran and oleic acid. Int. J. Biol. Macromol. 2011, 49, 378–384. [Google Scholar] [CrossRef]
- Motedayen, A.A.; Khodaiyan, F.; Salehi, E.A. Development and characterisation of composite films made of kefiran and starch. Food Chem. 2013, 136, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Babaei-Ghazvini, A.; Shahabi-Ghahfarrokhi, I.; Goudarzi, V. Preparation of UV-protective starch/kefiran/ZnO nanocomposite as a packaging film: Characterization. Food Packag. Shelf Life 2018, 16, 103–111. [Google Scholar] [CrossRef]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. The improvement of characteristics of biodegradable films made from kefiran-whey protein by nanoparticle incorporation. Carbohydr. Polym. 2014, 109, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, M.A.; Castro Vidaurre, E.F.; Armada, M.; Gottifredi, J.C. Water vapor permeability of edible starch based films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Smith, S.A. Polyethylene, low density. In The Wiley Encyclopedia of Packaging Technology; Bakker, M., Ed.; John Wiley & Sons: New York, NY, USA, 1986; pp. 514–523. [Google Scholar]
- Radhouani, H.; Bicho, D.; Gonçalves, C.; Maia, F.R.; Reis, R.L.; Oliveira, J.M. Kefiran cryogels as potential scaffolds for drug delivery and tissue engineering applications. Mater. Today Commun. 2019, 20, 100554. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Mokhena, T.C.; John, M.J. Cellulose Nanomaterials: New Generation Materials for Solving Global Issues; Springer: Dordrecht, The Netherlands, 2019; Volume 0123456789, ISBN 1057001902. [Google Scholar]
- Brown, A.J. Brown on acetic ferment which forms cellulose. J. Chem. Soc. Trans. 1886, 49, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Prescott, S.C.; Dunn, C.G. Industrial Microbiology, 2nd ed.; McGraw-Hill: Madrid, Spain, 1940. [Google Scholar]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Dima, S.O.; Panaitescu, D.M.; Orban, C.; Ghiurea, M.; Doncea, S.M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.A.; Trica, B.; et al. Bacterial nanocellulose from side-streams of kombucha beverages production: Preparation and physical-chemical properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Gullo, M.; La China, S.; Falcone, P.M.; Giudici, P. Biotechnological production of cellulose by acetic acid bacteria: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 6885–6898. [Google Scholar] [CrossRef]
- Rozenberga, L.; Skute, M.; Belkova, L.; Sable, I.; Vikele, L.; Semjonovs, P.; Saka, M.; Ruklisha, M.; Paegle, L. Characterisation of films and nanopaper obtained from cellulose synthesised by acetic acid bacteria. Carbohydr. Polym. 2016, 144, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Mamlouk, D.; Gullo, M. Acetic Acid Bacteria: Physiology and Carbon Sources Oxidation. Indian J. Microbiol. 2013, 53, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshk, S.M. Bacterial Cellulose Production and its Industrial Applications. J. Bioprocess. Biotech. 2014, 4, 1–10. [Google Scholar] [CrossRef]
- Dourado, F.; Ryngajllo, M.; Bielecki, S.; Gama, M. Taxonomic review and microbial ecology in bacterial nanocellulose Fermentation. In Bacterial Nanocellulose; Gama, M., Dourado, F., Bielecki, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–17. [Google Scholar]
- Gullo, M.; Sola, A.; Zanichelli, G.; Montorsi, M.; Messori, M.; Giudici, P. Increased production of bacterial cellulose as starting point for scaled-up applications. Appl. Microbiol. Biotechnol. 2017, 101, 8115–8127. [Google Scholar] [CrossRef] [PubMed]
- Ryngajłło, M.; Kubiak, K.; Jędrzejczak-Krzepkowska, M.; Jacek, P.; Bielecki, S. Comparative genomics of the Komagataeibacter strains—Efficient bionanocellulose producers. Microbiologyopen 2018, 8, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhsa, P.; Narain, R.; Manuspiya, H. Physical structure variations of bacterial cellulose produced by different Komagataeibacter xylinus strains and carbon sources in static and agitated conditions. Cellulose 2018, 25, 1571–1581. [Google Scholar] [CrossRef]
- Semjonovs, P.; Ruklisha, M.; Paegle, L.; Saka, M.; Treimane, R.; Skute, M.; Rozenberga, L.; Vikele, L.; Sabovics, M.; Cleenwerck, I. Cellulose synthesis by Komagataeibacter rhaeticus strain P 1463 isolated from Kombucha. Appl. Microbiol. Biotechnol. 2016, 101, 1003–1012. [Google Scholar] [CrossRef]
- Molina-Ramírez, C.; Castro, M.; Osorio, M.; Torres-Taborda, M.; Gómez, B.; Zuluaga, R.; Gómez, C.; Gañán, P.; Rojas, O.J.; Castro, C. Effect of different carbon sources on bacterial nanocellulose production and structure using the low pH resistant strain Komagataeibacter medellinensis. Materials 2017, 10, 639. [Google Scholar] [CrossRef]
- Prudnikova, S.V.; Shidlovsky, I.P. The New Strain of Acetic Acid Bacteria Komagataeibacter xylinus B-12068—Producer of Bacterial Cellulose for Biomedical Applications. J. Sib. Fed. Univ. Biol. 2017, 10, 246–254. [Google Scholar] [CrossRef]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2016, 23, 57–91. [Google Scholar] [CrossRef]
- Gama, F.M.; Gatenholm, P.; Klemm, D. Bacterial Nanocellulose: A Sophisticated Multifunctional Material; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781439869925. [Google Scholar]
- De Filippis, F.; Troise, A.D.; Vitaglione, P.; Ercolini, D. Different temperatures select distinctive acetic acid bacteria species and promotes organic acids production during Kombucha tea fermentation. Food Microbiol. 2018, 73, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, J.; Cal, T.; Bennett, J.W. The Kombucha consortia of yeasts and bacteria. Mycologist 2000, 14, 166–170. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Fu, C.; Yan, F.; Cao, Z.; Xie, F.; Lin, J. Antioxidant activities of kombucha prepared from three different substrates and changes in content of probiotics during storage. Food Sci. Technol. 2014, 34, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Sievers, M.; Lanini, C.; Weber, A.; Schuler-Schmid, U.; Teuber, M. Microbiology and Fermentation Balance in a Kombucha Beverage Obtained from a Tea Fungus Fermentation. Syst. Appl. Microbiol. 1995, 18, 590–594. [Google Scholar] [CrossRef]
- AL-Kalifawi, E.; Hassan, I. Factors Influence on the yield of Bacterial Cellulose of Kombucha (Khubdat Humza). Baghdad Sci. J. 2014, 11, 1420–1428. [Google Scholar]
- Goh, W.N.; Rosma, A.; Kaur, B.; Fazilah, A.; Karim, A.A.; Bhat, R. Fermentation of black tea broth (kombucha): I. effects of sucrose concentration and fermentation time on the yield of microbial cellulose. Int. Food Res. J. 2012, 19, 109–117. [Google Scholar]
- Ramírez-Tapias, Y.A.; Di Monte, M.V.; Delgado, J.F.; Peltzer, M.A.; Salvay, A.G. Producción y caracterización de películas obtenidas a partir del subproducto de la bebida de Kombucha. In Proceedings of the XIII Simposio Argentino de Polímeros, Buenos Aires, Argentina, 9–12th October 2019. [Google Scholar]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Padrão, J.; Gonçalves, S.; Silva, J.P.; Sencadas, V.; Lanceros-Méndez, S.; Pinheiro, A.C.; Vicente, A.A.; Rodrigues, L.R.; Dourado, F. Bacterial cellulose-lactoferrin as an antimicrobial edible packaging. Food Hydrocoll. 2016, 58, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Gorgieva, S.; Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Olyveira, G.M.; Costa, L.M.M.; Riccardi, C.D.S.; Dos Santos, M.L.; Daltro, P.B.; Basmaji, P.; Daltro, G.D.C.; Guastaldi, A.C. Bacterial cellulose for advanced medical materials. In Nanobiomaterials in Soft Tissue Engineering: Applications of Nanobiomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–82. ISBN 9780323428651. [Google Scholar]
- Mohite, B.V.; Koli, S.H.; Patil, S.V. Bacterial Cellulose-Based Hydrogels: Synthesis, Properties, and Applications; Springer: Cham, Switzerland, 2019; pp. 1255–1276. [Google Scholar]
- Cerrutti, P.; Roldán, P.; García, R.M.; Galvagno, M.A.; Vázquez, A.; Foresti, M.L. Production of bacterial nanocellulose from wine industry residues: Importance of fermentation time on pellicle characteristics. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Han, Y.H.; Mao, H.L.; Wang, S.S.; Deng, J.C.; Chen, D.L.; Li, M. Ecofriendly green biosynthesis of bacterial cellulose by Komagataeibacter xylinus B2-1 using the shell extract of Sapindus mukorossi Gaertn. as culture medium. Cellulose 2019, 1, 1255–1272. [Google Scholar] [CrossRef]


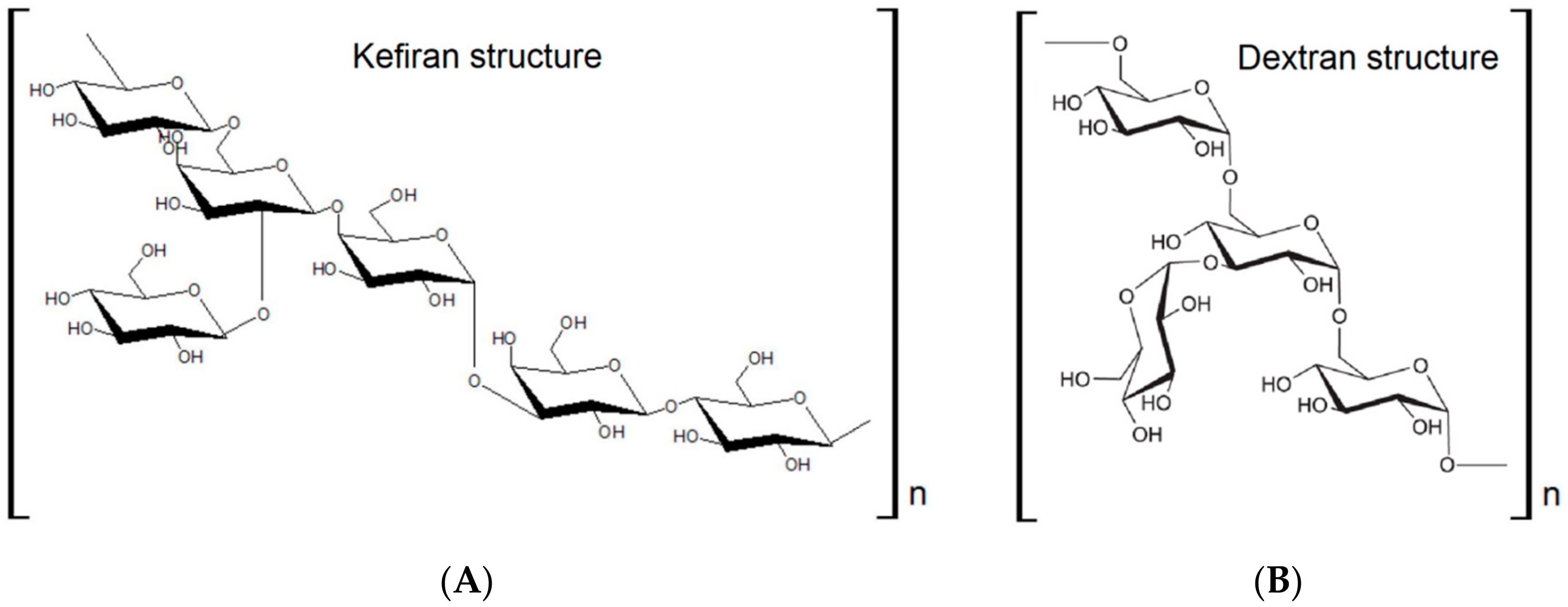



| Fungal Biomass | Applications | Details | References |
|---|---|---|---|
| Yeast Biomass | Encapsulation of bioactive compounds | Encapsulation of volatile molecules as flavors to guarantee permanence during the industrial process and probiotics in order to optimize its viability. | [59,61] |
| Drug delivery system | Yeast microcapsule used in the delivery of charged nanoparticles: quantum dots, gallium nanoparticles, and various fluorescent nanoparticles | [66] | |
| Wound dressing sheets | Yeast β-glucan for development of dressing sheets for wound healing (tested on mouse skin) | [67] | |
| Mycelium Biomass | Automotive applications | Replace foams in bumpers, doors, roofs, engine bays, etc. | [28,68] |
| Packaging materials | Packaging materials that are environmentally responsible, food wrapping. Alternative to traditional polystyrene and polyurethane | [69] | |
| Electrical circuit boards | Sheet of mycelium containing metal salts of CuSO4, CuCl2, or Al2O2 | [28] | |
| Construction and Building Materials | Insulation, structural insulating panels (SIPs), acoustical tiles | [28,68] | |
| Textile and paper industry | Potential use of fungal pulp in the production of textiles | [28] | |
| Home and garden | Containers, garden planters, wine shippers, candle holders | [68] |
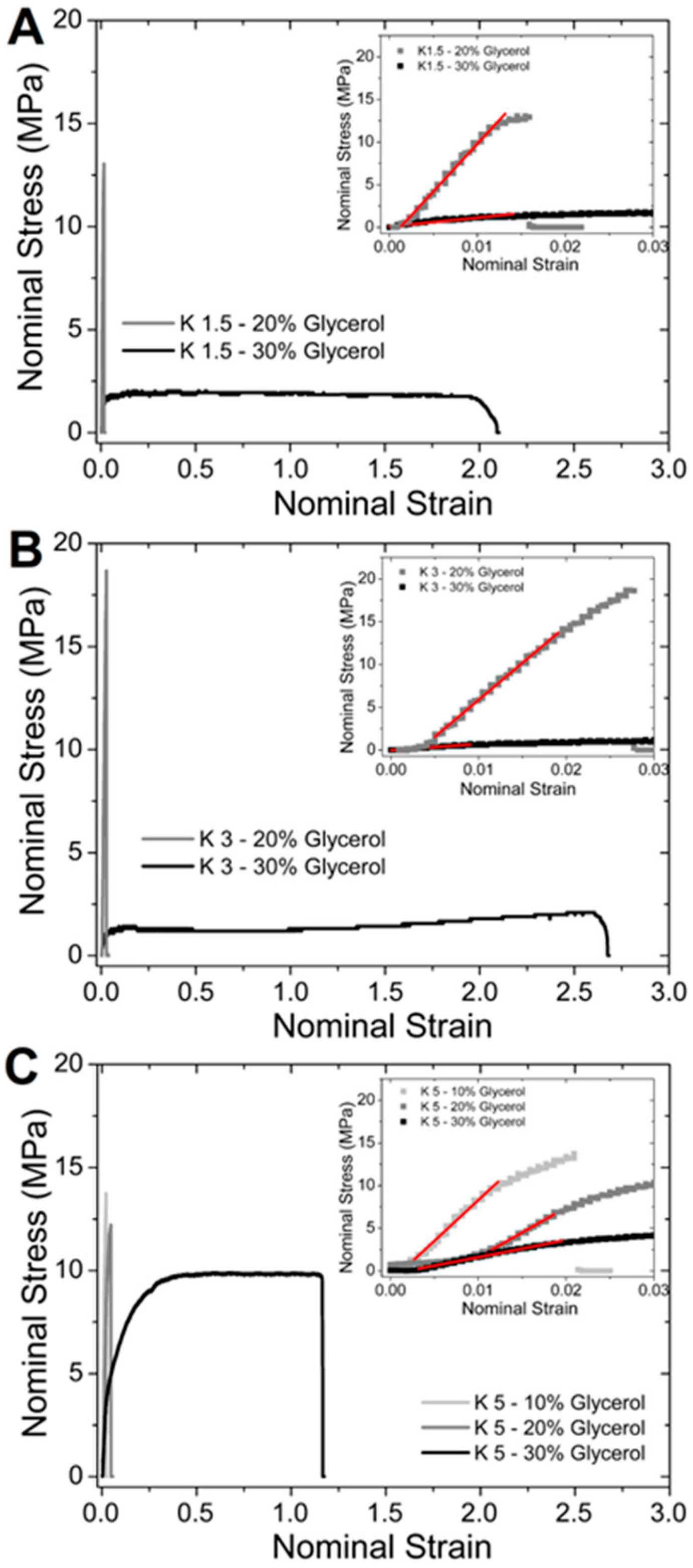
| Formulation | % wt Glycerol | YM [MPa] | TS [MPa] | e% |
|---|---|---|---|---|
| K1.5 | 20 | 1118 ± 27 | 13 ± 1 | 1.6 ± 0.2 |
| K1.5 | 30 | 103 ± 8 | 2.0 ± 0.4 | 215 ± 14 |
| K3 | 20 | 900 ± 15 | 13 ± 1 | 2.5 ± 0.2 |
| K3 | 30 | 54 ± 6 | 1.9 ± 0.3 | 275 ± 15 |
| K5 | 10 | 956 ± 21 | 14 ± 1 | 2.0 ± 0.2 |
| K5 | 20 | 585 ± 16 | 12 ± 1 | 4.8 ± 0.4 |
| K5 | 30 | 201 ± 11 | 10 ± 1 | 71 ± 11 |
| Komagataeibacter Species | Glucose | Temperature | Culture Time | Cellulose | Yield | Productivity | Reference |
|---|---|---|---|---|---|---|---|
| (g/L) | (°C) | (days) | (g/L) | (g/g) | (g/L·d) | ||
| K. xylinus | 100 | 28 | 10 | 19.6 | 0.61 | 1.96 | [109] |
| K. hansenii | 20 | 30 | 3 | 6.5 | 0.33 | 2.17 | [110] |
| K. xylinus | 50 | 30 | 7 | 1.8 | ND | 0.26 | [111] |
| K. rhaeticus | 20 | 30 | 5 | 4.4 | 0.25 | 0.88 | [112] |
| K. medellinensis | 20 | ND | 8 | 2.8 | 0.20 | 0.35 | [113] |
| K. xylinus | 20 | 28 | 7 | 17.0 | 0.85 | 2.43 | [114] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cottet, C.; Ramirez-Tapias, Y.A.; Delgado, J.F.; de la Osa, O.; Salvay, A.G.; Peltzer, M.A. Biobased Materials from Microbial Biomass and Its Derivatives. Materials 2020, 13, 1263. https://doi.org/10.3390/ma13061263
Cottet C, Ramirez-Tapias YA, Delgado JF, de la Osa O, Salvay AG, Peltzer MA. Biobased Materials from Microbial Biomass and Its Derivatives. Materials. 2020; 13(6):1263. https://doi.org/10.3390/ma13061263
Chicago/Turabian StyleCottet, Celeste, Yuly A. Ramirez-Tapias, Juan F. Delgado, Orlando de la Osa, Andrés G. Salvay, and Mercedes A. Peltzer. 2020. "Biobased Materials from Microbial Biomass and Its Derivatives" Materials 13, no. 6: 1263. https://doi.org/10.3390/ma13061263
APA StyleCottet, C., Ramirez-Tapias, Y. A., Delgado, J. F., de la Osa, O., Salvay, A. G., & Peltzer, M. A. (2020). Biobased Materials from Microbial Biomass and Its Derivatives. Materials, 13(6), 1263. https://doi.org/10.3390/ma13061263








