Segregation of Maghemite Nanoparticles within Symmetric Diblock Copolymer and Triblock Terpolymer Patterns under Solvent Vapor Annealing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.2.1. Block Copolymer Synthesis
2.2.2. Polystyrene Grafted γ-Fe2O3 Nanoparticles Functionalization
2.2.3. Preparation of IONPs/BCP Composite Films
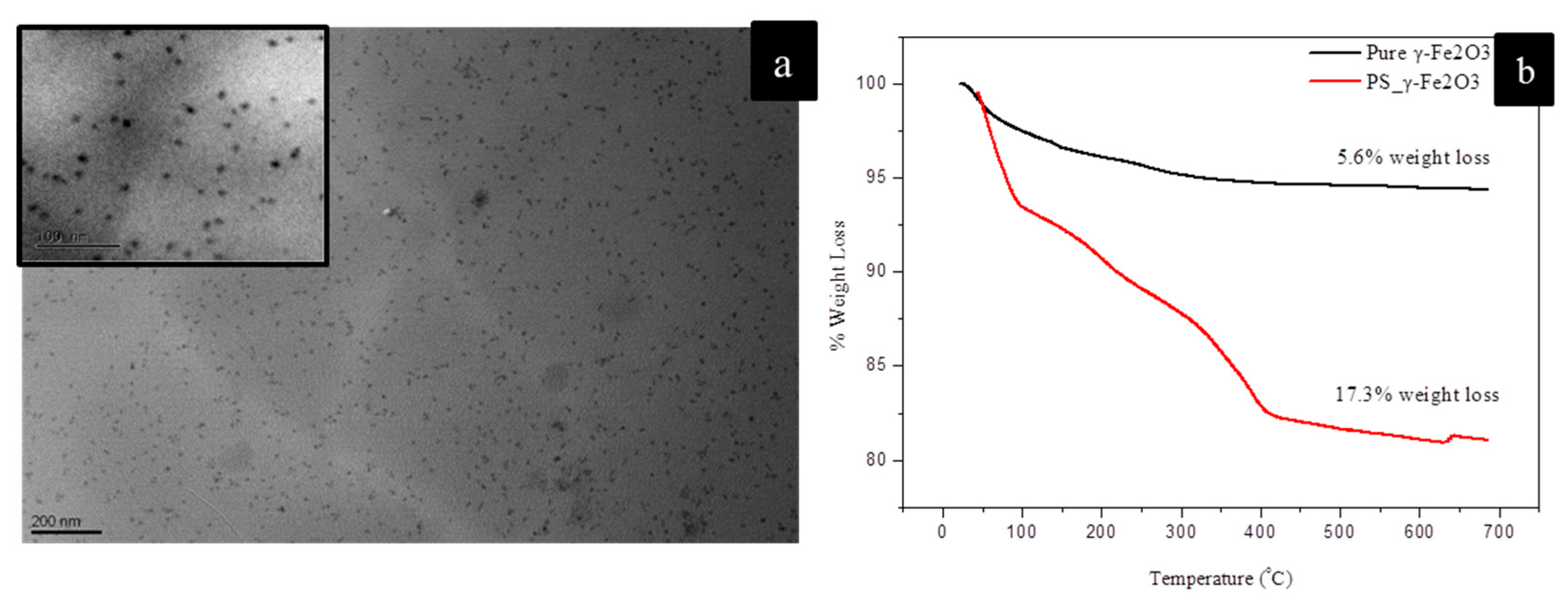
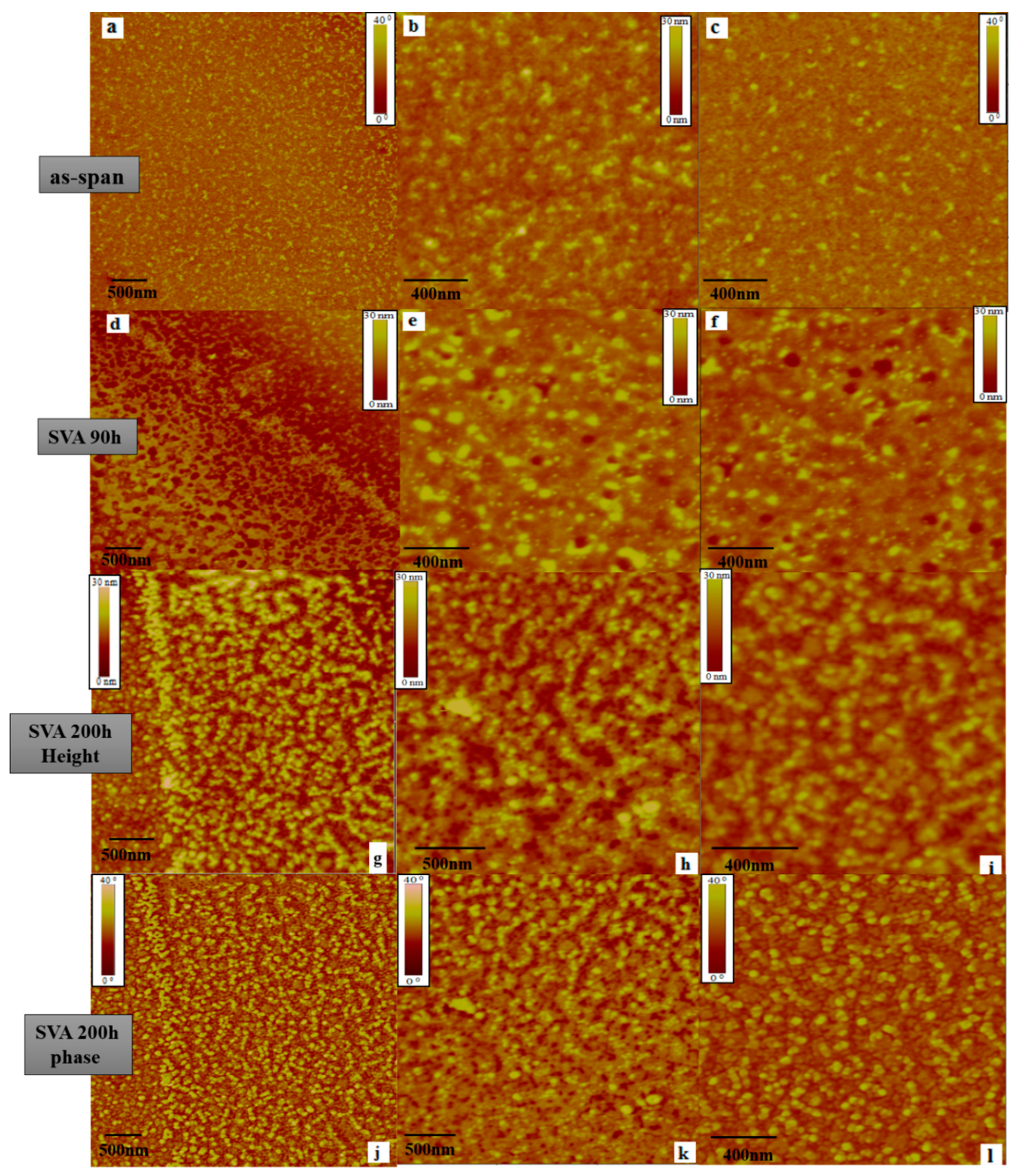
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.; Lin, M.M.; Toprak, M.S.; Kim, D.K.; Muhammed, M. Nanocomposites of polymer and inorganic nanoparticles for optical and magnetic applications. Nano Rev. 2010, 1, 5214. [Google Scholar] [CrossRef]
- Behrens, S.; Appel, I. Magnetic nanocomposites. Curr. Opin. Biotechnol. 2016, 39, 89–96. [Google Scholar] [CrossRef]
- Riggs, B.C.; Adireddy, S.; Rehm, C.H.; Puli, V.S.; Elupula, R.; Chrisey, D.B. Polymer nanocomposites for energy storage applications. Mater. Today Proc. 2015, 2, 3853–3863. [Google Scholar] [CrossRef]
- Liao, G.; Fang, J.; Li, Q.; Li, S.; Xu, Z.; Fang, B. Ag-Based nanocomposites: Synthesis and applications in catalysis. Nanoscale 2019, 11, 7062–7096. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, B.A.; Tenne, R. Polymer-assisted fabrication of nanoparticles and nanocomposites. Prog. Polym. Sci. 2008, 33, 40–112. [Google Scholar] [CrossRef]
- Sarkar, B.; Alexandridis, P. Block copolymer–nanoparticle composites: Structure, functional properties, and processing. Prog. Polym. Sci. 2015, 40, 33–62. [Google Scholar] [CrossRef]
- Folarin, O.M.; Sadiku, E.R.; Maity, A. Polymer-noble metal nanocomposites. Int. J. Phys. Sci. 2011, 21, 4869–4882. [Google Scholar]
- Arias-Zapata, J.; Cordeiro, J.; Böhme, S.; Girardot, C.; Garnier, J.; Bézard, P.; Ntetsikas, K.; Liontos, G.; Avgeropoulos, A.; Peyrade, D.; et al. High throughput sub-10 nm metallic particles organization on templates made by block copolymer self-assembly and nanoimprint. Microelectron. Eng. 2015, 141, 155–159. [Google Scholar] [CrossRef]
- Cheong Chan, Y.N.; Schrock, R.R.; Cohen, R.R. Synthesis of silver and gold nanoclusters within microphase-separated diblock copolymers. Chem. Mater. 1992, 4, 24–27. [Google Scholar] [CrossRef]
- Spatz, J.P.; Roescher, A.; Möller, M. Gold nanoparticles in micellar poly (styrene)-b-poly (ethylene oxide) films—Size and interparticle distance control in monoparticulate films. Adv. Mater. 1996, 8, 337–340. [Google Scholar]
- Li, Q.; He, J.; Glogowski, E.; Li, X.; Wang, J.; Emrick, T.; Russell, T.P. Responsive assemblies: Gold nanoparticles with mixed ligands in microphase separated block copolymers. Adv. Mater. 2008, 20, 1462–1466. [Google Scholar] [CrossRef]
- Sohn, B.H.; Choi, J.M.; Yoo, S.I.; Yun, S.H.; Zin, W.C.; Jung, J.C.; Kanehara, M.; Hirata, T.; Teranishi, T. Directed self-assembly of two kinds of nanoparticles utilizing monolayer films of diblock copolymer micelles. J. Am. Chem. Soc. 2003, 125, 6368–6369. [Google Scholar] [CrossRef] [PubMed]
- Horechyy, A.; Nandan, B.; Zafeiropoulos, N.E.; Jehnichen, D.; Göbel, M.; Stamm, M.; Pospiech, D. Nanoparticle directed domain orientation in thin films of asymmetric block copolymers. Colloid Polym. Sci. 2014, 292, 2249–2260. [Google Scholar] [CrossRef]
- Horechyy, A.; Nandan, B.; Zafeiropoulos, N.E.; Formanek, P.; Oertel, U.; Bigall, N.C.; Eychmüller, A.; Stamm, M. A Step-Wise Approach for Dual Nanoparticle Patterning via Block Copolymer Self-Assembly. Adv. Funct. Mater. 2013, 23, 483–490. [Google Scholar] [CrossRef]
- Kim, B.J.; Chiu, J.J.; Yi, G.R.; Pine, D.J.; Kramer, E.J. Nanoparticle-Induced Phase Transitions in Diblock-Copolymer Films. Adv. Mater. 2005, 17, 2618–2622. [Google Scholar] [CrossRef]
- García, I.; Tercjak, A.; Rueda, L.; Mondragon, I. Self-assembled nanomaterials using magnetic nanoparticles modified with polystyrene brushes and poly (styrene-b-butadiene-b-styrene). Macromolecules 2008, 41, 9295–9298. [Google Scholar] [CrossRef]
- Horechyy, A.; Zafeiropoulos, N.E.; Nandan, B.; Formanek, P.; Simon, F.; Kiriy, A.; Stamm, M. Highly ordered arrays of magnetic nanoparticles prepared via block copolymer assembly. J. Mater. Chem. 2010, 20, 7734–7741. [Google Scholar] [CrossRef]
- Bockstaller, M.R.; Lapetnikov, Y.; Margel, S.; Thomas, E.L. Size-selective organization of enthalpic compatibilized nanocrystals in ternary block copolymer/particle mixtures. J. Am. Chem. Soc. 2003, 125, 5276–5277. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block copolymer thermodynamics: Theory and experiment. Annu. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef]
- Yao, Y.; Metwalli, E.; Su, B.; Kōrstgens, V.; Moseguí González, D.; Miasnikova, A.; Laschewsky, A.; Opel, M.; Santoro, G.; Roth, S.V.; et al. Arrangement of maghemite nanoparticles via wet chemical self-assembly in PS-b-PNIPAM diblock copolymer films. ACS Appl. Mater. Interfaces 2015, 7, 13080–13091. [Google Scholar] [CrossRef]
- Xu, C.; Ohno, K.; Ladmiral, V.; Composto, R.J. Dispersion of polymer-grafted magnetic nanoparticles in homopolymers and block copolymers. Polymer 2008, 49, 3568–3577. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Wu, S.; Huang, G.; Xing, W.; Tang, M.; Fu, Q. Influence of magnetic nanoparticle size on the particle dispersion and phase separation in an ABA triblock copolymer. J. Phys. Chem. B 2014, 118, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Barandiaran, I.; Kortaberria, G. Magnetic nanocomposites based on poly (styrene-b-butadiene-b-methyl methacrylate) and modified Fe2O3 nanoparticles. Eur. Polym. J. 2016, 78, 340–351. [Google Scholar] [CrossRef]
- Xu, C.; Ohno, K.; Ladmiral, V.; Milkie, D.E.; Kikkawa, J.M.; Composto, R.J. Simultaneous block copolymer and magnetic nanoparticle assembly in nanocomposite films. Macromolecules 2009, 42, 1219–1228. [Google Scholar] [CrossRef]
- Metwalli, E.; Körstgens, V.; Schlage, K.; Meier, R.; Kaune, G.; Buffet, A.; Couet, S.; Roth, S.V.; Röhlsberger, R.; Müller-Buschbaum, P. Cobalt nanoparticles growth on a block copolymer thin film: A time-resolved GISAXS study. Langmuir 2013, 29, 6331–6340. [Google Scholar] [CrossRef] [PubMed]
- Barandiaran, I.; Grana, E.; Katsigiannopoulos, D.; Avgeropoulos, A.; Kortaberria, G. Nanocomposites based on nanostructured PI-b-PMMA copolymer and selectively placed PMMA-modified magnetic nanoparticles: Morphological and magnetic characterization. Eur. Polym. J. 2016, 75, 514–524. [Google Scholar] [CrossRef]
- Etxeberria, H.; Tercjak, A.; Mondragon, I.; Eceiza, A.; Kortaberria, G.; Science, P. Electrostatic force microscopy measurements of CdSe-PS nanoparticles and CdSe-PS/poly (styrene-b-butadiene-b-styrene) nanocomposites. Colloid Polym. Sci. 2014, 292, 229–234. [Google Scholar] [CrossRef]
- Czaniková, K.; Ilčíková, M.; Krupa, I.; Mičušík, M.; Kasák, P.; Pavlova, E.; Mosnáček, J.; Chorvat, D.; Omastová, M. Elastomeric photo-actuators and their investigation by confocal laser scanning microscopy. Smart Mater. Struct. 2013, 22, 104001. [Google Scholar] [CrossRef]
- Löbling, T.I.; Hiekkataipale, P.; Hanisch, A.; Bennet, F.; Schmalz, H.; Ikkala, O.; Gröschel, A.H.; Müller, A.H.E. Bulk morphologies of polystyrene-block-polybutadiene-block-poly (tert-butyl methacrylate) triblock terpolymers. Polymer 2015, 72, 479–489. [Google Scholar] [CrossRef]
- Hückstädt, H.; Göpfert, A.; Abetz, V. Influence of the block sequence on the morphological behavior of ABC triblock copolymers. Polymer 2000, 41, 9089–9094. [Google Scholar]
- Elbs, H.; Drummer, C.; Abetz, V.; Krausch, G. Thin film morphologies of ABC triblock copolymers prepared from solution. Macromolecules 2002, 35, 5570–5577. [Google Scholar] [CrossRef]
- Toombes, G.E.; Mahajan, S.; Thomas, M.; Du, P.; Tate, M.W.; Gruner, S.M.; Wiesner, U. Hexagonally patterned lamellar morphology in ABC triblock copolymer/aluminosilicate nanocomposites. Chem. Mater. 2008, 20, 3278–3287. [Google Scholar] [CrossRef]
- Barandiaran, I.; Gutierrez, J.; Tercjak, A.; Kortaberria, G. Thin Film Nanocomposites Based on SBM Triblock Copolymer and Silver Nanoparticles: Morphological and Dielectric Analysis. Macromol. Mater. Eng. 2017, 302, 1700169. [Google Scholar] [CrossRef]
- Schmalz, H.; Böker, A.; Lange, R.; Krausch, G.; Abetz, V. Synthesis and properties of ABA and ABC triblock copolymers with glassy (A), elastomeric (B), and crystalline (C) blocks. Macromolecules 2001, 34, 8720–8729. [Google Scholar] [CrossRef]
- Avgeropoulos, A.; Paraskeva, S.; Hadjichristidis, N.; Thomas, E.L. Synthesis and microphase separation of linear triblock terpolymers of polystyrene, high 1, 4-polybutadiene, and high 3, 4-polyisoprene. Macromolecules 2002, 35, 4030–4035. [Google Scholar] [CrossRef]
- Zapsas, G.; Moschovas, D.; Ntetsikas, K.; Rangou, S.; Lee, J.H.; Thomas, E.L.; Zafeiropoulos, N.E.; Avgeropoulos, A. Immiscible polydiene blocks in linear copolymer and terpolymer sequences. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1238–1246. [Google Scholar] [CrossRef]
- Ntaras, C.; Polymeropoulos, G.; Zapsas, G.; Ntetsikas, K.; Liontos, G.; Karanastasis, A.; Moschovas, D.; Rangou, S.; Stewart-Sloan, C.; Hadjichristidis, N.; et al. Synthesis, characterization and self-assembly of well-defined linear heptablock quaterpolymers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1443–1449. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Iatrou, H.; Pispas, S.; Pitsikalis, M. Anionic polymerization: High vacuum techniques. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3211–3234. [Google Scholar] [CrossRef]
- Uhrig, D.; Mays, J.M. Experimental techniques in high-vacuum anionic polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6179–6222. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Rubio-Retama, J.; Zafeiropoulos, N.E.; Serafinelli, C.; Rojas-Reyna, R.; Voit, B.; Cabarcos, E.L.; Stamm, M. Synthesis and Characterization of Thermosensitive PNIPAM Microgels Covered with Superparamagnetic γ-Fe2O3 Nanoparticles. Langmuir 2007, 23, 10280–10285. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, D.; Rangou, S.; Avgeropoulos, A.; Zafeiropoulos, N.E.; López-Cabarcos, E.; Rubio-Retama, J. Synthesis and chemical modification of magnetic nanoparticles covalently bound to polystyrene-SiCl2-poly (2-vinylpyridine). J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1668–1675. [Google Scholar] [CrossRef]
- Lauter, V.; Müller-Buschbaum, P.; Lauter, H.; Petry, W. Morphology of thin nanocomposite films of asymmetric diblock copolymer and magnetite nanoparticles. J. Phys. Condens. Matter 2011, 23, 254215. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, R.; Li, Y.; Shen, J. Understanding tapping-mode atomic force microscopy data on the surface of soft block copolymers. Surf. Sci. 2003, 530, 136–148. [Google Scholar] [CrossRef]
- García, I.; Tercjak, A.; Zafeiropoulos, N.E.; Stamm, M.; Mondragon, I. Self-Assembling Nanomaterials using Magnetic Nanoparticles Modified with Polystyrene Brushes. Macromol. Rapid Commun. 2007, 28, 2361–2365. [Google Scholar] [CrossRef]
- Etxeberria, H.; Zalakain, I.; Mondragon, I.; Eceiza, A.; Kortaberria, G. Generation of nanocomposites based on polystyrene-grafted CdSe nanoparticles by grafting through and block copolymer. Colloid Polym. Sci. 2013, 291, 633–640. [Google Scholar] [CrossRef]
- Jehnichen, D.; Pospiech, D.; Friedel, P.; He, G.; Sepe, A.; Zhang, J.; Papadakis, C.M.; Taurino, R.; Perlich, J. Thin-film morphologies of block copolymers with nanoparticles. Power Diffr. J. 2015, 30, 1–9. [Google Scholar] [CrossRef]
- Ganguly, A.; De Sarkar, M.; Bhowmick, A.K. Thermoplastic elastomeric nanocomposites from poly [styrene–(ethylene-co-butylene)–styrene] triblock copolymer and clay: Preparation and characterization. SEBS Clay Nanocomposites 2006, 100, 2040–2052. [Google Scholar] [CrossRef]






| Samples | (Κg/mol) | (Κg/mol) | (Κg/mol) | (Κg/mol) | Ð b | fPS c | fPB c | fPI c | NPS/NPB/NPI d |
|---|---|---|---|---|---|---|---|---|---|
| PS40-b-PB 40 | 40.5 | 41.3 | - | 81.8 | 1.07 | 0.50 | 0.50 | - | 389/765/- |
| PS50-b-PB50 | 48.2 | 50.6 | - | 98.8 | 1.08 | 0.49 | 0.51 | - | 463/937/- |
| PS45-b-PB34-b-PI74 | 45.0 | 34.2 | 74.1 | 153.3 | 1.09 | 0.30 | 0.21 | 0.49 | 433/633/1090 |
| PS26-b-PB23-b-PI12 | 25.8 | 23.4 | 12.2 | 61.4 | 1.10 | 0.43 | 0.37 | 0.20 | 248/433/179 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapsas, G.; Moschovas, D.; Ntetsikas, K.; Karydis-Messinis, A.; Chalmpes, N.; Kouloumpis, A.; Gournis, D.; Zafeiropoulos, N.E.; Avgeropoulos, A. Segregation of Maghemite Nanoparticles within Symmetric Diblock Copolymer and Triblock Terpolymer Patterns under Solvent Vapor Annealing. Materials 2020, 13, 1286. https://doi.org/10.3390/ma13061286
Zapsas G, Moschovas D, Ntetsikas K, Karydis-Messinis A, Chalmpes N, Kouloumpis A, Gournis D, Zafeiropoulos NE, Avgeropoulos A. Segregation of Maghemite Nanoparticles within Symmetric Diblock Copolymer and Triblock Terpolymer Patterns under Solvent Vapor Annealing. Materials. 2020; 13(6):1286. https://doi.org/10.3390/ma13061286
Chicago/Turabian StyleZapsas, George, Dimitrios Moschovas, Konstantinos Ntetsikas, Andreas Karydis-Messinis, Nikolaos Chalmpes, Antonios Kouloumpis, Dimitrios Gournis, Nikolaos E. Zafeiropoulos, and Apostolos Avgeropoulos. 2020. "Segregation of Maghemite Nanoparticles within Symmetric Diblock Copolymer and Triblock Terpolymer Patterns under Solvent Vapor Annealing" Materials 13, no. 6: 1286. https://doi.org/10.3390/ma13061286
APA StyleZapsas, G., Moschovas, D., Ntetsikas, K., Karydis-Messinis, A., Chalmpes, N., Kouloumpis, A., Gournis, D., Zafeiropoulos, N. E., & Avgeropoulos, A. (2020). Segregation of Maghemite Nanoparticles within Symmetric Diblock Copolymer and Triblock Terpolymer Patterns under Solvent Vapor Annealing. Materials, 13(6), 1286. https://doi.org/10.3390/ma13061286







