Synthesis and Characterization of Nitrogen-doped Carbon Nanotubes Derived from g-C3N4
Abstract
:1. Introduction
2. Details Experimental
2.1. Materials and Procedures
2.1.1. Synthesis of Exfoliated Graphitic Carbon Nitride
2.1.2. Synthesis of Functionalized Exfoliated Graphitic Carbon Nitride with Nickel Oxides
2.1.3. Synthesis of Nitrogen-doped Carbon Nanotubes
2.2. Characterization
3. Results and Discussion
3.1. Microscopic Analysis
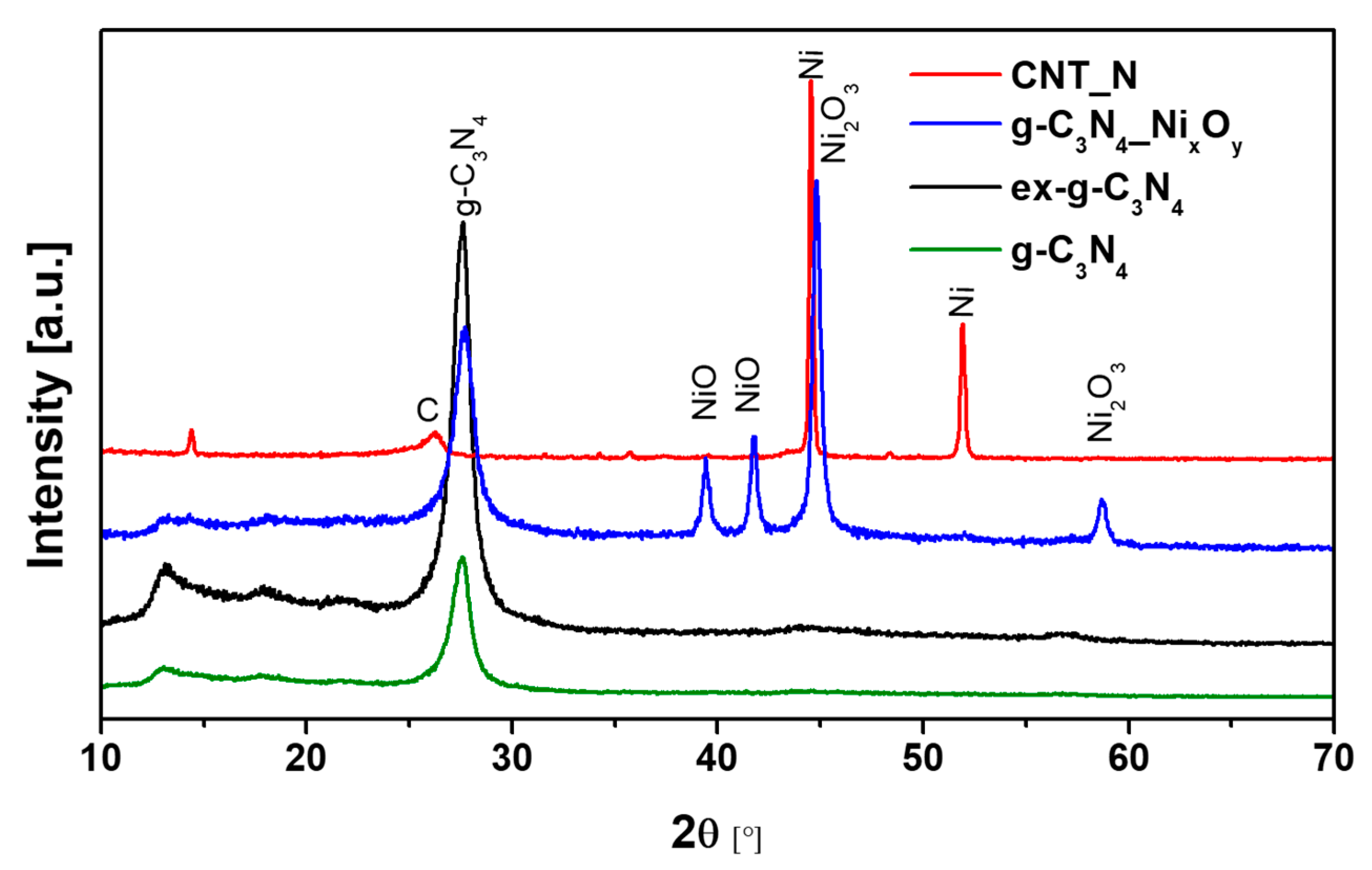
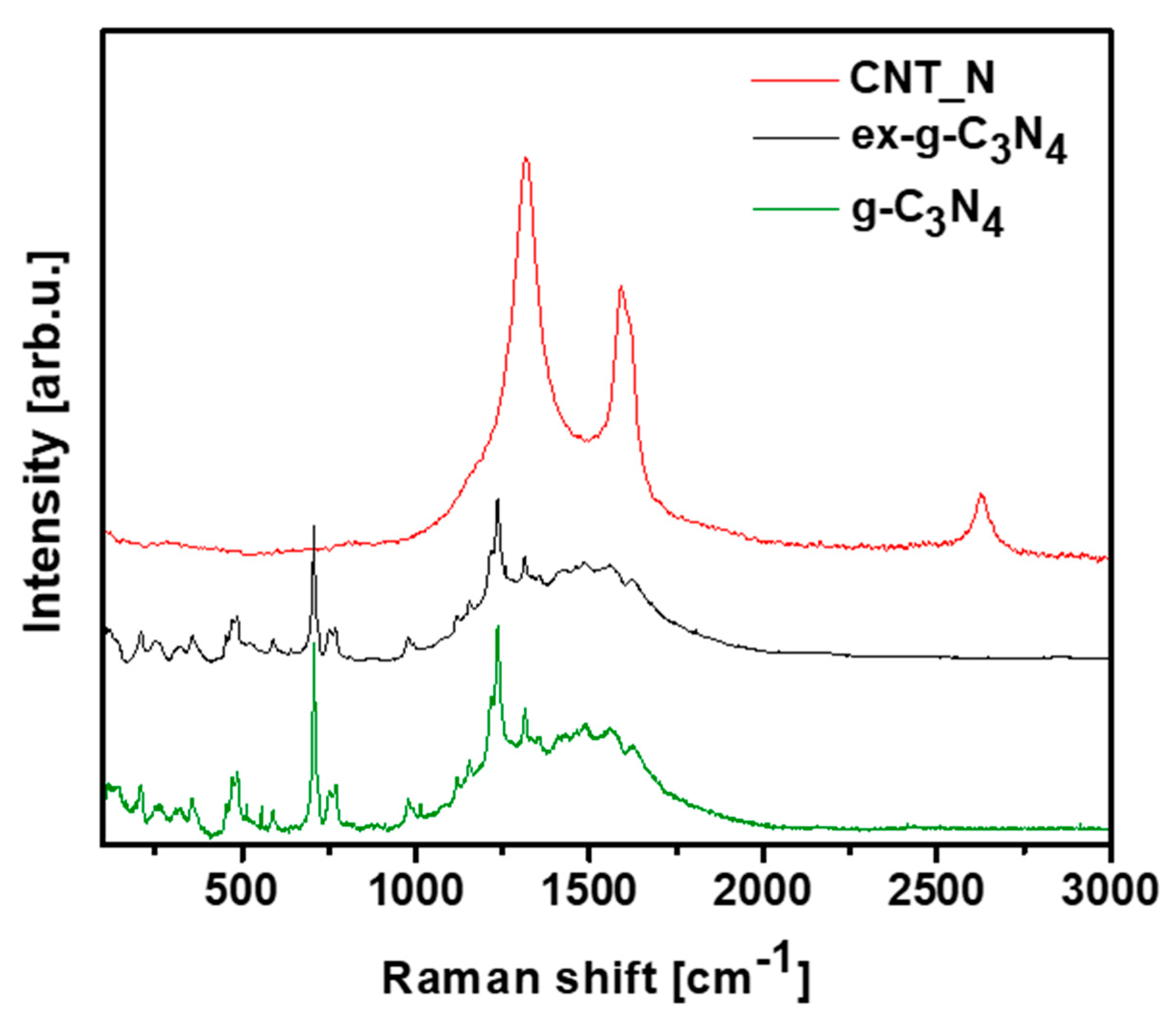
3.2. Spectroscopic, Crystal and Thermogravimetric Studies
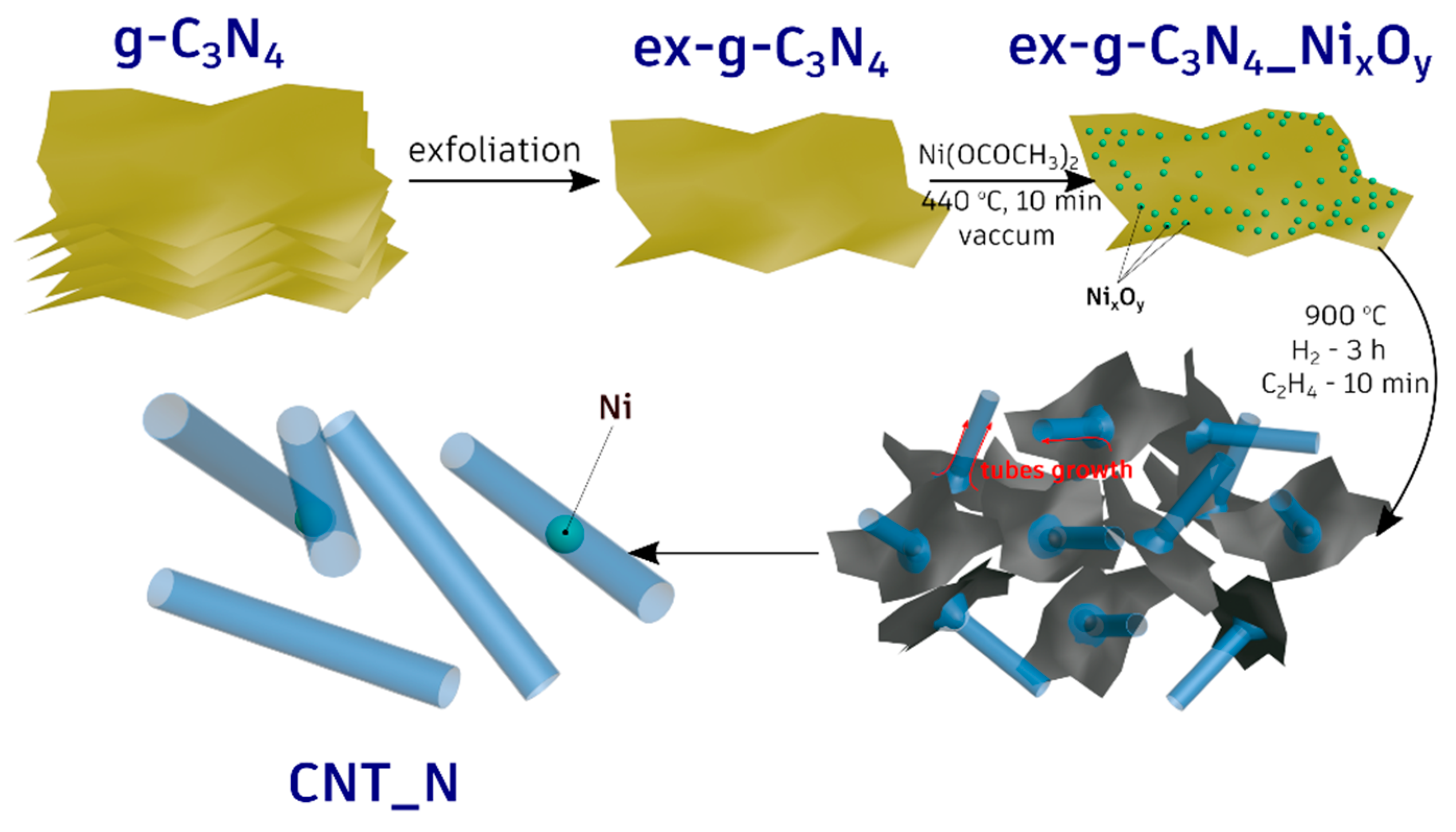
3.3. Mechanism of the CNT-N Formation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mozetič, M. Surface Modification to Improve Properties of Materials. Materials 2019, 12, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalia, S.; Boufi, S.; Celli, A. Nanofibrillated cellulose: Surface modification and potential applications. Colloid Polym. Sci. 2014, 292, 5–31. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Kukulka, W.; Cendrowski, K.; Mijowska, E. Electrochemical performance of MOF-5 derived carbon nanocomposites with 1D, 2D and 3D carbon structures. Electrochem. Acta 2019, 307, 582–594. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, L.; Xin, Z.; Yu, Y.; Wang, L.; Zhang, W. Visible light response and heterostructure of composite CdS@ZnS–ZnO to enhance its photocatalytic activity. J. Alloys Compd. 2020, 813, 152190. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Chen, C.; Li, H.; Xia, Y.; Zhou, T. MoS2 -CNFs composites to enhance the anticorrosive and mechanical performance of epoxy coating. Prog. Org. Coat. 2019, 129, 178–186. [Google Scholar] [CrossRef]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2019, 219, 499–511. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Ang, H.M.; Tadé, M.O. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Li, H.F.; Wu, F.; Wang, C.; Zhang, P.X.; Hu, H.Y.; Xie, N.; Pan, M.; Zeng, Z.; Deng, S.; Wu, M.H.; et al. One-Step Chemical Vapor Deposition Synthesis of 3D N-doped Carbon Nanotube/N-doped Graphene Hybrid Material on Nickel Foam. Nanomaterials 2018, 8, 700. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Zhang, H.; Sun, H.; Suvorova, A.; Saunders, M.; Tade, M.; Wang, S. Heteroatom (N or N-S)-Doping Induced Layered and Honeycomb Microstructures of Porous Carbons for CO2 Capture and Energy Applications. Adv. Funct. Mater. 2016, 26, 8651–8661. [Google Scholar] [CrossRef]
- Liu, J.; Shen, A.; Wei, X.; Zhou, K.; Chen, W.; Chen, F.; Xu, J.; Wang, S.; Dai, L. Ultrathin Wrinkled N Doped Carbon Nanotubes for Noble-Metal Loading and Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 20507–20512. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, 1804799. [Google Scholar] [CrossRef] [PubMed]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Yang, W.; Peng, Z.; Zhang, C.; Huang, Z.; Shi, W. A Nitrogen Doping Method for CoS2 Electrocatalysts with Enhanced Water Oxidation Performance. ACS Catal. 2017, 7, 4214–4220. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Y.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Controllable Nitrogen Doping of High-Surface-Area Microporous Carbons Synthesized from an Organic–Inorganic Sol–Gel Approach for Li–S Cathodes. ACS Appl. Mater. Interfaces 2015, 7, 21188–21197. [Google Scholar] [CrossRef]
- Ariharan, A.; Viswanathan, B.; Nandhakumar, W. Nitrogen-incorporated carbon nanotube derived from polystyrene and polypyrrole as hydrogen storage material. Int. J. Hydrogen Energy 2018, 43, 5077–5088. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Liu, X.; Chen, J.; Liu, W.; Liu, T.; Sun, M.; Zhao, L.; Ding, F.; Lu, Z.; et al. CuCo2O4/N-Doped CNTs loaded with molecularly imprinted polymer for electrochemical sensor: Preparation, characterization and detection of metronidazole. Biosens. Bioelectron. 2019, 142, 111483. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, J.-S.; Chen, X.-B.; Xiong, H.-M. Heteroatom-doped carbon dots based catalysts for oxygen reduction reactions. J. Colloid Interface Sci. 2019, 537, 716–724. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Li, R.; Tu, K.; Li, J.; Xie, Z.; Lei, J.; Liu, D.; Qu, D. Self-assembled N-doped carbon with a tube-in-tube nanostructure for lithium-sulfur batteries. J. Colloid Interface Sci. 2020, 559, 224–253. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Hsu, W.-L.; Lin, S.-Y.; Juang, R.-S. Enhanced CO2 adsorption on activated carbon fibers grafted with nitrogen-doped carbon nanotubes. Materials 2017, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Adjizian, L.-L.; Leghrib, R.; Koos, A.A.; Suarez-Martinez, I.; Crossley, A.; Wagner, P.; Grobert, N.; Llobet, E.; Ewels, C.P. Boron- and nitrogen-doped multi-wall carbon nanotubes for gas detection. Carbon 2014, 66, 662–673. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhang, Y.; Wang, B.; Peng, H. The recent progress of nitrogen-doped carbon nanomaterials for electrochemical batteries. J. Mater. Chem. A 2018, 6, 12932–12944. [Google Scholar] [CrossRef]
- Liu, Q.; Zeng, C.; Xie, Z.; Ai, L.; Liu, Y.; Zhou, Q.; Jiang, J.; Sun, H.; Wang, S. Cobalt@nitrogen-doped bamboo-structured carbon nanotube to boost photocatalytic hydrogen evolution on carbon nitride. Appl. Catal. B Environ. 2019, 254, 443–451. [Google Scholar] [CrossRef]
- Liu, Q.; Pu, Z.; Asiri, A.M.; Sun, X. Bamboo-like nitrogen-doped carbon nanotubes toward fluorescence recovery assay for DNA detection. Sens. Actuators B Chem. 2015, 206, 37–42. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Lian, C.; Wei, F.; Zhang, D.; Wu, G.; Chen, B.; Wang, S. Fe, Co, Ni nanocrystals encapsulated in nitrogen-doped carbon nanotubes as Fenton-like catalysts for organic pollutant removal. J. Hazard. Mater. 2016, 314, 129–139. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Chen, Z. Nitrogen doped carbon nanotubes and their impact on the oxygen reduction reaction in fuel cells. Carbon 2010, 48, 3057–3065. [Google Scholar] [CrossRef]
- Nxumalo, E.N.; Nyamori, V.O.; Coville, N.J. CVD synthesis of nitrogen doped carbon nanotubes using ferrocene/aniline mixtures. J. Organomet. Chem. 2008, 693, 2942–2948. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Y.; He, S.; Hao, F.; Yang, Y.; Lv, Y.; Zhang, W.; Liu, P.; Luo, H. Nitrogen-doped carbon nanotubes as a highly active metal-free catalyst for nitrobenzene hydrogenation. Appl. Catal. B Environ. 2020, 260, 118105. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Golberg, D.; Xu, F. Structure and nitrogen incorporation of carbon nanotubes synthesized by catalytic pyrolysis of dimethylformamide. Carbon 2004, 42, 2625–2633. [Google Scholar] [CrossRef]
- Chetty, R.; Kundu, S.; Xia, W.; Bron, M.; Schuhmann, W.; Chirila, V.; Brandl, W.; Reincecke, T.; Muhler, M. PtRu nanoparticles supported on nitrogen-doped multiwalled carbon nanotubes as catalyst for methanol electrooxidation. Electrochim. Acta 2009, 54, 4208–4215. [Google Scholar] [CrossRef]
- Kumar, M. Carbon Nanotube Synthesis and Growth Mechanism. In Carbon Nanotubes-Synthesis, Characterization, Applications; Web of science: Philadelphia, PA, USA, 2011; pp. 147–170. ISBN 978-953-307-497-9. [Google Scholar]
- Shukrullah, S.; Naz, M.Y.; Mohamed, N.M.; Ibrahim, K.A.; Abdel-Salam, N.M.; Ghaffar, A. CVD Synthesis, Functionalization and CO2 Adsorption Attributes of Multiwalled Carbon Nanotubes. Processes 2019, 7, 634. [Google Scholar] [CrossRef] [Green Version]
- Manawi, Y.M.; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Pan, Q.; Chai, G.; Liang, M.; Dong, G.; Zhang, Q.; Qiu, J. Synthesis and luminescence mechanism of multicolor-emitting g-C3N4 nanopowders by low temperature thermal condensation of melamine. Sci. Rep. 2013, 3, 1943. [Google Scholar] [CrossRef]
- Day, S.; Bhattacharjee, S.; Chaudhuri, M.G.; Bose, R.S.; Halder, S.; Ghosh, C.K. Synthesis of pure nickel(III) oxide nanoparticles at room temperature for Cr(VI) ion removal. RSC Adv. 2015, 5, 54717–54726. [Google Scholar] [CrossRef]
- Rifaya, M.N.; Theivasanthi, T.; Alagar, M. Chemical Capping Synthesis of Nickel Oxide Nanoparticles and their Characterizations Studies. Nanosci. Nanotechnol. 2012, 5, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Arghavanian, R.; Bostani, B.; Parvini-Ahmadi, N. Characterisation of coelectrodeposited Ni–Al composite coating. Surf. Eng. 2015, 31, 189–193. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic Carbon Nitride: Synthesis, Properties, and Applications in Catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Liu, D.; Wang, J.; Zhu, Y. Enhanced catalytic activity of potassium-doped graphitic carbon nitride induced by lower valence position. Appl. Catal. B Environ. 2015, 164, 77–81. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yang, W.J.; Chen, X.J.; Wang, J.; Zhu, Y.F. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 layer. Appl. Catal. B Environ. 2018, 220, 337–347. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Kandula, S.; Shanker, V. Fe-doped and -mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J. Mater. Chem. A 2014, 2, 6772–6780. [Google Scholar] [CrossRef]
- Hu, S.W.; Yang, L.W.; Tian, Y.; Wei, X.L.; Ding, J.W.; Zhong, J.X.; Chu, P.K. Simultaneous nanostructure and heterojunction engineering of graphitic carbon nitride via in situ Ag doping for enhanced photoelectrochemical activity. Appl. Catal. B Environ. 2015, 163, 611–622. [Google Scholar] [CrossRef]
- Liu, S.; Ke, J.; Sun, H.; Liu, J.; Tade, M.O.; Wang, S. Size dependence of uniformed carbon spheres in promoting graphitic carbon nitride toward enhanced photocatalysis. Appl. Catal. B Environ. 2017, 204, 358–364. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.G.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Zinin, P.V.; Ming, L.C.; Sharma, S.K.; Khabashesku, V.N.; Liu, X.; Hong, S.; Endo, S.; Acosta, T. Ultraviolet and near-infrared Raman spectroscopy of graphitic C3N4 phase. Chem. Phys. Lett. 2009, 472, 69–73. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maślana, K.; Kaleńczuk, R.J.; Zielińska, B.; Mijowska, E. Synthesis and Characterization of Nitrogen-doped Carbon Nanotubes Derived from g-C3N4. Materials 2020, 13, 1349. https://doi.org/10.3390/ma13061349
Maślana K, Kaleńczuk RJ, Zielińska B, Mijowska E. Synthesis and Characterization of Nitrogen-doped Carbon Nanotubes Derived from g-C3N4. Materials. 2020; 13(6):1349. https://doi.org/10.3390/ma13061349
Chicago/Turabian StyleMaślana, Klaudia, Ryszard J. Kaleńczuk, Beata Zielińska, and Ewa Mijowska. 2020. "Synthesis and Characterization of Nitrogen-doped Carbon Nanotubes Derived from g-C3N4" Materials 13, no. 6: 1349. https://doi.org/10.3390/ma13061349
APA StyleMaślana, K., Kaleńczuk, R. J., Zielińska, B., & Mijowska, E. (2020). Synthesis and Characterization of Nitrogen-doped Carbon Nanotubes Derived from g-C3N4. Materials, 13(6), 1349. https://doi.org/10.3390/ma13061349






