Development by Mechanochemistry of La0.8Sr0.2Ga0.8Mg0.2O2.8 Electrolyte for SOFCs
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microstructural Analysis and Thermal Stability
3.2. Electrolyte-Electrode Interface
3.3. Electrical Properties and Conductivity Measurements
4. Conclusions
- (1)
- Two components of a SOFC were synthesized by mechanochemistry in a significant short time; cathode (La0.8Sr0.2MnO3) and electrolyte (La0.8Sr0.2Ga0.8Mg0.2O3−δ), both possessing a perovskite structure with pseudo-cubic symmetry and crystalline domains of nanometric character.
- (2)
- Mechanochemistry, which is a scalable process and is also considered cost-effective since no heat input is necessary, is an alternative method to synthesize in a powder form SOFC components based on solid solution oxides with perovskite structure.
- (3)
- The synthesized La0.8Sr0.2Ga0.8Mg0.2O3−δ electrolyte is chemically and thermally stable up to 800 °C. At 900 °C, two secondary phases start to appear, LaSrGaO4 and LaSrGa3O7; however, the first one disappears at 1400 °C, this being the optimum sintering temperature of the electrolyte.
- (4)
- The HRTEM analysis of the LSGM electrolyte after heating at 1400 °C indicates that the symmetry of the structure is kept, and although the nanocrystals have grown, the size of the crystalline domain is still quite small.
- (5)
- The La0.8Sr0.2Ga0.8Mg0.2O3−δ electrolyte is chemically compatible with the electrode La0.8Sr0.2MnO3 at the operating temperature of 800 °C, and the analysis of the SEM micrographs shows good adhesion at the cathode-electrolyte interface.
- (6)
- Obtaining the different SOFC components with materials having the same perovskite structural type and using the same synthesis method can represent an advantage, since both factors should minimize the problems associated with chemical and structural compatibility, leading usually to failure in SOFCs.
- (7)
- The complex impedance measurement of LSGM indicates that it is a good candidate to be used as an electrolyte in SOFC, with an Ea value of 0.9 eV. The results of the conductivity measurements of the LSM//LSGM half-cell indicate that LSM sample is a good candidate to be used as a cathode when LSGM acts as electrolyte.
Author Contributions
Funding
Conflicts of Interest
References
- Singh, P. Solid Oxide Fuel Cells: Technology Status. Int. J. Appl. Ceram. Technol. 2004, 1, 5–15. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Aguadero, A.; Fawcett, L.; Taub, S.; Woolley, R.; Wu, K.T.; Xu, N.; Kilner, J.A.; Skinner, S.J. Materials development for intermediate-temperature solid oxide electrochemical devices. J. Mater. Sci. 2012, 47, 3925–3948. [Google Scholar] [CrossRef]
- Madhavan, B.; Ashok, A. Review on nanoperovskites: Materials, synthesis, and applications for proton and oxide ion conductivity. Ionics 2015, 21, 601–610. [Google Scholar] [CrossRef]
- Fergus, J.W. Oxide anode materials for solid oxide fuel cells. Solid State Ionics 2006, 177, 1529–1541. [Google Scholar] [CrossRef]
- Hsu, M.; Wu, L.; Wu, J.; Shiu, Y.; Lin, K. Solid Oxide Fuel Cell Fabricated Using All-Perovskite. Electrochem. Solid-State Lett. 2006, 9, A193–A195. [Google Scholar] [CrossRef]
- Jacobson, A.J. Materials for Solid Oxide Fuel Cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Sun, C.; Hui, R.; Roller, J. Cathode materials for solid oxide fuel cells: A review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; Takita, Y. Doped LaGaO3 Perovskite Type Oxide as a New Oxide Ionic Conductor. J. Am. Chem. Soc. 1994, 116, 3801–3803. [Google Scholar] [CrossRef]
- Huang, K.; Tichy, R.S.; Goodenough, J.B. Superior Perovskite Oxide-Ion Conductor; Strontium- and Magnesium-Doped LaGaO3: I, Phase Relationships and Electrical Properties. J. Am. Chem. Soc. 1998, 81, 2565–2575. [Google Scholar] [CrossRef]
- Skowron, A.; Huang, P.; Petric, A. Structural Study of La0.8Sr0.2Ga0.85Mg0.15O2.825. J. Solid State Chem. 1999, 143, 202–209. [Google Scholar] [CrossRef]
- Liu, N.; Shi, M.; Wang, C.; Yuan, Y.P.; Majewski, P.; Aldinger, F. Microstructure and ionic conductivity of Sr- and Mg-doped LaGaO3. J. Mater. Sci. 2006, 41, 4205–4213. [Google Scholar] [CrossRef]
- Ishikawa, H.; Enoki, M.; Ishihara, T.; Akiyama, T. Mechanically activated self-propagating high-temperature synthesis of La0.9Sr0.1Ga0.8Mg0.2O3−δ as an electrolyte for SOFC. J. Alloys Compd. 2009, 488, 238–242. [Google Scholar] [CrossRef]
- Nobuta, A.; Hsieh, F.F.; Shin, T.H.; Hosokai, S.; Yamamoto, S.; Okinaka, N.; Ishihara, T.; Akiyama, T. Self-propagating high-temperature synthesis of La(Sr)Ga(Mg,Fe)O3-δ with planetary ball-mill treatment for solid oxide fuel cell electrolytes. J. Alloys Compd. 2011, 509, 8387–8391. [Google Scholar] [CrossRef]
- Wu, Y.C.; Lee, M.Z. Properties and microstructural analysis of La1-xSrxGa1-yMgyO3-δ solid electrolyte ceramic. Ceram. Int. 2013, 39, 9331–9341. [Google Scholar] [CrossRef]
- Biswal, R.C.; Biswas, K. Novel way of phase stability of LSGM and its conductivity enhancement. Int. J. Hydrog. Energy 2015, 40, 509–518. [Google Scholar] [CrossRef]
- Morales, M.; Roa, J.J.; Tartaj, J.; Segarra, M. A review of doped lanthanum gallates as electrolytes for intermediate temperature solid oxides fuel cells: From materials processing to electrical and thermo-mechanical properties. J. Eur. Ceram. Soc. 2016, 36, 1–16. [Google Scholar] [CrossRef]
- Ishihara, T.; J., Tabuchi; Ishikawa, S.; Yan, J.; Enoki, M.; Matsumoto, H. Recent progress in LaGaO3 based solid electrolyte for intermediate temperature SOFCs. Solid State Ionics 2006, 177, 1949–1953. [Google Scholar] [CrossRef]
- Majewski, P.; Rozumek, M.; Aldinger, F. Phase diagram studies in the systems La2O3–SrO–MgO–Ga2O3 at 1350–1400 °C in air with emphasis on Sr and Mg substituted LaGaO3. J. Alloys Compd. 2001, 329, 253–258. [Google Scholar] [CrossRef]
- Majewski, P.; Rozumek, M.; Schluckwerder, H.; Aldinger, F. Phase diagram studies in the systems La2O3–SrO–Ga2O3 and La2 O3–MgO–Ga2O3 at 1400 8 C in air. Int. J. Inorg. Mater. 2001, 3, 1343–1344. [Google Scholar] [CrossRef]
- Majewski, P.J.; Rozumek, M.; Schluckwerder, H.; Aldinger, F. Phase Diagram Studies in the Systems La2O3-SrO-Ga2O3, La2O3-MgO-Ga2O3, and SrO-MgO-Ga2O3 at 1400 °C in Air. J. Am. Ceram. Soc. 2001, 84, 1093–1096. [Google Scholar] [CrossRef]
- Rozumek, M.; Majewski, P.; Aldinger, F. Metastable Crystal Structure of Strontium- and Magnesium-substituted LaGaO3. J. Am. Ceram. Soc. 2004, 87, 656–661. [Google Scholar] [CrossRef]
- Datta, P.; Majewski, P.; Aldinger, F. Structural studies of Sr- and Mg-doped LaGaO3. J. Alloys Compd. 2007, 438, 232–237. [Google Scholar] [CrossRef]
- Sammells, A.F.; Cook, R.L.; White, J.H.; Osborne, J.J.; MacDuff, R.C. Rational selection of advanced solid electrolytes for intermediate temperature fuel cells. Solid State Ionics 1992, 52, 111–123. [Google Scholar] [CrossRef]
- Hayashi, H.; Inaba, H.; Matsuyama, M.; Lan, N.G.; Dokiya, M.; Tagawa, H. Structural consideration on the ionic conductivity of perovskite-type oxides. Solid State Ionics 1999, 122, 1–15. [Google Scholar] [CrossRef]
- Huang, K.; Goodenough, J.B. A solid oxide fuel cell based on Sr- And Mg-doped LaGaO3 electrolyte: The role of a rare-earth oxide buffer. J. Alloys Compd. 2000, 303, 454–464. [Google Scholar] [CrossRef]
- Gonçalves, P.; Figueiredo, F.M. Mechanosynthesis of La1–xSrxGa1–yMgyO3–δ materials. Solid State Ionics 2008, 179, 991–994. [Google Scholar] [CrossRef]
- Moure, A.; Castro, A.; Tartaj, J.; Moure, C. Mechanosynthesis of perovskite LaGaO3and its effect on the sintering of ceramics. Ceram. Int. 2009, 35, 2659–2665. [Google Scholar] [CrossRef]
- Sayagués, M.J.; Córdoba, J.M.; Gotor, F.J. Room temperature mechanosynthesis of the La1-xSrxMnO3±δ (0≤x≤1) system and microstructural study. J. Solid State Chem. 2012, 188, 11–16. [Google Scholar] [CrossRef]
- Moriche, R.; Marrero-López, D.; Gotor, F.J.; Sayagués, M.J. Chemical and electrical properties of LSM cathodes prepared by mechanosynthesis. J. Power Sources 2014, 252, 43–50. [Google Scholar] [CrossRef]
- Sayagués, M.J.; Gotor, F.J.; Pueyo, M.; Poyato, R.; Garcia-Garcia, F.J. Mechanosynthesis of Sr1-xLaxTiO3 anodes for SOFCs: Structure and electrical conductivity. J. Alloys Compd. 2018, 763, 679–686. [Google Scholar] [CrossRef]
- Minakshi, M.; Sharma, N.; Ralph, D.; Appadoo, D.; Nallathamby, K. Synthesis and Characterization of Li(Co05Ni0.5)PO4 Cathode for Li-Ion Aqueous Battery Applications. Electrochem Solid State Lett. 2011, 14, A86–A89. [Google Scholar] [CrossRef]
- Reis, S.L.; Muccillo, E.N.S. Microstructure and electrical conductivity of fast fired Sr- and Mg-doped lanthanum gallate. Ceram. Int. 2016, 42, 7270–7277. [Google Scholar] [CrossRef]
- Yamaji, K.; Horita, T.; Ishikawa, M.; Sakai, N.; Yokokawa, H. Chemical stability of the La0.9Sr0.1Ga0.8Mg0.2O2.85 electrolyte in a reducing atmosphere. Solid State Ionics 1999, 121, 217–224. [Google Scholar] [CrossRef]
- Lee, D.; Han, J.H.; Chun, Y.; Song, R.H.; Shin, D.R. Preparation and characterization of strontium and magnesium doped lanthanum gallates as the electrolyte for IT-SOFC. J. Power Sources 2007, 166, 35–40. [Google Scholar] [CrossRef]
- Marreo-López, D.; Ruiz-Morales, J.C.; Peña-Martínez, J.; Martín-Sedeño, M.C.; Ramos-Barrado, J.R. Influence of phase segregation on the bulk and grain boundary conductivity of LSGM electrolytes. Solid State Ionics 2011, 186, 44–52. [Google Scholar] [CrossRef]
- Raghvendra, R.; Singh, R.K.; Singh, P. Synthesis of La0.9Sr0.1Ga0.8Mg0.2O3-δ electrolyte via ethylene glycol route and its characterizations for IT-SOFC. Ceram. Int. 2014, 40, 7177–7184. [Google Scholar] [CrossRef]
- Cong, L.G.; He, T.M.; Ji, Y.; Guan, P.F.; Huang, Y.L.; Su, W.H. Synthesis and characterization of IT-electrolyte with perovskite structure La0.8Sr0.2Ga0.85Mg0.15O3−δ by glycine-nitrate combustion method. J. Alloys Compd. 2003, 348, 325–331. [Google Scholar] [CrossRef]
- Xu, S.; Lin, X.; Ge, B.; Ai, D.; Ma, J.; Peng, Z. Microstructure and electrical conductivity of La0.9Sr0.1Ga0.8Mg0.2O2.85-Ce0.8Gd0.2O1.9 composite electrolytes for SOFCs. Int. J. Appl. Ceram. Technol. 2019, 16, 108–118. [Google Scholar] [CrossRef]
- Chae, N.S.; Park, K.S.; Yoon, Y.S.; Yoo, I.S.; Kim, J.S.; Yoon, H.H. Sr- and Mg-doped LaGaO3 powder synthesis by carbonate coprecipitation. Colloids Surf. A 2008, 313, 154–155. [Google Scholar] [CrossRef]
- Huang, K.Q.; Feng, M.; Goodenough, J.B. Sol–gel synthesis of a new oxide-ion conductor Sr-doped and Mg-doped LaGaO3 perovskite. J. Am. Ceram. Soc. 1996, 79, 1100–1104. [Google Scholar] [CrossRef]
- Chen, T.Y.; Fung, K.Z. Synthesis and densification of oxygen-conducting La0.8Sr0.2Ga0.8Mg0.2O2.8 nanopowder prepared from a low temperature hydrothermal urea precipitation process. J. Eur. Ceram. Soc. 2008, 28, 803–810. [Google Scholar] [CrossRef]
- Zhai, Y.; Ye, C.; Xia, F.; Xiao, J.; Dai, L.; Yang, Y.; Wang, Y. Preparation of La0.8Sr0.2Ga0.83Mg0.17O2.815 powders by microwave induced polyvinyl alcohol solution polymerization. J. Power Sources 2006, 162, 146–150. [Google Scholar] [CrossRef]

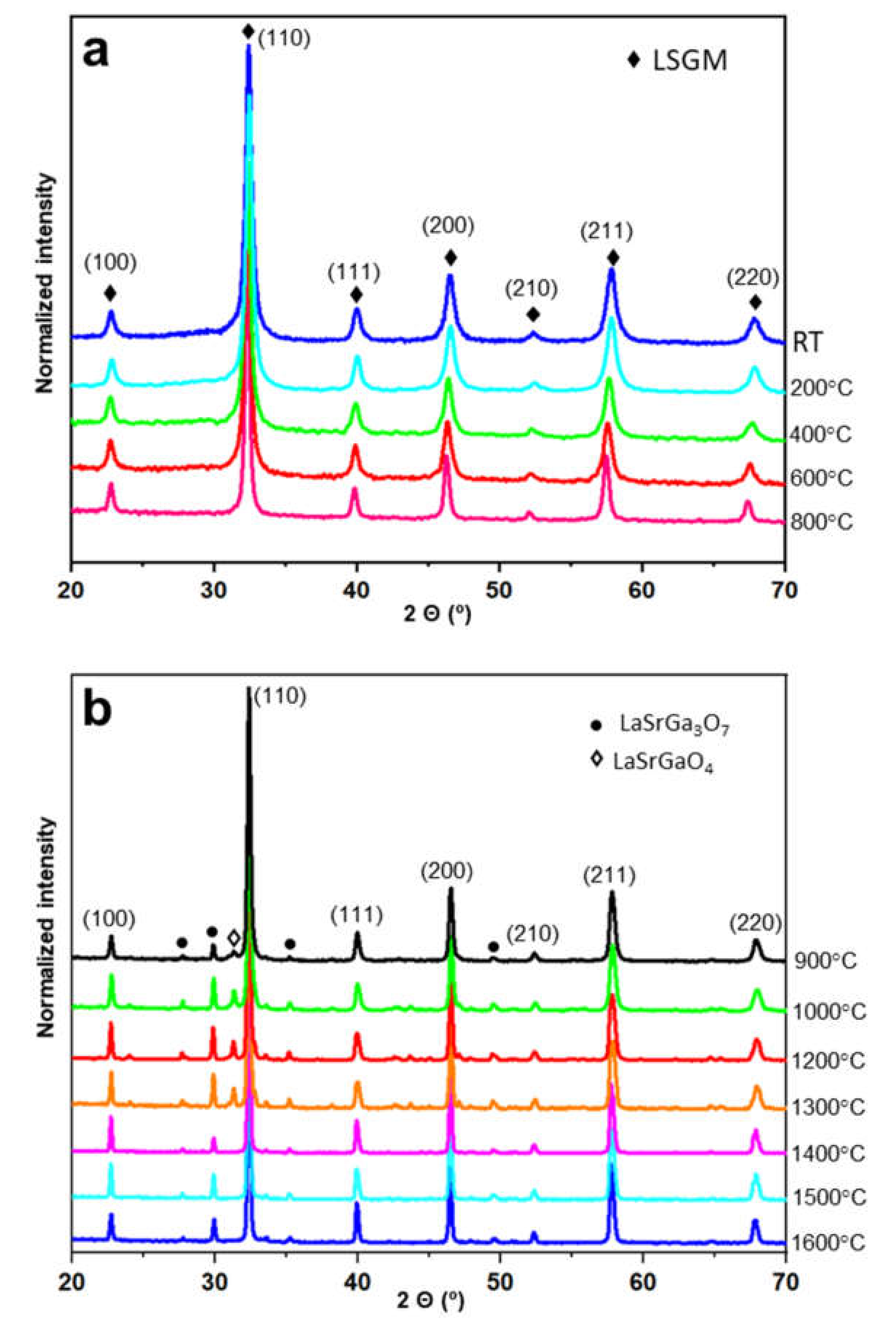

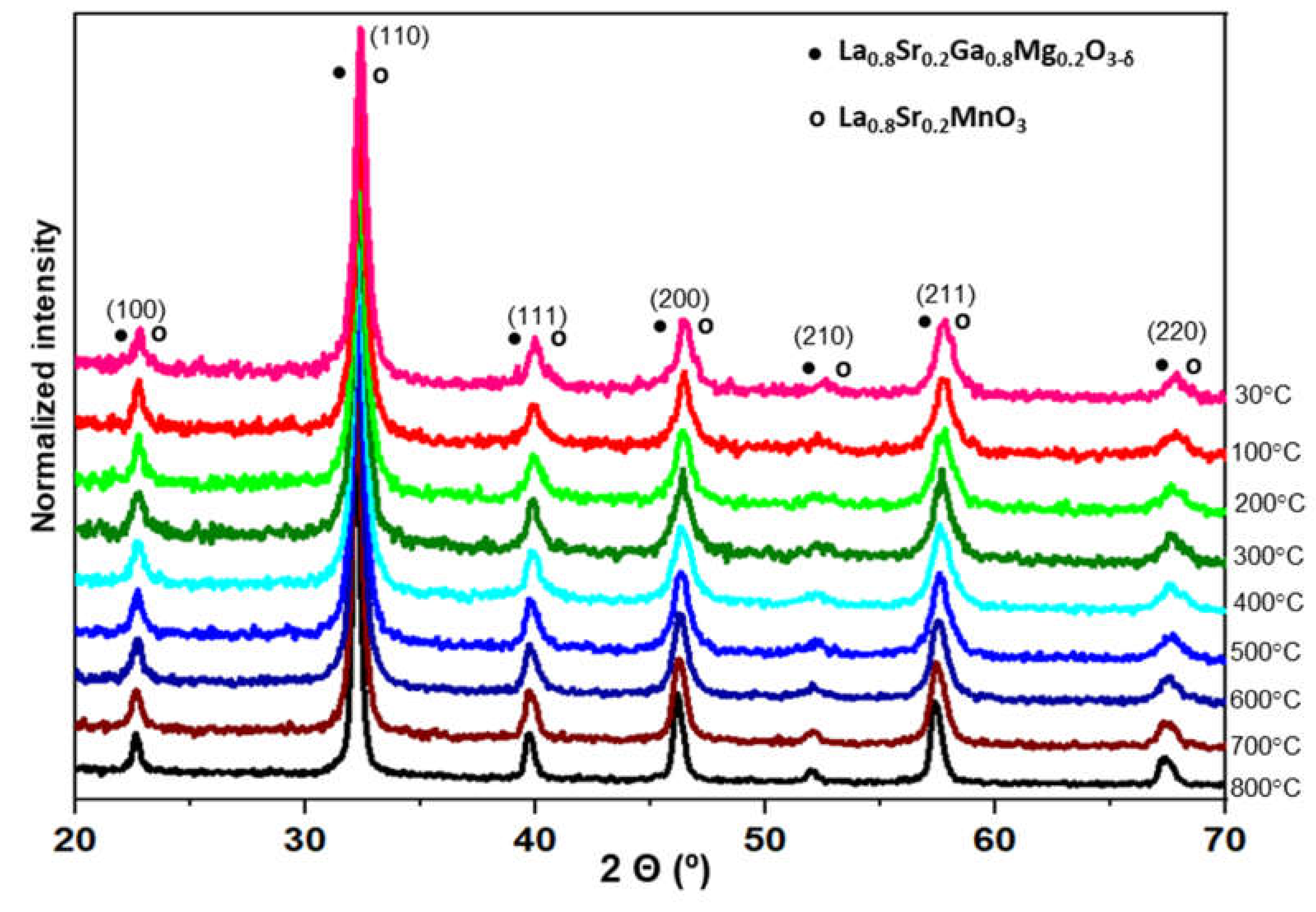




| T (°C) | LSMG | LaSrGaO4 | LaSrGa3O7 | Goodness of Fit |
|---|---|---|---|---|
| 900 | 89.2 | 5.2 | 5.7 | 1.24 |
| 1000 | 81.2 | 8.1 | 10.7 | 1.32 |
| 1200 | 81.9 | 6.7 | 11.4 | 2.14 |
| 1300 | 81.5 | 6.3 | 12.2 | 1.53 |
| 1400 | 93.7 | 0 | 6.3 | 1.76 |
| 1500 | 89.9 | 0 | 10.1 | 1.78 |
| 1600 | 89.5 | 0 | 10.5 | 1.84 |
| Synthesis Method | Electrolyte Composition | σ (S cm−1) | Ref. |
|---|---|---|---|
| Ethylene glycol | La0.9Sr0.1Ga0.8Mg0.2O2.85 | 0.056 | [37] |
| Glycine-nitrate | La0.8Sr0.2Ga0.85Mg0.15O3−δ | 0.06 | [38] |
| Solid state | La0.9Sr0.1Ga0.8Mg0.2O2.85 | 0.03 | [39] |
| Carbonate co-precipitation | La0.9Sr0.1Ga0.8Mg0.2O2.85 | 0.045 | [40] |
| Sol-gel method | La0.9Sr0.1Ga0.8Mg0.2O2.85 | 0.11 | [41] |
| Hydrothermal urea-precipitation process | La0.8Sr0.2Ga0.8Mg0.2O2.8 | 0.056 | [42] |
| Cellulose templating method | La0.8Sr0.2Ga0.83Mg0.17O2.815 | 0.042 | [43] |
| Mechanosynthesis | La0.8Sr0.2Ga0.8Mg0.2O3−δ | 0.093 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Garcia, F.J.; Tang, Y.; Gotor, F.J.; Sayagués, M.J. Development by Mechanochemistry of La0.8Sr0.2Ga0.8Mg0.2O2.8 Electrolyte for SOFCs. Materials 2020, 13, 1366. https://doi.org/10.3390/ma13061366
Garcia-Garcia FJ, Tang Y, Gotor FJ, Sayagués MJ. Development by Mechanochemistry of La0.8Sr0.2Ga0.8Mg0.2O2.8 Electrolyte for SOFCs. Materials. 2020; 13(6):1366. https://doi.org/10.3390/ma13061366
Chicago/Turabian StyleGarcia-Garcia, Francisco J., Yunqing Tang, Francisco J. Gotor, and María J. Sayagués. 2020. "Development by Mechanochemistry of La0.8Sr0.2Ga0.8Mg0.2O2.8 Electrolyte for SOFCs" Materials 13, no. 6: 1366. https://doi.org/10.3390/ma13061366
APA StyleGarcia-Garcia, F. J., Tang, Y., Gotor, F. J., & Sayagués, M. J. (2020). Development by Mechanochemistry of La0.8Sr0.2Ga0.8Mg0.2O2.8 Electrolyte for SOFCs. Materials, 13(6), 1366. https://doi.org/10.3390/ma13061366






