In Situ Spinel Formation in a Smart Nano-Structured Matrix for No-Cement Refractory Castables
Abstract
:1. Introduction
2. Experimental Sections
2.1. Sample Preparation and Analytical Techniques
2.2. Measurement Conditions
3. Results and Discussion
3.1. Mechanism of Hydrotalcite Formation in the nano-MgO–nano-Al2O3 Blended Paste
3.2. Heat Transport Parameters and Mechanical Properties
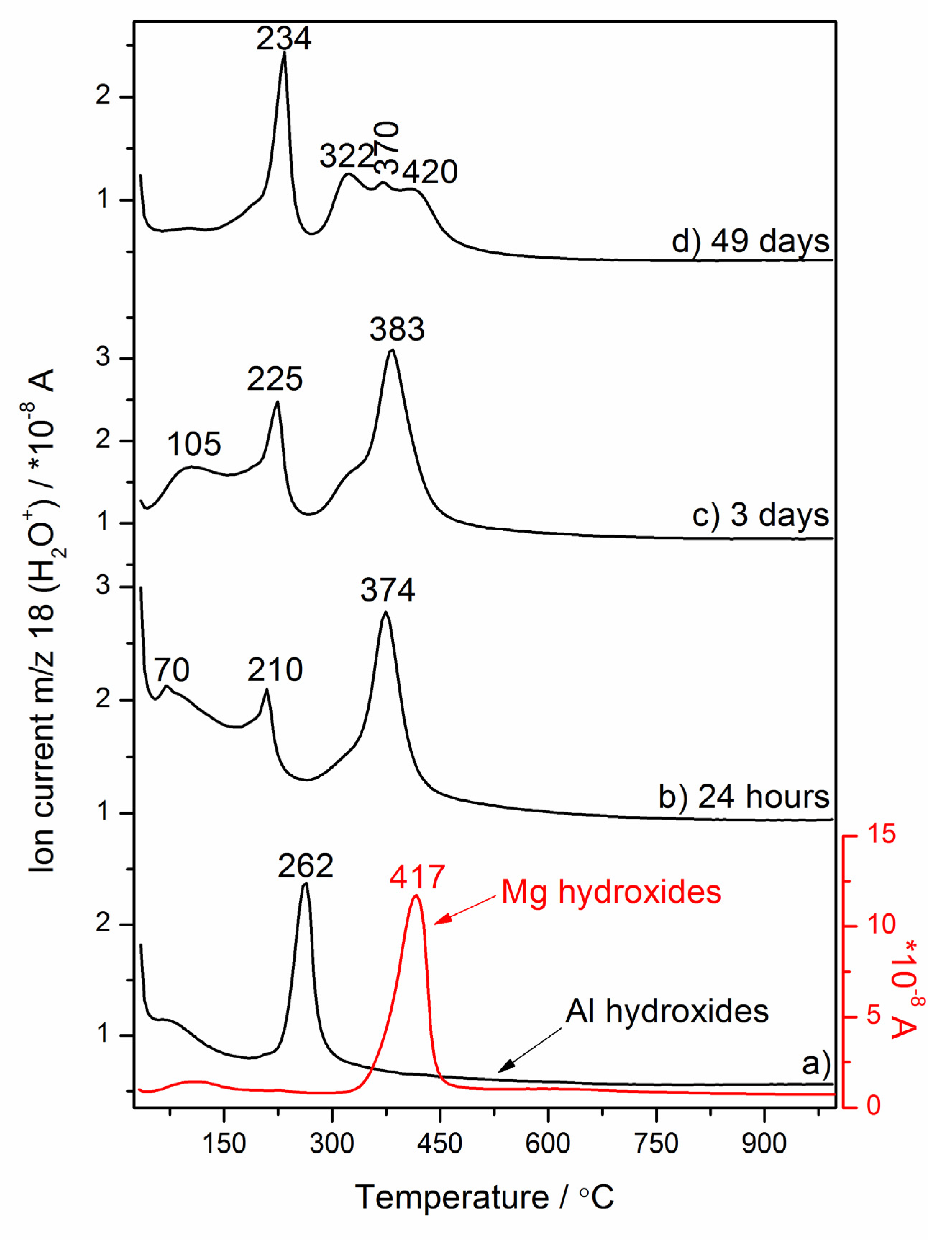
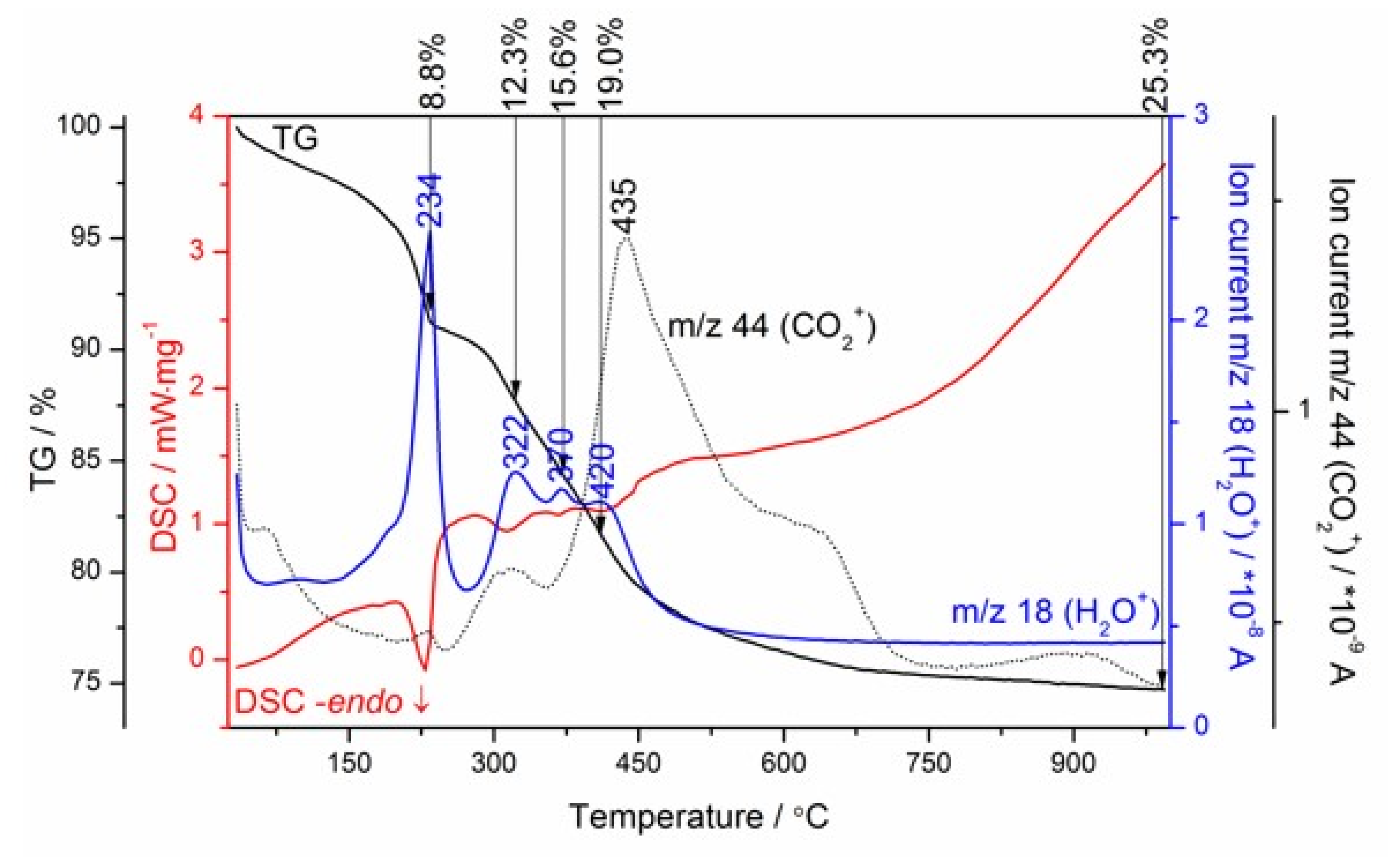
3.3. Thermal Decomposition Mechanism of Mg–Al Layered Double Hydroxide as a Magnesia-Alumina Spinel Precursor
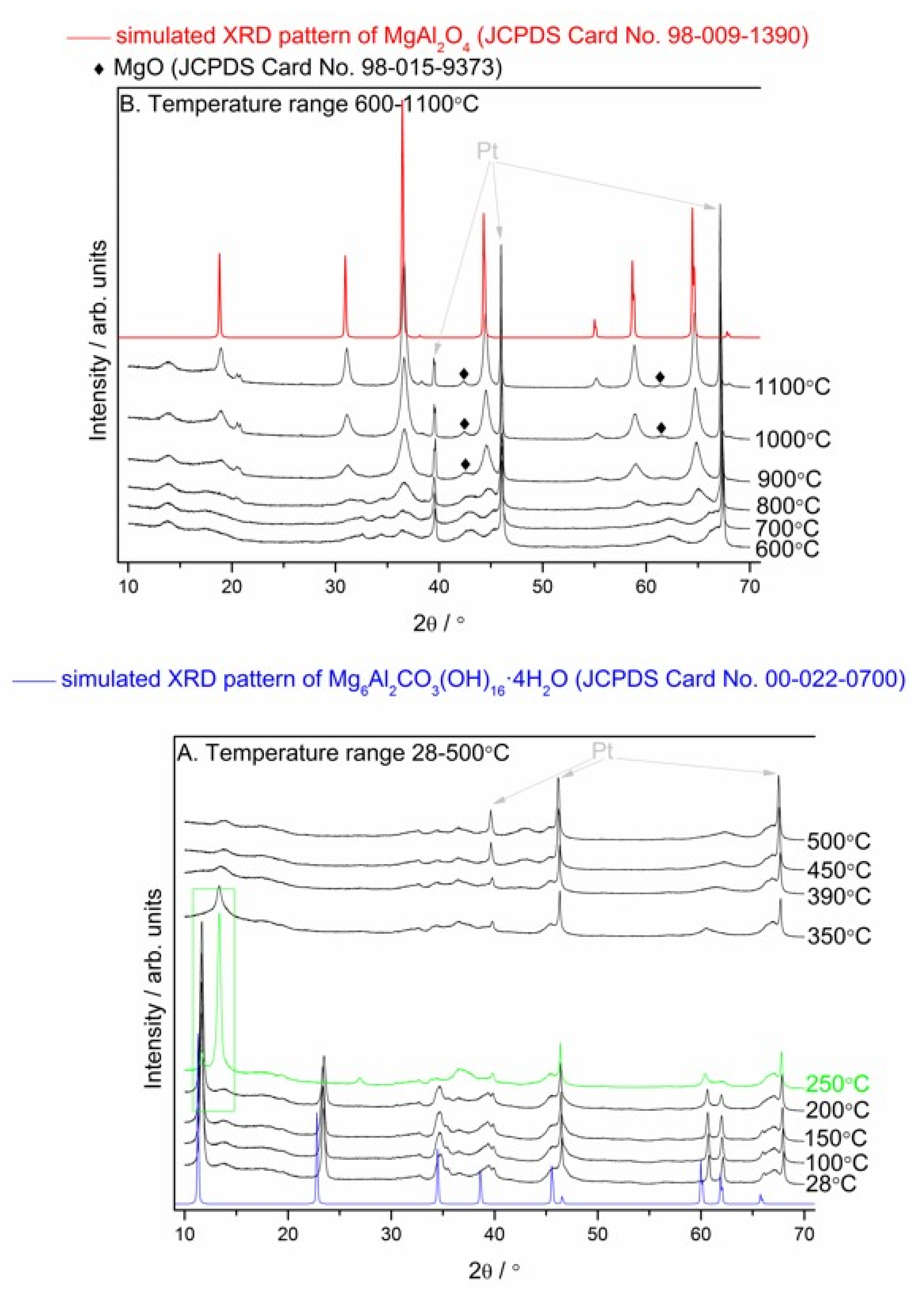
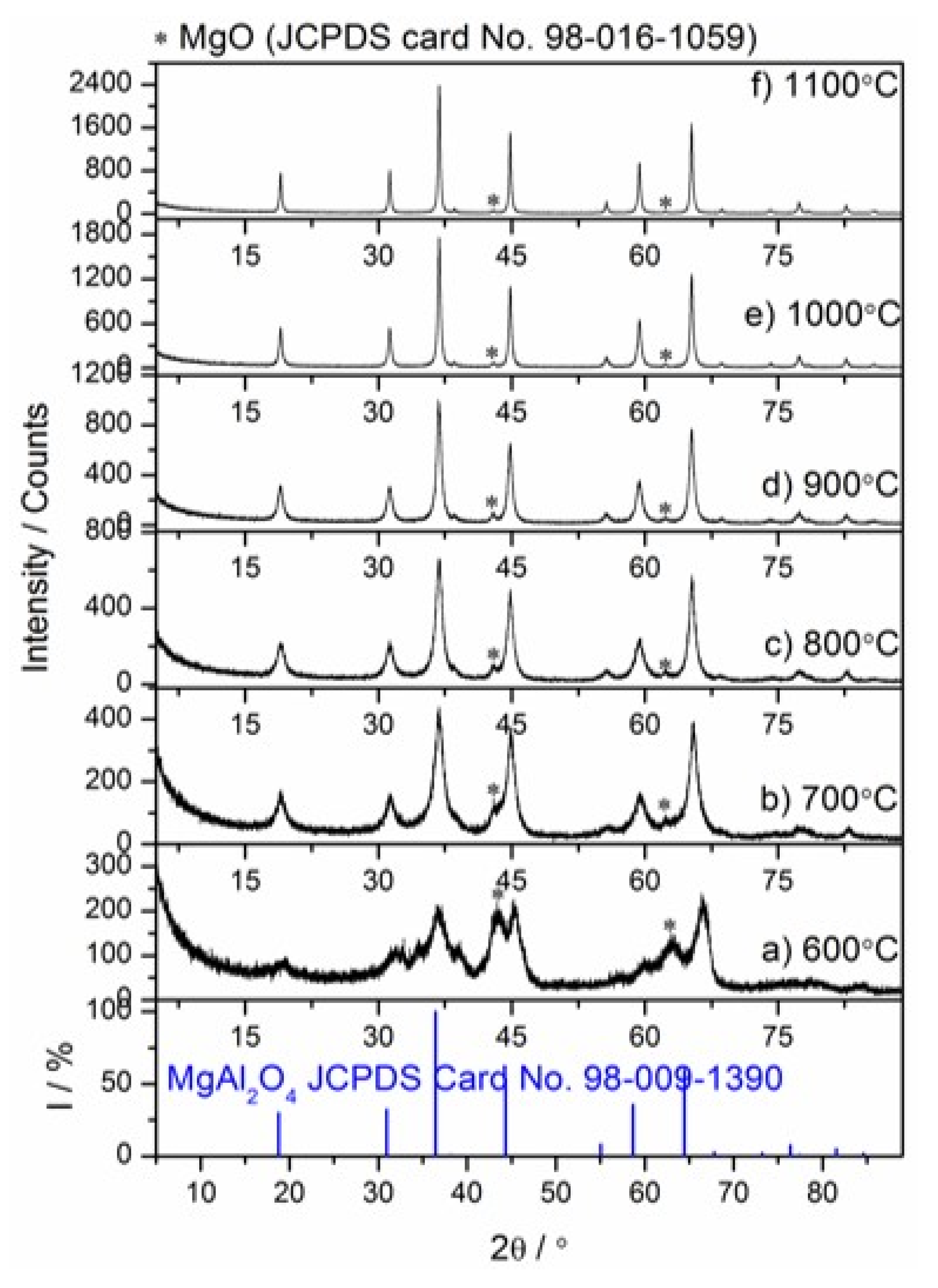
3.3.1. In Situ High-Temperature X-Ray Diffraction (HT–XRD) Studies of the Mg–Al layered Double Hydroxide
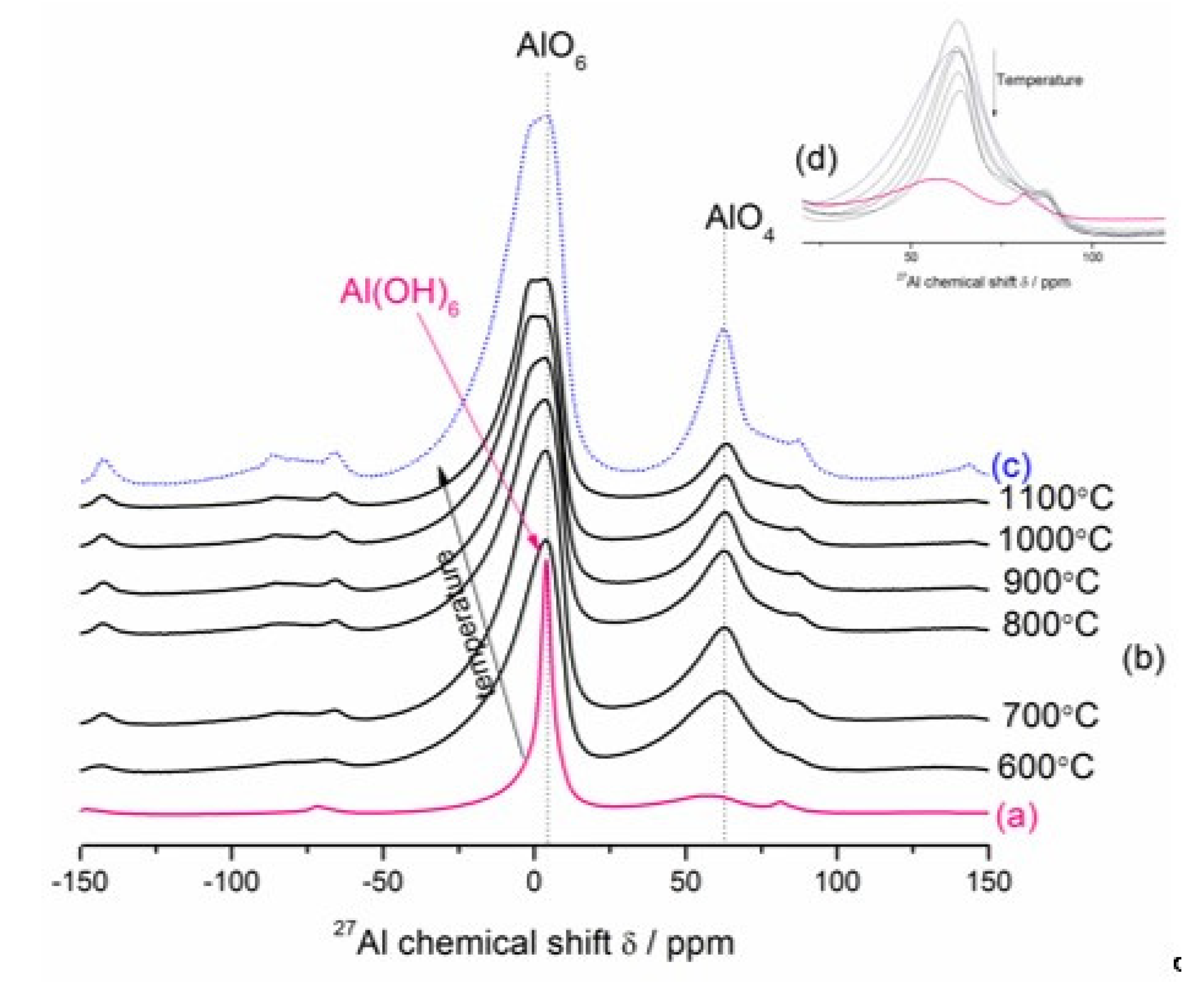
3.3.2. Ex-situ LT–XRD, FT–IR and NMR Investigations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghosh, S.; Majumdar, R.; Sinhamahapatra, B.K.; Nandy, R.N.; Mukherjee, M.; Mukhopadhyay, S. Microstructures of refractory castables prepared with sol-gel additives. Ceram. Int. 2003, 29, 671–677. [Google Scholar] [CrossRef]
- Nouri-Khezrabad, M.; Braulio, M.A.L.; Pandolfelli, V.C.; Golestani-Fard, F.; Rezaie, H.R. Nano-bonded refractory castables. Ceram. Int. 2013, 39, 3479–3497. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Ghosh, S.; Mahapatra, M.K.; Mazumder, R.; Barick, P.; Gupta, S.; Chakraborty, S. Easy-to-use mullite and spinel sols as bonding agents in a high-alumina based ultra low cement castable. Ceram. Int. 2002, 28, 719–729. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xu, Y.; Sang, S.; Jin, S. Enhanced formation of magnesium silica hydrates (M-S-H) using sodium metasilicate and caustic magnesia in magnesia castables. Ceram. Int. 2017, 43, 9110–9116. [Google Scholar] [CrossRef]
- Nied, D.; Enemark-Rasmussen, K.; L’Hopital, E.; Skibsted, J.; Lothenbach, B. Properties of magnesium silicate hydrates (M-S-H). Cem. Conc. Res. 2016, 79, 323–332. [Google Scholar] [CrossRef]
- Esteban-Cubillo, A.; Pina-Zapardiel, R.; Moya, J.S.; Barba, M.F.; Pecharromán, C. The role of magnesium on the stability of crystalline sepiolite structure. J. Eur. Ceram. Soc. 2008, 28, 1763–1768. [Google Scholar] [CrossRef]
- Ghanbari Ahari, K.; Sharp, J.H.; Lee, W.E. Hydration of refractory oxides in castable bond systems-I: Alumina, magnesia, and alumina-magnesia mixtures. J. Eur. Ceram. Soc. 2002, 22, 495–503. [Google Scholar] [CrossRef]
- Madej, D. Examination of dehydration and dehydroxylation of synthetic layered (oxy)hydroxides through thermal analysis (TG-DSC-EGA-MS) and a discussion to the second Pauling’s rule. Inorg. Chim. Acta 2018, 482, 402–410. [Google Scholar] [CrossRef]
- Madej, D.; Prorok, R.; Wiśniewska, K. An experimental investigation of hydration mechanism of the binary cementitious pastes containing MgO and Al2O3 micro-powders. J. Therm. Anal. Calorim. 2018, 134, 1481–1492. [Google Scholar] [CrossRef]
- Madej, D. Size-dependent hydration mechanism and kinetics for reactive MgO and Al2O3 powders with respect to the calcia-free hydraulic binder systems designed for refractory castables. J. Mater. Sci. 2017, 52, 7578–7590. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, A.; Ding, D.; Gao, S.; Liu, X.; Ye, G.; Liao, G. Trace nanoscale Al2O3 in Al2O3-MgAl2O4 castable for improved thermal shock performance. Ceram. Int. 2019, 45, 23029–23036. [Google Scholar] [CrossRef]
- dos Anjos, R.D.; Ismael, M.R.; de Oliveira, I.R.; Pandolfelli, V.C. Workability and setting parameters evaluation of colloidal silica bonded refractory suspensions. Ceram. Int. 2008, 34, 165–171. [Google Scholar] [CrossRef]
- Tang, X.; Guo, L.; Chen, C.; Liu, Q.; Li, T.; Zhu, Y. The analysis of magnesium oxide hydration in three-phase reaction system. J. Solid. State. Chem. 2014, 213, 32–37. [Google Scholar] [CrossRef]
- Ma, W.; Brown, P.W. Mechanisms of reaction of hydratable aluminas. J. Am. Cer. Soc. 1999, 82, 453–456. [Google Scholar] [CrossRef]
- dos Santos, T.; Pinola, F.G.; Luz, A.P.; Pagliosa, C.; Pandolfelli, V.C. Al2O3-MgO refractory castables with enhanced explosion resistance due to in situ formation of phases with lamellar structure. Ceram. Int. 2018, 44, 8048–8056. [Google Scholar] [CrossRef]
- Ye, G.; Troczynski, T. Effect of magnesia on strength of hydratable alumina-bonded castable refractories. J. Mater. Sci. 2005, 40, 3921–3926. [Google Scholar] [CrossRef]
- Salomão, R.; Pandolfelli, V.C. The role of hydraulic binders on magnesia containing refractory castables: Calcium aluminate cement and hydratable alumina. Ceram. Int. 2009, 35, 3117–3124. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S. Mechanochemical approach for synthesis of layered double hydroxides. Appl. Surf. Sci. 2013, 274, 158–163. [Google Scholar] [CrossRef]
- Chubar, N.; Gerda, V.; Megantari, O.; Mičušík, M.; Omastova, M.; Heister, K.; Man, P.; Fraissard, J. Applications versus properties of Mg-Al layered double hydroxides provided by their syntheses methods: Alkoxide and alkoxide-free sol-gel syntheses and hydrothermal precipitation. Chem. Eng. J. 2013, 234, 284–299. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Yu, J. Influences of polyhydric alcohol co-solvents on the hydration and thermal stability of MgAl-LDH obtained via hydrothermal synthesis. Appl. Clay Sci. 2013, 72, 37–43. [Google Scholar] [CrossRef]
- Komlev, A.A.; Gusarov, V.V. Mechanism of the nanocrystals formation of the spinel structure in the MgO-Al2O3-H2O system under the hydrothermal conditions. Russ. J. Gen. Chem. 2011, 81, 2222–2230. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, Q.; Li, X.; He, X.; Song, S. Mechanochemical approaches to synthesize layered double hydroxides: A review. Appl. Clay Sci. 2016, 119, 185–192. [Google Scholar] [CrossRef]
- Gabrovska, M.; Edreva-Kardjieva, R.; Angelov, V.; Crişan, D.; Millet, J.-M.M. Mg-Al and Mg-In oxide compounds as catalyst components for the oxidative dehydrogenation of propane. Part II Characterization of the calcined materials. Rev. Roum. Chim. 2007, 52, 527–532. [Google Scholar]
- Xu, X.; Li, D.; Song, J.; Lin, Y.; Lv, Z.; Wei, M.; Duan, X. Synthesis of Mg-Al-carbonate layered double hydroxide by an atom-economic reaction. Particuology 2010, 8, 198–201. [Google Scholar] [CrossRef]
- Kovanda, F.; Jindova, E.; Doušová, B.; Kolouskova, S. Layered double hydroxides intercalated with organic anions and their application in preparation of LDH/polymer nanocomposites. Acta Geodyn. Geomater. 2009, 6, 111–119. [Google Scholar]
- Oh, J.-M.; Hwang, S.-H.; Choy, J.-H. The effect of synthetic conditions on tailoring the size of hydrotalcite particles. Solid State Ion. 2002, 151, 285–291. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Meinhold, R.H.; Sherriff, B.L.; Xu, Z. 27Al and 25Mg solid-state magic-angle spinning nuclear magnetic resonance study of hydrotalcite and its thermal decomposition sequence. J. Mater. Chem. 1993, 3, 1263–1269. [Google Scholar] [CrossRef]
- Miyata, S. Physico-chemical properties of synthetic hydrotalcites in relation to composition. Clay Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Crystal structures of some double hydroxide minerals. Mineral. Mag. 1973, 39, 377–389. [Google Scholar] [CrossRef] [Green Version]
- Stanimirova, T.; Piperov, N.; Petrova, N.; Kirov, G. Thermal evolution of Mg-Al-CO3 hydrotalcites. Clay Miner. 2004, 39, 177–191. [Google Scholar] [CrossRef]
- Stanimirova, T.; Vergilov, I.; Kirov, G.; Petrova, N. Thermal decomposition products of hydrotalcite-like compounds: Low-temperature metaphases. J. Mater. Sci. 1999, 34, 4153–4161. [Google Scholar] [CrossRef]
- Sato, T.; Kato, K.; Endo, T.; Shimada, M. Preparation and chemical properties of magnesium aluminium oxide solid solutions. React. Solid. 1986, 2, 253–260. [Google Scholar] [CrossRef]
- Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Berthet, P.; Huntz, A.M.; Roy, P.; Tétot, R. Transition alumina phases induced by heat treatment of boehmite: An X-ray diffraction and infrared spectroscopy study. J. Solid State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schuchardt, U. On the paradox of transition metal-free alumina-catalyzed epoxidation with aqueous hydrogen peroxide. J. Catal. 2005, 236, 335–345. [Google Scholar] [CrossRef]
- Krishna Priya, G.; Padmaja, P.; Warrier, K.G.K.; Damodaran, A.D.; Aruldhas, G. Dehydroxylation and high temperature phase formation in sol-gel boehmite characterized by Fourier transform infrared spectroscopy. J. Mater. Sci. Lett. 1997, 16, 1584–1587. [Google Scholar] [CrossRef]
- Mo, S.-D.; Ching, W.Y. Electronic structure of normal, inverse, and partially inverse spinels in the MgAl2O4 system. Phys. Rev. B 1996, 54, 16555–16561. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Zhou, X.; Liu, Z.; Liu, Q.; Li, X. Different dye removal mechanisms between monodispersed and uniform hexagonal thin plate-like MgAl––LDH and its calcined product in efficient removal of Congo red from water. J. Alloys Compd. 2016, 673, 265–271. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L. Infrared emission spectroscopic study of the thermal transformation of Mg-, Ni- and Co-hydrotalcite catalysts. Appl. Catal. A General 1999, 184, 61–71. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L. Fourier transform infrared and Raman spectroscopic study of the local structure of Mg-, Ni-, and Co-hydrotalcites. J. Solid State Chem. 1999, 146, 506–515. [Google Scholar] [CrossRef]
- Tao, Q.; Reddy, B.J.; He, H.; Frost, R.L.; Yuan, P.; Zhu, J. Synthesis and infrared spectroscopic characterization of selected layered double hydroxides containing divalent Ni and Co. Mater. Chem. Phys. 2008, 112, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Mora, M.; López, M.I.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Study of organo-hybrid layered double hydroxides by medium and near infrared spectroscopy. Spectrochim. Acta. A 2011, 78, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, H.; Zhou, J.; Qian, G.; Xu, Z.; Xi, Y.; Xu, Y.; Theiss, F.L.; Frost, R. Mid- and near-infrared spectroscopic investigation of homogeneous cation distribution in Mgx- ZnyAl(x+y)/2-layered double hydroxide (LDH). J. Colloid Interface Sci. 2013, 411, 240–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sideris, P.J.; Nielsen, U.G.; Gan, Z.; Grey, C.P. Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy. Science 2008, 321, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloprogge, J.T.; Hickey, L.; Frost, R.L. The effects of synthesis pH and hydrothermal treatment on the formation of zinc aluminium hydrotalcites. J. Solid State Chem. 2004, 177, 4047–4057. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, S.; Takagaki, A.; Ebitani, K. Characterization, synthesis and catalysis of hydrotalcite-related materials for highly efficient materials transformations. Green Chem. 2013, 15, 2026–2042. [Google Scholar] [CrossRef]
- Pesic, L.; Salipurovic, S.; Markovic, V.; Vueselic, D.; Kagunya, W.; Jones, W. Thermal characteristics of a synthetic hydrotalcite-like mineral. J. Mater. Chem. 1992, 2, 1069–1073. [Google Scholar] [CrossRef]
- Occelli, M.L.; Olivier, J.P.; Auroux, A.; Kalwei, M.; Eckert, H. Basicity and porosity of a calcined hydrotalcite-type material from nitrogen porosimetry and adsorption microcalorimetry methods. Chem. Mater. 2003, 15, 4231–4238. [Google Scholar] [CrossRef]
- Tarte, P. Infra-red spectra of inorganic aluminates and characteristic vibrational frequencies of AlO4 tetrahedra and AlO6 octahedra. Spectrochim. Acta A Mol. Spectrosc. 1967, 23, 2127–2143. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Feng, Z.; Deng, X.; Li, Y.Q. Activation of Mg-Al hydrotalcite catalysts for transesterification of rape oil. Fuel 2008, 87, 3071–3076. [Google Scholar] [CrossRef]
- Santosa, S.J.; Kunarti, E.S. Karmanto. Synthesis and utilization of Mg/Al hydrotalcite for removing dissolved humic acid. App. Surface Sci. 2008, 254, 7612–7617. [Google Scholar] [CrossRef]
- Šepelák, V.; Indris, S.; Bergmann, I.; Feldhoff, A.; Becker, K.D. Nonequilibriumcation distribution in nanocrystalline MgAl2O4 spinel studied by 27Al magic-angle spinning NMR. Solid State Ion. 2006, 177, 2487–2490. [Google Scholar] [CrossRef]
- Harris, R.K.; Wasylishen, R.E.; Duer, M.J. (Eds.) NMR Crystallography; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Park, T.-J.; Choi, S.-S.; Kim, Y. 27Al solid-state NMR structural studies of hydrotalcite compounds calcined at different temperatures. Bull. Korean Chem. Soc. 2009, 30, 149–152. [Google Scholar]
- Shylesh, S.; Kim, D.; Gokhale, A.A.; Canlas, C.G.; Struppe, J.O.; Ho, C.R.; Jadhav, D.; Yeh, A.; Bell, A.T. Effects of composition and structure of Mg/Al oxides on their activity and selectivity for the condensation of methyl ketones. Ind. Eng. Chem. Res. 2016, 55, 10635–10644. [Google Scholar] [CrossRef]
- Béres, A.; Pálinkó, I.; Bertrand, J.-C.; Nagy, J.B.; Kiricsi, I. Dehydration-rehydration behaviour of layered double hydroxides: A study by X-ray diffractometry and MAS NMR spectroscopy. J. Mol. Struct. 1997, 410–411, 13–16. [Google Scholar] [CrossRef]
- Prescott, H.A.; Li, Z.-J.; Kemnitz, E.; Trunschke, A.; Deutsch, J.; Lieske, H.; Auroux, A. Application of calcined Mg-Al hydrotalcites for Michael additions: An investigation of catalytic activity and acid-base properties. J. Catal. 2005, 234, 119–130. [Google Scholar] [CrossRef]
- Lopes, T.R.; Gonçalves, G.R.; de Barcellos, E., Jr.; Schettino, M.A., Jr.; Cunha, A.G.; Emmerich, F.G.; Freitas, J.C.C. Solid state 27Al NMR and X-ray diffraction study of alumina-carbon composites. Carbon 2015, 93, 751–761. [Google Scholar] [CrossRef]
- O’Dell, L.A.; Savin, S.L.P.; Chadwick, A.V.; Smith, M.E. A 27Al MAS NMR study of a sol-gel produced alumina: Identification of the NMR parameters of the θ-Al2O3 transition alumina phase. Solid State Nucl. Magn. 2007, 31, 169–173. [Google Scholar] [CrossRef]
- Chagas, L.H.; De Carvalho, G.S.G.; San Gil, R.A.S.; Chiaro, S.S.X.; Leitão, A.A.; Diniz, R. Obtaining aluminas from the thermal decomposition of their different precursors: An 27Al MAS NMR and X-ray powder diffraction studies. Mater. Res. Bull. 2014, 49, 216–222. [Google Scholar] [CrossRef]
- Jackson, S.D.; Hargreaves, J.S.J. Metal Oxide Catalysis; Wiley-VCH Verlag GmbH: Weinheim, Germany; Co. KGaA, John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- vanBokhoven, J.A.; Roelofs, J.C.A.A.; de Jong, K.P.; Koningsberger, D.C. Unique structural properties of the Mg-Al hydrotalcite solid base catalyst: An in situ study using Mg and Al K-Edge XAFS during calcination and rehydration. Chem. Eur. J. 2001, 7, 1258–1265. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Wu, J.; Yi, X.; Zheng, A.; Umar, A.; O’Hare, D.; Wang, Q. Comprehensive investigation of CO2 adsorption on Mg-Al-CO3 LDH-derived mixed metal oxides. J. Mater. Chem. A. 2013, 1, 12782–12790. [Google Scholar] [CrossRef]
- Ono, Y.; Hattori, H. Solid Base Catalysis; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Valente, J.S.; Sánchez-Cantú, M.; Lima, E.; Figueras, F. Method for large-scale production of multimetallic layered double hydroxides: Formation mechanism discernment. Chem. Mater. 2009, 21, 5809–5818. [Google Scholar] [CrossRef]
- Takehira, K.; Kawabata, T.; Shishido, T.; Murakami, K.; Ohi, T.; Shoro, D.; Honda, M.; Takaki, K. Mechanism of reconstitution of hydrotalcite leading to eggshell-type Ni loading on Mg-Al mixed oxide. J. Catal. 2005, 231, 92–104. [Google Scholar] [CrossRef]
- Valente, J.S.; Pfeiffer, H.; Lima, E.; Prince, J.; Flores, J. Cyanoethylation of alcohols by activated Mg-Al layered double hydroxides: Influence of rehydration conditions and Mg/Al molar ratio on Brönsted basicity. J. Catal. 2011, 279, 196–204. [Google Scholar] [CrossRef]
- Díez, V.K.; Apesteguía, C.R.; Di Cosimo, J.I. Effect of the chemical composition on the catalytic performance of MgyAlOx catalysts for alcohol elimination reactions. J. Catal. 2003, 215, 220–233. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Smith, M.E. Multinuclear Solid-State Nuclear Magnetic Resonance of Inorganic Materials, 1st ed.; Pergamon Materials Series; Elsevier: London, UK, 2002. [Google Scholar]
- Di Cosimo, J.I.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Rebours, B.; d’Espinose de la Caillerie, J.-B.; Clause, O. Decoration of nickel and magnesium oxide crystallites with spinel-type phases. J. Am. Chem. Soc. 1994, 116, 1707–1717. [Google Scholar] [CrossRef]
- Bellotto, M.; Rebours, B.; Clause, O.; Lynch, J.; Bazin, D.; Elkaïm, E. Hydrotalcite decomposition mechanism: A clue to the structure and reactivity of spinel-like mixed oxides. J. Phys. Chem. 1996, 100, 8535–8542. [Google Scholar] [CrossRef]
- Reichle, W.T.; Kangand, S.Y.; Everhardt, D.S. The nature of the thermal decomposition of a catalytically active anionic clay mineral. J. Catal. 1986, 101, 352–359. [Google Scholar] [CrossRef]
- Rocha, J.; del Arco, M.; Rives, V.; Ulibarri, M.A. Reconstruction of layered double hydroxides from calcined precursors: A powder XRD and 27Al MAS NMR study. J. Mater. Chem. 1999, 9, 2499–2503. [Google Scholar] [CrossRef]
- McCarty, R.; Stebbins, J.F. Constraints on aluminum and scandium substitution mechanisms in forsterite, periclase, and larnite: High-resolution NMR. Am. Mineral. 2017, 102, 1244–1253. [Google Scholar] [CrossRef]
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Layered Material, 1st ed.; CRC Press: New York, NY, USA, 2004. [Google Scholar]
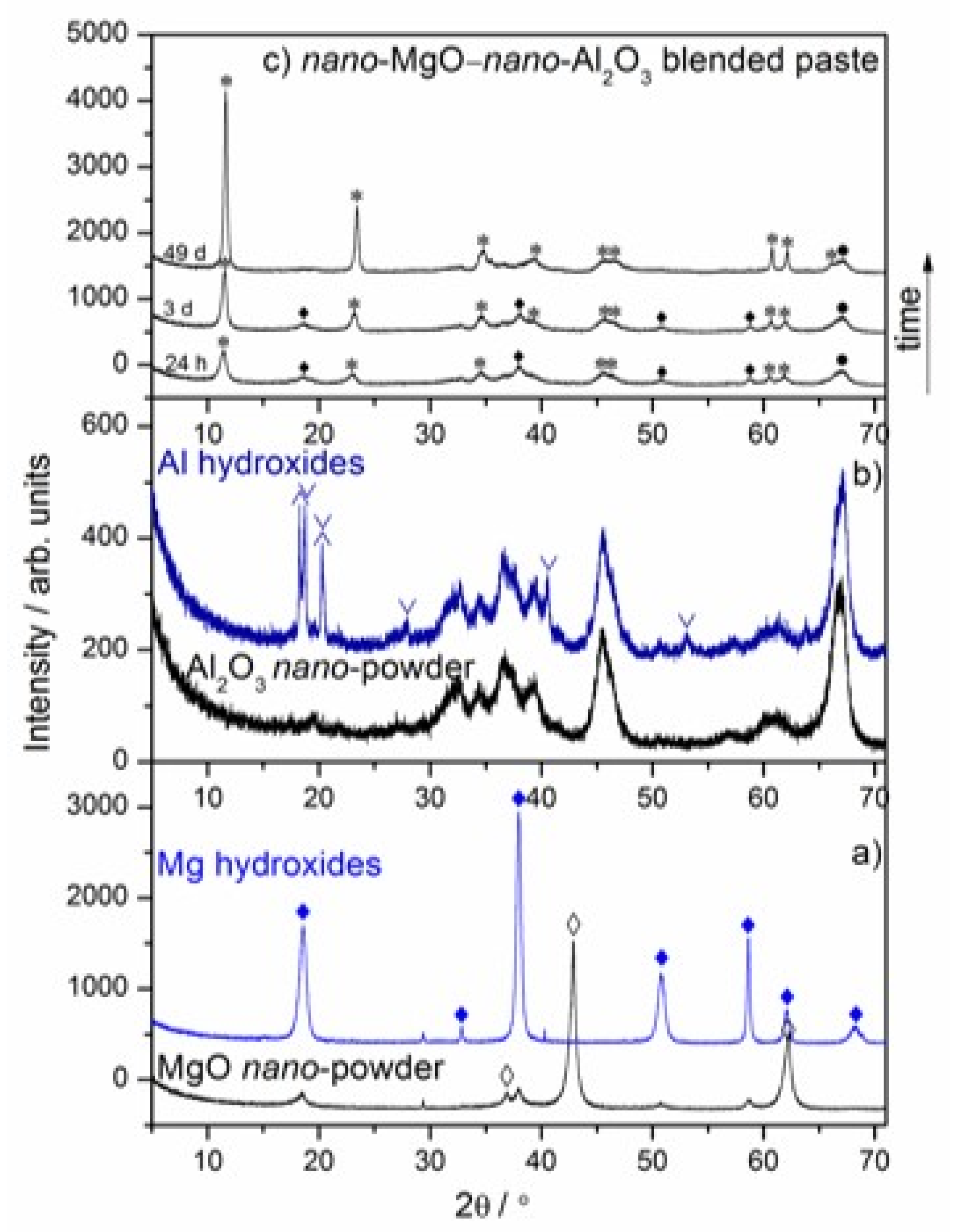
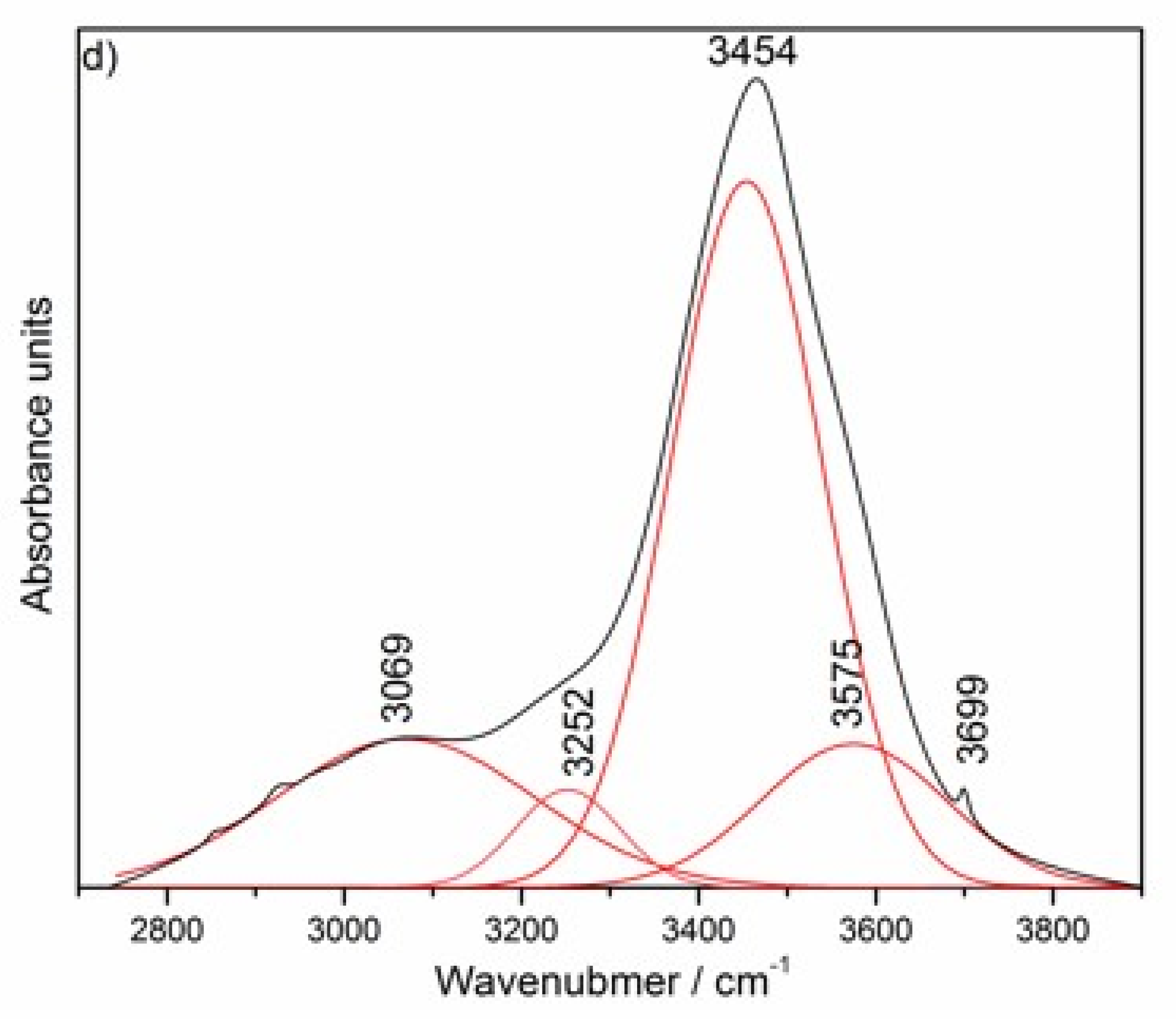
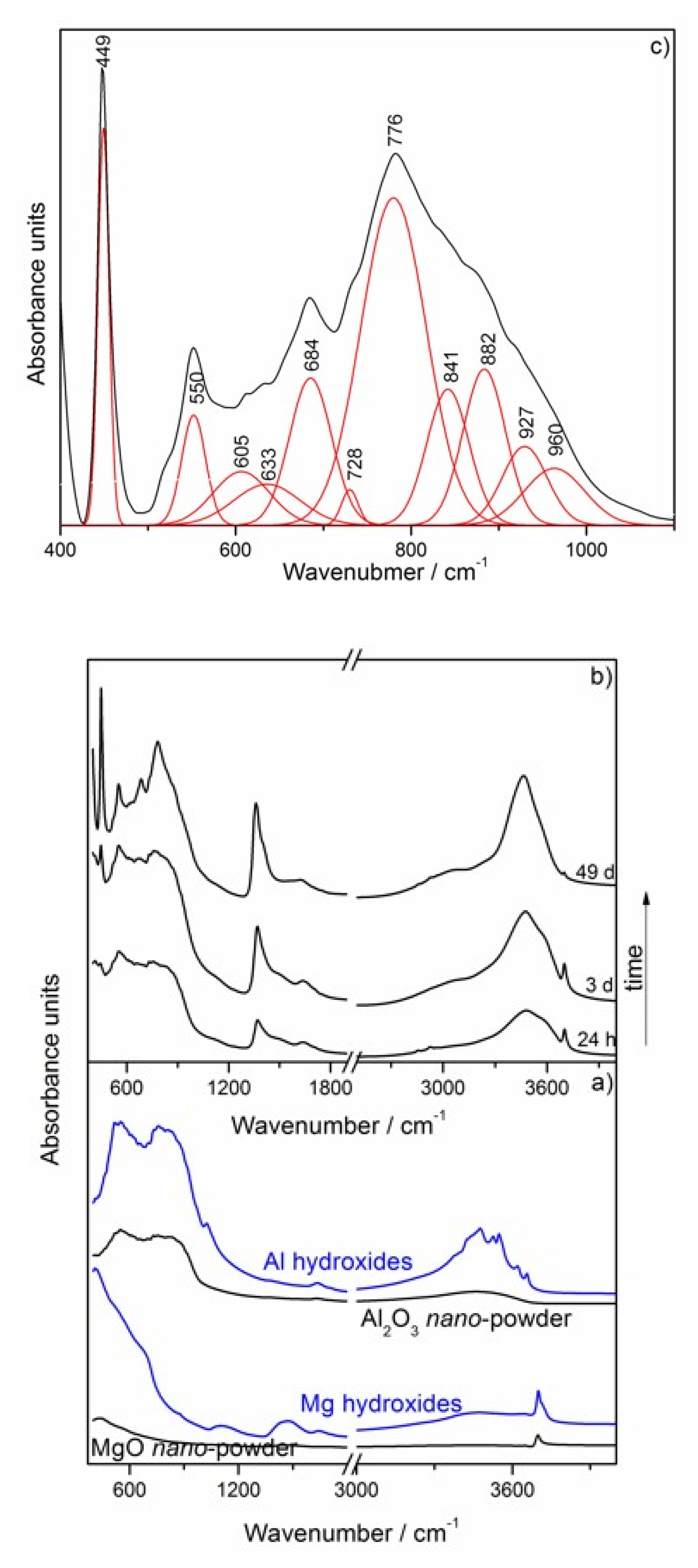
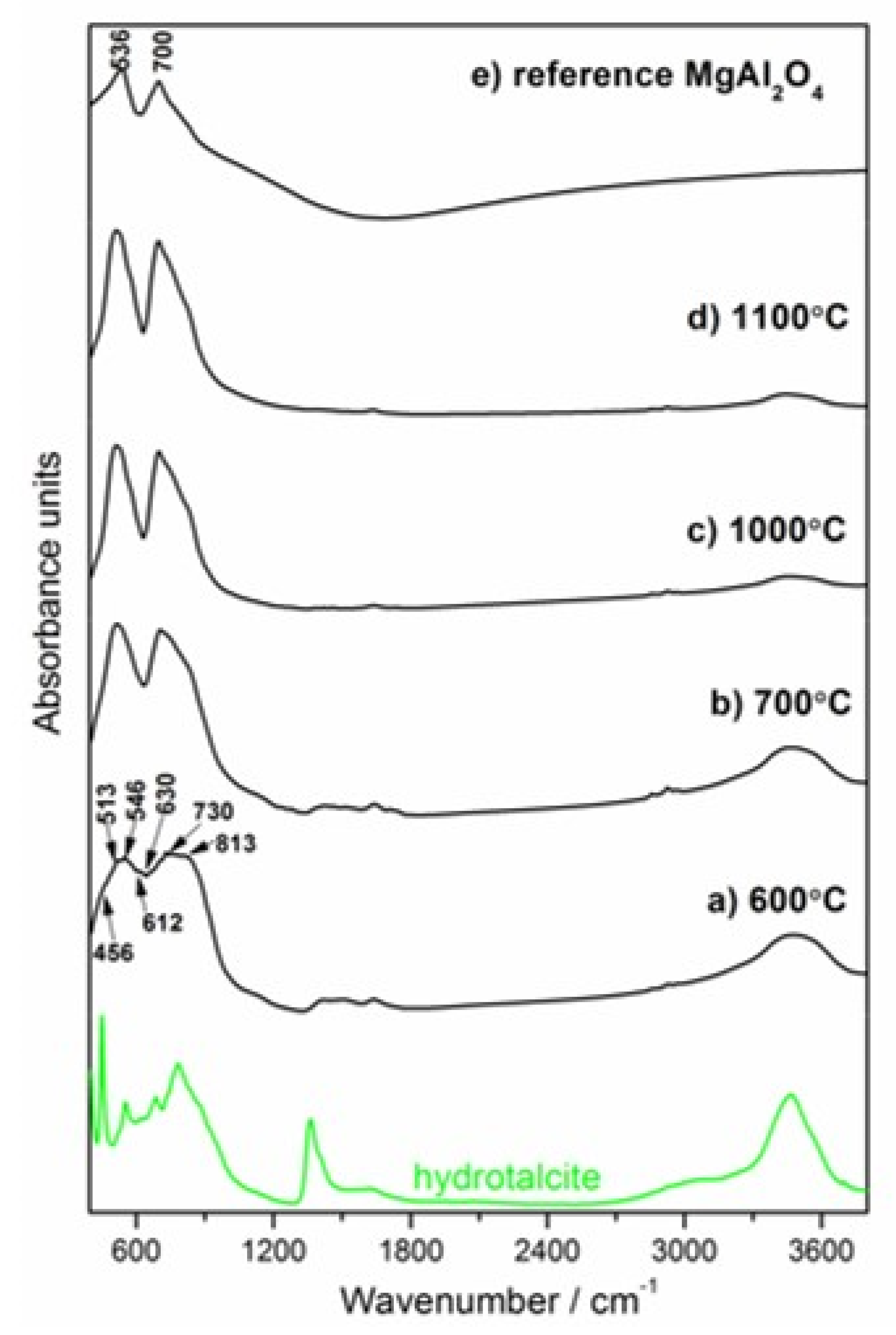
| Uncalcined Mg–Al–CO3–Hydrotalcite Band Positions [cm−1] | Assignment | |
|---|---|---|
| Bands Presented in this Work | Reference Bands | |
| 449 | 447, 449 [8,10,37] | M–O stretching and M–OH bending vibrations (M = Mg, Al) in the octahedral host layers |
| 550 | 552, 557 [8,37] | Al–OH translation modes |
| 605 | 629 [8,10] | Mg–OH translation modes |
| 684 | 667, 683 [8,10] | Antisymmetric deformation mode (ν4) of hydrotalcite ions |
| 776 | 783 or 772, 783 [8,10,37,38] | Strong out-of-plane symmetric deformation mode (ν2) of hydrotalcite ions or Al–OH translation modes |
| 1362 | 1358, 1365 [8,10,39] | Antisymmetric stretching vibration (ν3) of carbonate anion |
| 1628 | 1622, 1655 [10,39] | Bending vibration of interlayer water molecules (dO–H) |
| 3069 | 3045 [10,42] | H-bonded modes |
| 3252 | 3250 [8,10,40,42] | bridging mode |
| 3454 | 3450 [8,10] | The stretching vibrations of hydroxyl –OH groups attached to both Mg and Al in brucite-like layers (OH–Mg2Al) |
| 3575 | 3546 [8,10] | The stretching vibrations of hydroxyl –OH groups attached to Mg in brucite-like layers (OH–Mg3) |
| 3700 | 3700 [10] | O–H stretching vibration in brucite |
| Mortar | Heat Transport Parameters | ||
|---|---|---|---|
| Thermal Conductivity λ W/(mK) | Thermal Diffusivity a 106 J/(m3K) | Volume Heat Capacity Cv 10−6 m2/s | |
| CAC | 2.584 | 1.739 | 1.486 |
| MgO–Al2O3 | 2.063 | 1.546 | 1.335 |
| Mortar | Time of Curing | Cold Crushing Strength/MPa | Bending Strength/MPa |
|---|---|---|---|
| CAC | 3 days | 81.33 | 10.59 |
| 7 days | 101.82 | 13.52 | |
| MgO–Al2O3 | 3 days | 1.26 | 0.50 |
| 7 days | 1.54 | 0.74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madej, D.; Tyrała, K. In Situ Spinel Formation in a Smart Nano-Structured Matrix for No-Cement Refractory Castables. Materials 2020, 13, 1403. https://doi.org/10.3390/ma13061403
Madej D, Tyrała K. In Situ Spinel Formation in a Smart Nano-Structured Matrix for No-Cement Refractory Castables. Materials. 2020; 13(6):1403. https://doi.org/10.3390/ma13061403
Chicago/Turabian StyleMadej, Dominika, and Karina Tyrała. 2020. "In Situ Spinel Formation in a Smart Nano-Structured Matrix for No-Cement Refractory Castables" Materials 13, no. 6: 1403. https://doi.org/10.3390/ma13061403
APA StyleMadej, D., & Tyrała, K. (2020). In Situ Spinel Formation in a Smart Nano-Structured Matrix for No-Cement Refractory Castables. Materials, 13(6), 1403. https://doi.org/10.3390/ma13061403






