Study on Rheological Behavior of Micro/Nano-Silicon Carbide Particles in Ethanol by Selecting Efficient Dispersants
Abstract
:1. Introduction
2. Materials and Methods
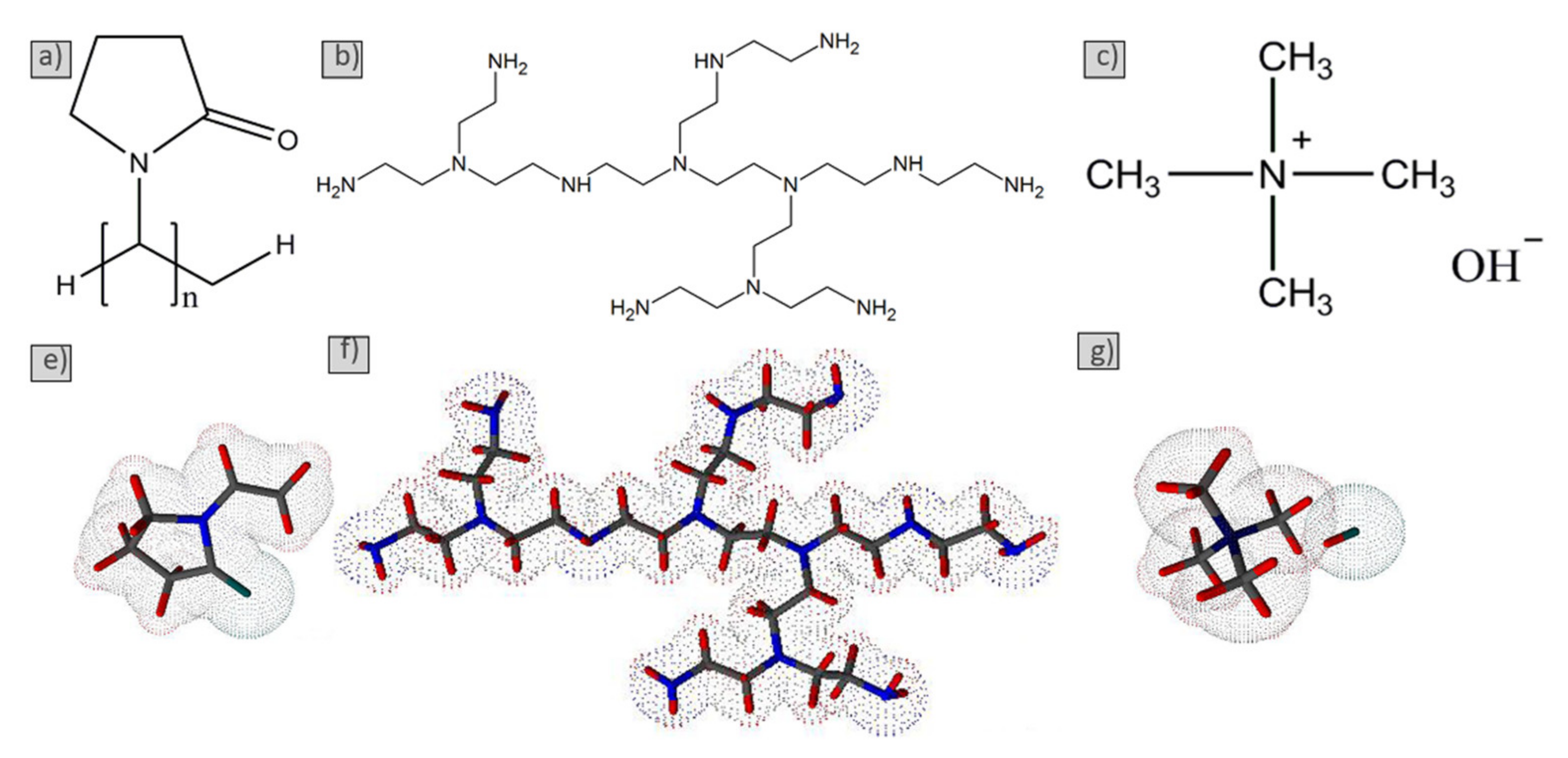
2.1. Starting Materials
2.2. Preparation of SiC Suspensions
2.3. Rheological Measurement
2.4. Adsorption Equilibrium of Dispersants on SiC Powder
2.5. FTIR and XPS Measurements
3. Results and Discussion
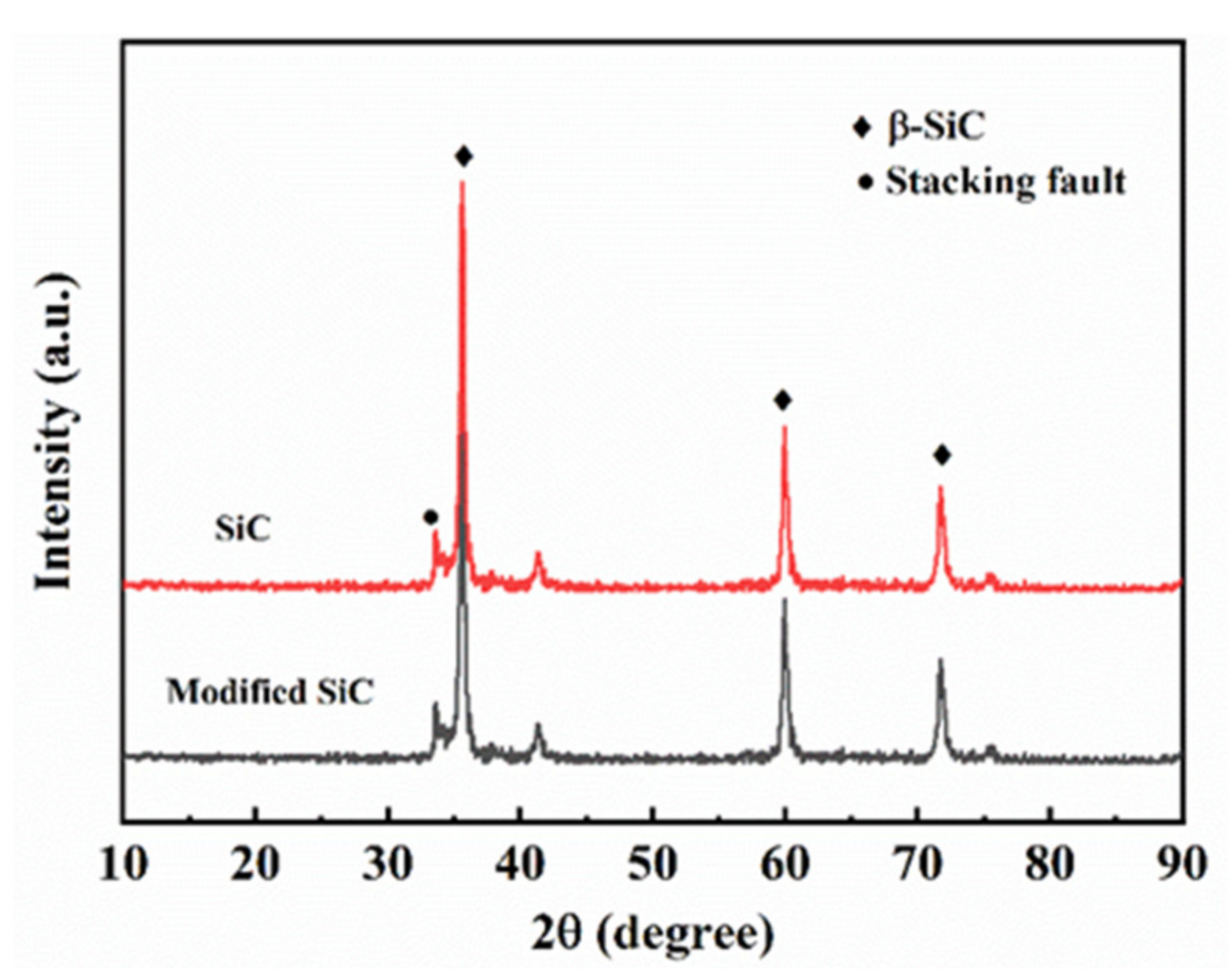
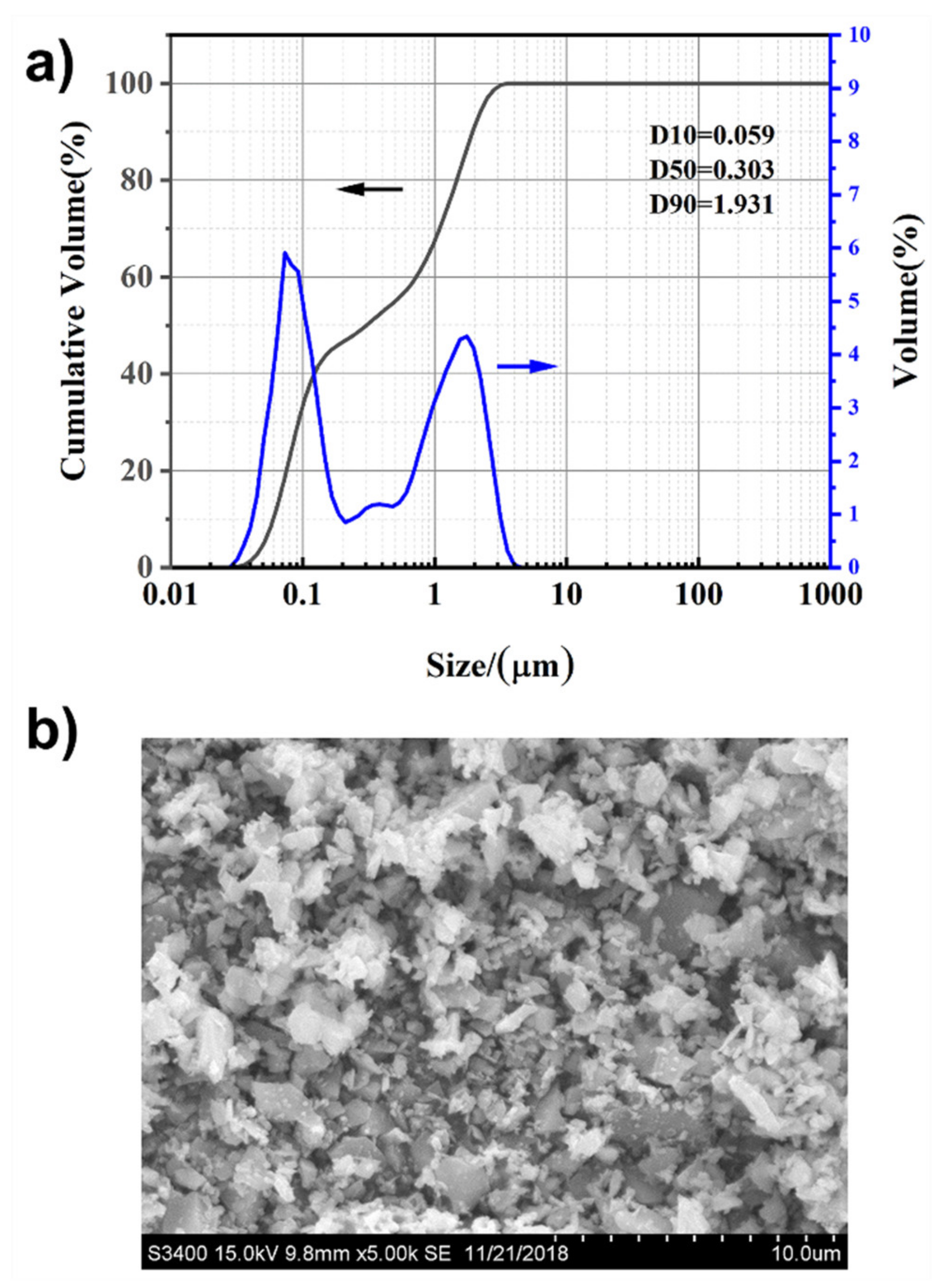
3.1. Characterization of the Silicon Carbide Powder
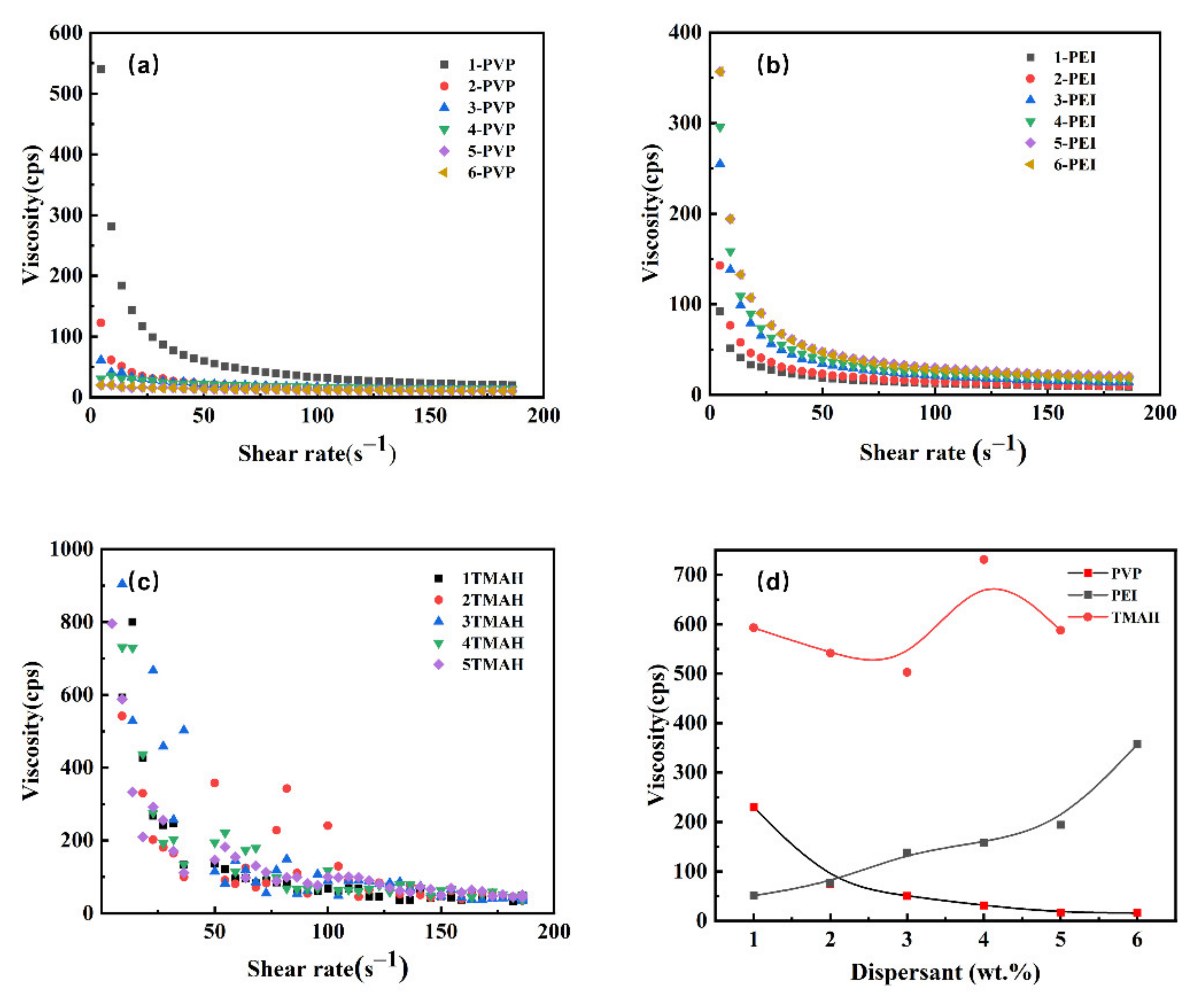
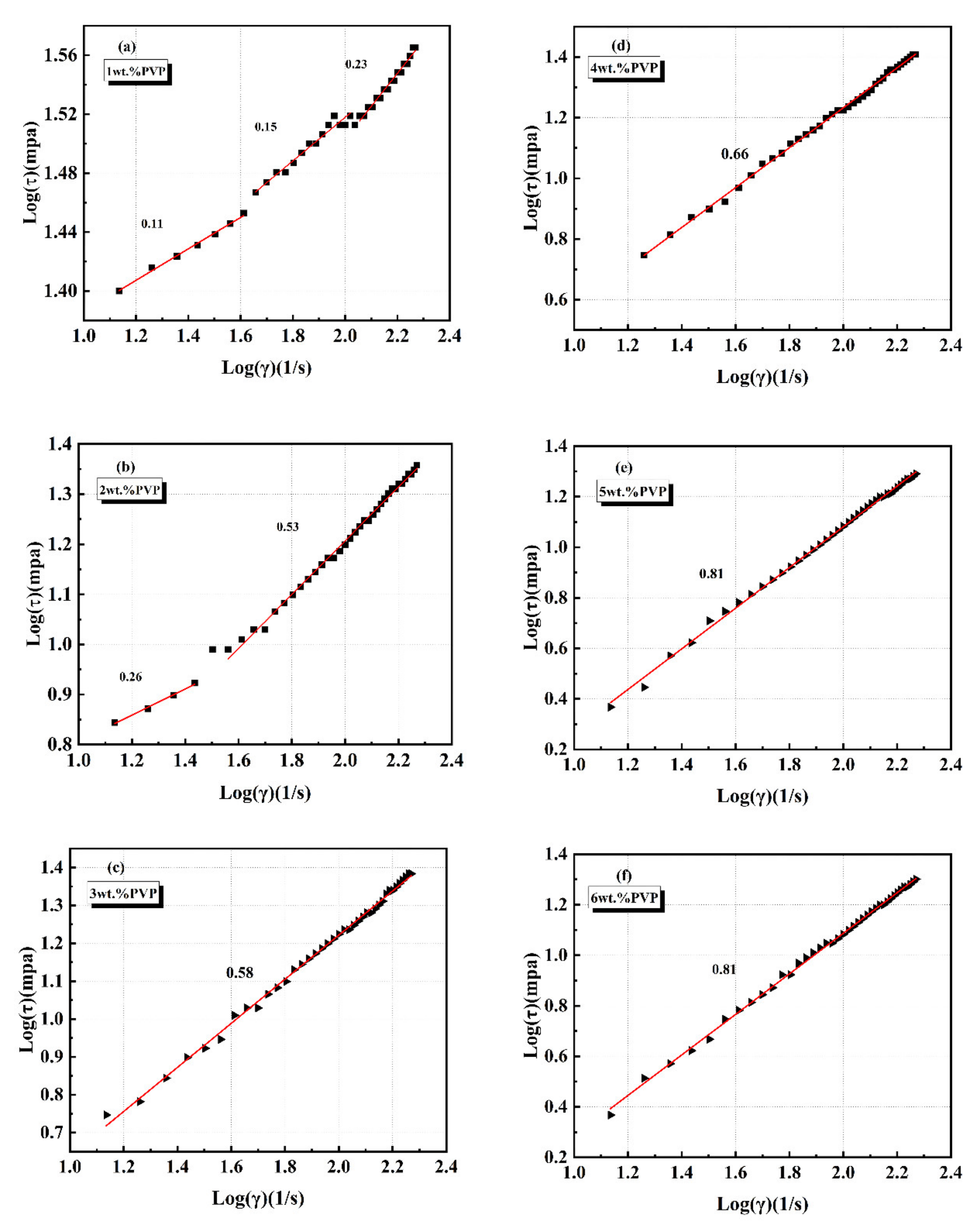
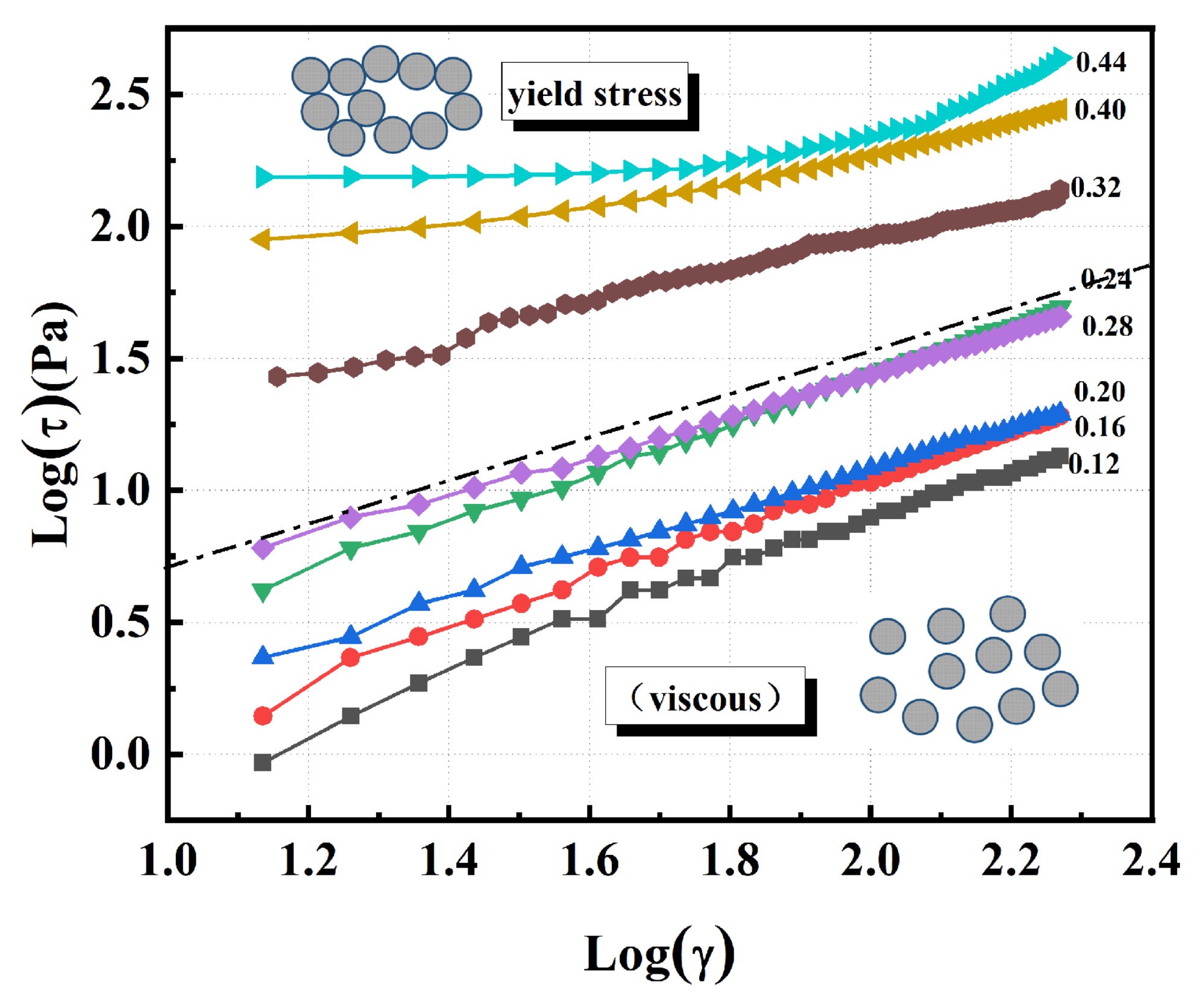
3.2. Rheological Measurement
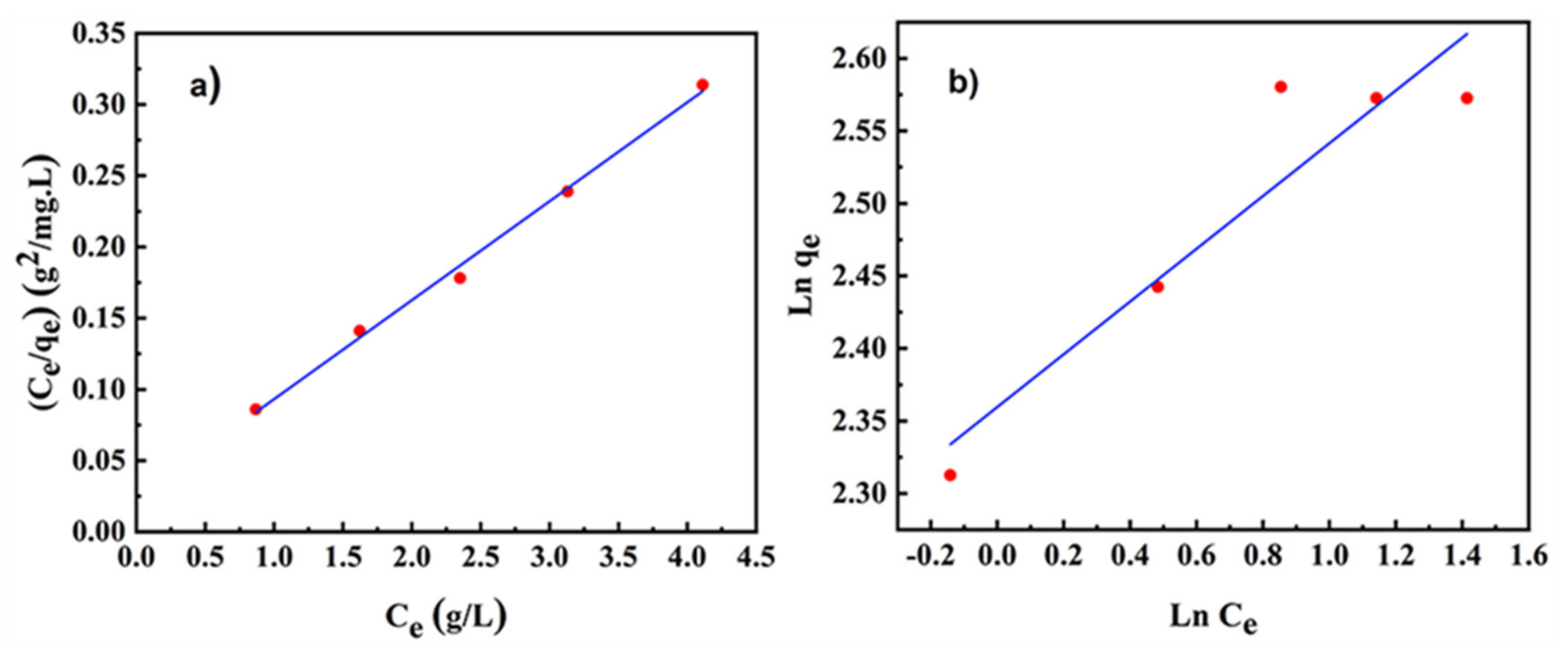
3.3. Adsorption Mechanism of Dispersants on SiC Powder
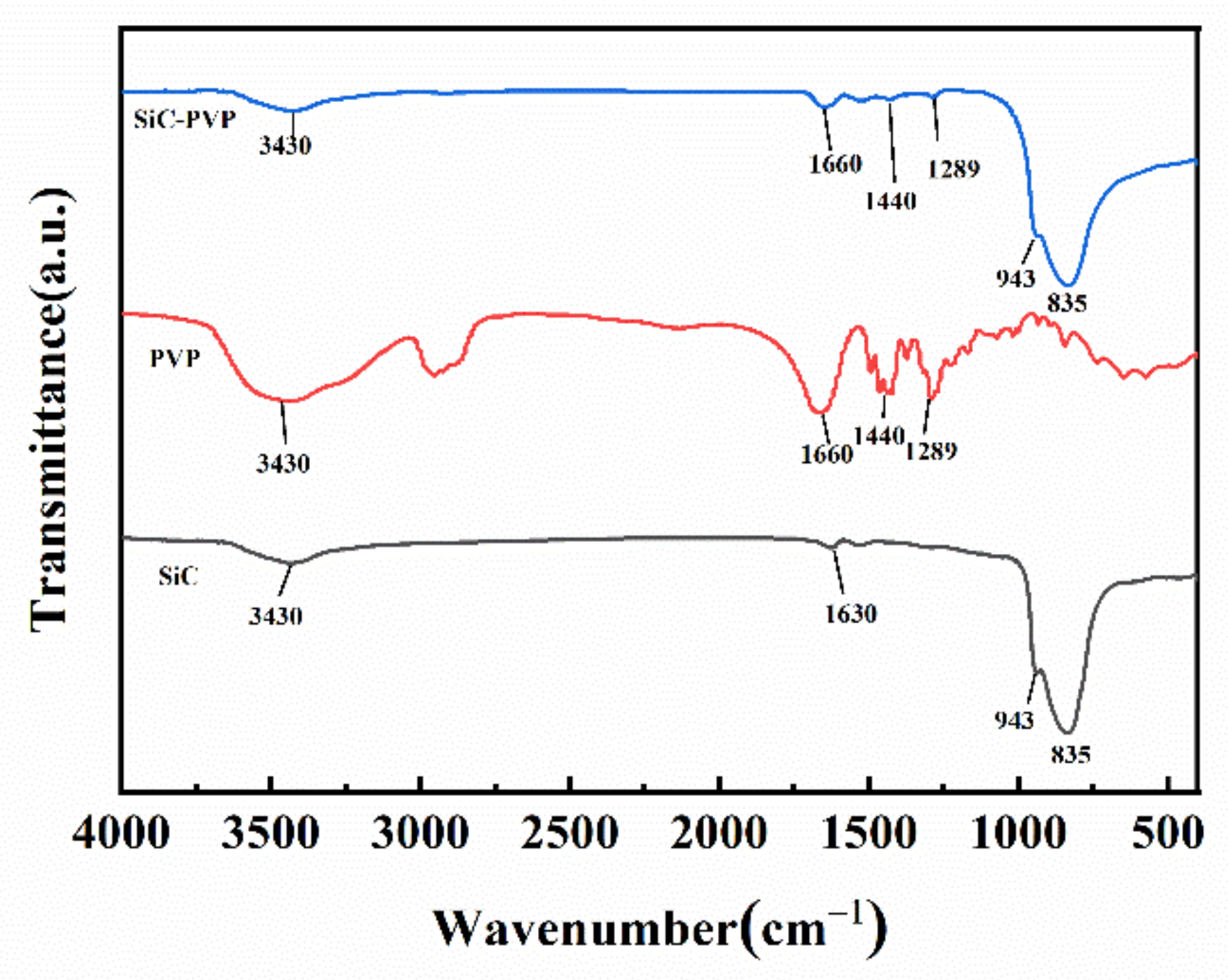
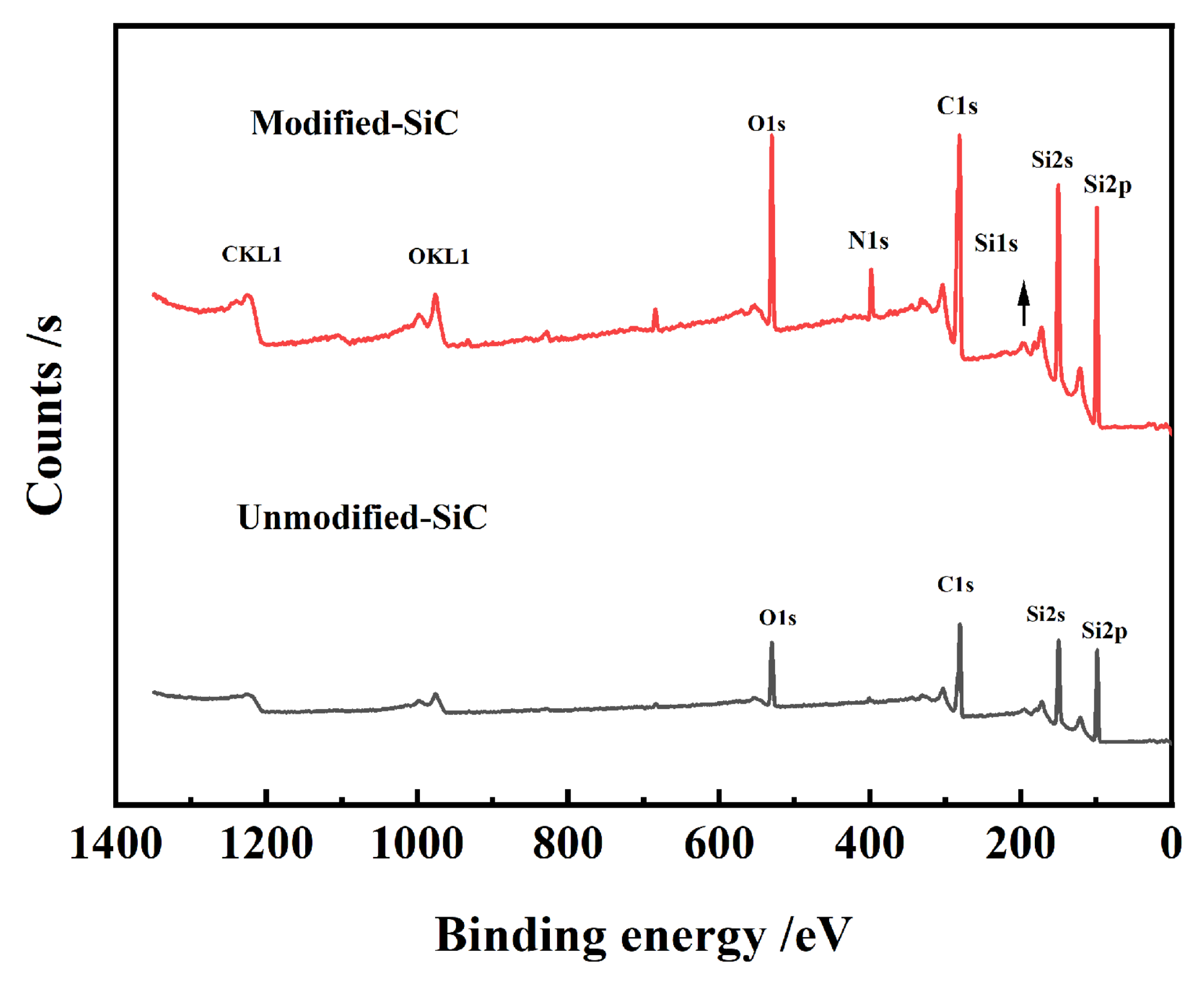
3.4. FTIR and XPS Characterization
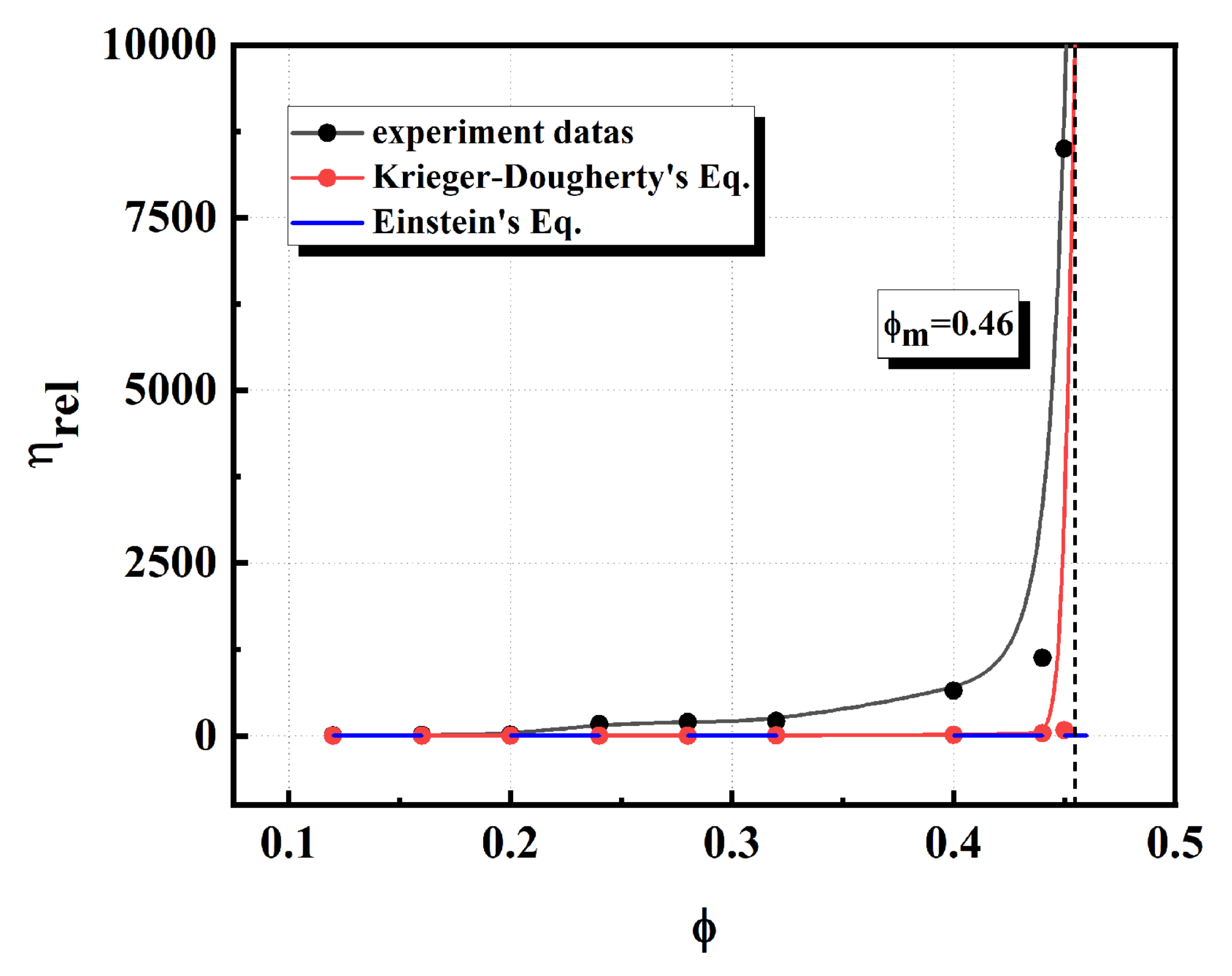
3.5. Maximum Ceramic Loading of the PVP Stabilized Systems
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Casady, R.W.J.B. Status of silicon carbide (SiC) as a wide bandgap semiconductor for high temperature applications: A review. J. Solid State Electron. 1996, 39, 1409–1422. [Google Scholar] [CrossRef]
- Nakamura, D.; Gunjishima, I.; Yamaguchi, S. Ultrahigh-Quality Silicon Carbide Single Crystals. Nature 2004, 430, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Madar, R. Material Science-Silicon carbide in contention. Nature 2004, 430, 974–975. [Google Scholar] [CrossRef] [PubMed]
- Lange, F.F. Powder Processing Science and Technology for Increased Reliability. J. Am. Ceram. Soc. 1989, 72, 3–15. [Google Scholar] [CrossRef]
- Fan, J.; Chu, P.K. Silicon Carbide Nanostructures: Fabrication, Structure, and Properties; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; p. 330. [Google Scholar]
- Hotza, D.; Greil, P. Review: Aqueous tape casting of ceramic powders. Mater. Sci. Eng. A 1994, 202, 206–217. [Google Scholar] [CrossRef]
- Montanaro, L.; Coppola, B.; Palmero, P. A review on aqueous gelcasting: A versatile and low-toxic technique to shape ceramics. Ceram. Int. 2019, 45, 9653–9673. [Google Scholar] [CrossRef]
- Jabbari, M.; Bulatova, R.; Tok, A.I.Y. Ceramic tape casting: A review of current methods and trends with emphasis on rheological behaviour and flow analysis. Mater. Sci. Eng. B 2016, 212, 39–61. [Google Scholar] [CrossRef]
- Yu, J.; Yang, J.; Huang, Y. The transformation mechanism from suspension to green body and the development of colloidal forming. Ceram. Int. 2011, 37, 1435–1451. [Google Scholar] [CrossRef]
- Iijima, M. Surface modification techniques toward controlling the dispersion stability and particle-assembled structures of slurries. J. Ceram. Soc. Jpn. 2017, 125, 603–607. [Google Scholar] [CrossRef] [Green Version]
- Rödel, C.; Müller, M.; Glorius, M. Effect of varied powder processing routes on the stabilizing performance and coordination type of polyacrylate in alumina suspensions. J. Eur. Ceram. Soc. 2011, 32, 363–370. [Google Scholar] [CrossRef]
- Zhang, Y.; Binner, J. Effect of dispersants on the rheology of aqueous silicon carbide suspensions. Ceram. Int. 2008, 34, 1381–1386. [Google Scholar] [CrossRef] [Green Version]
- Makio Naito, T.Y.K.H. Nanoparticle Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ganguly, S.; Chakraborty, S. Sedimentation of nanoparticles in nanoscale colloidal suspensions. Phys. Lett. A 2011, 375, 2394–2399. [Google Scholar] [CrossRef]
- Vázquez, G.; Alvarez, E.; Navaza, J.M. Surface Tension of Alcohol + Water from 20 to 50 °C. J. Chem. Eng. Data. 1995, 40, 611–614. [Google Scholar] [CrossRef]
- Raiteri, P.; Laio, A.; Parrinello, M. Correlations among hydrogen bonds in liquid water. Phys. Rev. Lett. 2004, 93, 87801. [Google Scholar] [CrossRef] [PubMed]
- Luzar, A.; Chandler, D. Hydrogen-bond kinetics in liquid water. Nature 1996, 379, 55–57. [Google Scholar] [CrossRef]
- Riedel, R.; Chen, I.W. Ceramics Science and Technology: Volume3: Synthesis and Processing; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Chin, C.H.; Muchtar, A.; Azhari, C.H.; Razali, M. Optimization of pH and Dispersant Amount of Y-TZP Suspension for Colloidal Stability. Ceram Int. 2015, 41, 9939–9946. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Chen, Y. Efficient dispersants for the dispersion of gallium zinc oxide nanopowder in aqueous suspensions. J. Am. Ceram. Soc. 2017, 100, 920–928. [Google Scholar] [CrossRef]
- Foratirad, H.; Baharvandi, H.R.; Maragheh, M.G. Effects of dispersants on dispersibility of titanium carbide aqueous suspension. Int. J. Refract. Met. H 2016, 56, 96–103. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Ye, F. Effect of citric acid on the adsorption behavior of polyethylene imine (PEI) and the relevant stability of SiC slurries. Colloids Surf. A 2006, 276, 168–175. [Google Scholar] [CrossRef]
- Avadiar, L.; Leong, Y. Interactions of PEI (polyethylenimine)–silica particles with citric acid in dispersions. Colloid. Poly. Sci. 2011, 289, 237–245. [Google Scholar] [CrossRef]
- Barick, P.; Prasad, S.B.; Mitra, R. Effect of concentration and molecular weight of polyethylenimine on zeta potential, isoelectric point of nanocrystalline silicon carbide in aqueous and ethanol medium. Ceram. Int. 2015, 41, 4289–4293. [Google Scholar] [CrossRef]
- Li, W.; Chen, P.; Gu, M. Effect of TMAH on rheological behavior of SiC aqueous suspension. J. Eur. Ceram. Soc. 2004, 24, 3679–3684. [Google Scholar] [CrossRef]
- Iijima, M.; Okamura, N.; Tatami, J. Polyethyleneimine–Oleic Acid Complex as a Polymeric Dispersant for Si3N4 and Si3N4-Based Multicomponent Nonaqueous Slurries. Ind. Eng. Chem. Res. 2015, 54, 12847–12854. [Google Scholar] [CrossRef]
- Li, C.; Jhang, J.; Tsai, H. Water-soluble polyethylenimine as an efficient dispersant for gallium zinc oxide nanopowder in organic-based suspensions. Powder. Technol. 2017, 305, 226–231. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J. Study on modification effect and mechanism of binary modifier co-modified silicon carbide powder. Mater. Res. Express. 2018, 6, 35204. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Shimai, S. Effect of TMAH on the rheological behavior of alumina slurries for gelcasting. J. Asian. Ceram. Soc. 2018, 5, 261–265. [Google Scholar] [CrossRef] [Green Version]
- Vieira, L.E.; Neto, J.B.R.; Klein, A.N. Colloidal processing of highly concentrated aqueous copper suspensions. Powder. Technol. 2014, 256, 540–544. [Google Scholar] [CrossRef]
- Vilinska, A.; Ponnurangam, S.; Chernyshova, I. Stabilization of Silicon Carbide (SiC) micro- and nanoparticle dispersions in the presence of concentrated electrolyte. J. Colloid. Interf. Sci. 2014, 423, 48–53. [Google Scholar] [CrossRef]
- Gan, K.; Xu, J.; Lu, Y. Preparation of silicon carbide ceramics using chemical treated powder by DCC via dispersant reaction and liquid phase sintering. J. Eur. Ceram. Soc. 2017, 37, 891–897. [Google Scholar] [CrossRef]
- Barick, P.; Mitra, R.; Saha, B.P. Influence of a few important parameters on the rheological behaviour of silicon carbide nanoparticles dispersed aqueous suspension. Ceram. Int. 2018, 44, 9070–9075. [Google Scholar] [CrossRef]
- Marani, D.; Sudireddy, B.R.; Nielsen, L. Poly(vinylpyrrolidone) as dispersing agent for cerium-gadolinium oxide (CGO) suspensions. J. Mater. Sci. 2016, 51, 1098–1106. [Google Scholar] [CrossRef]
- Bailly, N.; Thomas, M.; Klumperman, B. Poly(N-vinylpyrrolidone)-block-poly(vinyl acetate) as a Drug Delivery Vehicle for HydrophobicDrugs. Biomacromolecules 2012, 13, 4109–4117. [Google Scholar] [CrossRef]
- Kim, D.; Park, I.; Lee, M. Tensile strength of aqueous-based alumina tapes using a PVP–PVA–gelatin cobinder. Ceram. Int. 2005, 31, 577–581. [Google Scholar] [CrossRef]
- Ong, B.C.; Leong, Y.K.; Chen, S.B. Interparticle forces in spherical monodispersed silica dispersions: Effects of branched polyethylenimine and molecular weight. J. Colloid. Interf. Sci. 2009, 337, 24–31. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Mottaghitalab, V.; Rezaei, M. Rheological and viscoelastic behavior of concentrated colloidal suspensions of silica nanoparticles: A response surface methodology approach. Adv. Powder. Technol. 2015, 26, 1570–1577. [Google Scholar] [CrossRef]
- Li, X.; Zou, C.; Wang, T. Rheological behavior of ethylene glycol-based SiC nanofluids. Int. J. Heat. Mass. Tran. 2015, 84, 925–930. [Google Scholar] [CrossRef]
- Cwalina, C.D.; Wagner, N.J. Rheology of non-Brownian particles suspended in concentrated colloidal dispersions at low particle Reynolds number. J. Rheol. 2016, 60, 47–59. [Google Scholar] [CrossRef]
- Jamali, S.; Boromand, A.; Wagner, N. Microstructure and rheology of soft to rigid shear-thickening colloidal suspensions. J. Rheol. 2015, 59, 1377–1395. [Google Scholar] [CrossRef] [Green Version]
- Marani, D.; Hjelm, J.; Wandel, M. Rheological analysis of stabilized cerium-gadolinium oxide (CGO) dispersions. J. Eur. Ceram. Soc. 2014, 34, 695–702. [Google Scholar] [CrossRef]
- Willenbacher, N.; Georgieva, K. Rheology of Disperse Systems; Wiley-VCH: Weinheim, Germany, 2013; pp. 7–46. [Google Scholar]
- Xiao, C.; Gao, L.; Lu, M. Effect of polyvinylpyrrolidone on rheology of aqueous SiC suspensions with polyethylene glycol as binder. Colloids Surf. A 2010, 368, 53–57. [Google Scholar] [CrossRef]
- Xiao, C.; Gao, L.; Lu, M. Synergistic effect of copolymer and poly(vinylpyrrolidone) mixtures on rheology of aqueous SiC suspensions. Colloids Surf. A 2010, 355, 104–108. [Google Scholar] [CrossRef]
- De Riccardis, M.F.; Carbone, D. Study of Polymer Particles Suspensions for Electrophoretic Deposition. J. Phys. Chem. B 2013, 117, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Kissa, E. Dispersions: Characterization, Testing, and Measurement; Dekker, M., Ed.; CRC Press: Boca Raton, FL, USA, 1999; p. 695. [Google Scholar]
- George, V.C.; Das, A.; Roy, M. Bias enhanced deposition of highly oriented β-SiC thin films using low pressure hot filament chemical vapour deposition technique. Thin Solid Films 2002, 410, 114–117. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Zhao, S. Effect of surface modification and medium on the rheological properties of silica nanoparticle suspensions. Ceram. Int. 2016, 42, 7767–7773. [Google Scholar] [CrossRef]
- Mewis, J.; Wagner, N.J. Colloidal Suspension Rheology; Cambridge University Press: Cambridge, UK, 2012; p. 415. [Google Scholar]
- Smith, T.L.; Bruce, C.A. Intrinsic viscosities and other rheological properties of flocculated suspensions of nonmagnetic and magnetic ferric oxides. J. Colloid Interf. Sci. 1979, 72, 13–26. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, G.; Zhang, Z.; Hu, J.; Zhang, J.; Sun, Y.; Shen, Q.; Zhang, L. Study on Rheological Behavior of Micro/Nano-Silicon Carbide Particles in Ethanol by Selecting Efficient Dispersants. Materials 2020, 13, 1496. https://doi.org/10.3390/ma13071496
Luo G, Zhang Z, Hu J, Zhang J, Sun Y, Shen Q, Zhang L. Study on Rheological Behavior of Micro/Nano-Silicon Carbide Particles in Ethanol by Selecting Efficient Dispersants. Materials. 2020; 13(7):1496. https://doi.org/10.3390/ma13071496
Chicago/Turabian StyleLuo, Guoqiang, Zhuang Zhang, Jianian Hu, Jian Zhang, Yi Sun, Qiang Shen, and Lianmeng Zhang. 2020. "Study on Rheological Behavior of Micro/Nano-Silicon Carbide Particles in Ethanol by Selecting Efficient Dispersants" Materials 13, no. 7: 1496. https://doi.org/10.3390/ma13071496
APA StyleLuo, G., Zhang, Z., Hu, J., Zhang, J., Sun, Y., Shen, Q., & Zhang, L. (2020). Study on Rheological Behavior of Micro/Nano-Silicon Carbide Particles in Ethanol by Selecting Efficient Dispersants. Materials, 13(7), 1496. https://doi.org/10.3390/ma13071496




