Abstract
Lithium tantalite (LiTaO3) is a common piezoelectric and ferroelectric crystal, but the LiTaO3 polycrystalline ceramics have rarely been reported, and their refractory character presents difficulties in their fabrication. In this study, LiTaO3-based ceramics with different amounts of CoO were prepared by pressureless sintering at 1250 °C, and the effects of the amount of sintering aid on the sinterability, microstructure, and dielectric properties of the ceramics were investigated. The relative densities of the LiTaO3-based ceramics were significantly improved by the addition of CoO powder. The LiTaO3-based ceramics achieved the highest relative density (89.4%) and obtained a well-grained microstructure when the added amount of CoO was 5 wt.%. Only the LiTaO3 phase in the ceramics was observed, indicating that the ions Co diffused into the LiTaO3 lattices and mainly existed in two forms: Co2+ and Co3+. The effects of the added amount of CoO on the dielectric properties of the LiTaO3-based ceramics were studied thoroughly. Consequently, the dielectric constant was enhanced, and the dielectric loss decreased in the LiTaO3-based ceramics with the addition of CoO. The optimal value was obtained at 5 wt.% of CoO-added LiTaO3-based ceramics.
1. Introduction
Lithium tantalite (LiTaO3), an excellent single crystal, has high planar electromechanical coupling, a mechanical quality factor, and low acoustic transmission loss and is used in various applications because of its low dielectric constant [1,2,3,4]. Many previous studies have focused on single crystal LiTaO3 [5,6,7,8]. However, relatively little attention has been paid to the fabrication and sintering of LiTaO3 ceramics or their microstructures and properties, because LiTaO3 is beset with difficulties regarding densification in the sintering process [9,10,11,12,13]. Previous studies have tried to facilitate the sintering of the LiTaO3 ceramics by adding oxides/fluorides or by doping with two steps of powder synthesis first, followed by sintering [14,15]. Meanwhile, there have been many studies on LiTaO3 solid solution systems such as Mg, CaTiO3, Ag, Cu-doped and LiF, MgF2 co-doped LiTaO3 systems [16,17,18,19,20]. Nowadays, lead zirconate titanate (PZT) is the most widely-used piezoelectric ceramic, but the production and use of lead-containing materials will produce serious lead volatilization, which is not conducive to environmental protection, and the toxicity of lead oxide is very large. Therefore, in the field of electronic ceramics, even though the piezoelectric properties of lead-free piezoelectric ceramics are less than those of lead-containing piezoelectric ceramics, many lead-free ceramic materials have been developed to replace these lead-based ceramic materials in recent years [21,22]. The research on LiTaO3-based piezoelectric ceramics is valuable for the application in potential engineering and is expected to lead to the development of a new lead-free piezoelectric ceramic. However, the sintering process, microstructures, and properties of LiTaO3-based ceramics are yet to be investigated systematically.
There are three main reasons why it is difficult to fabricate high-density LiTaO3-based ceramics. First, sintering densification is accompanied by grain growth, and an increased sintering temperature leads to abnormal grain growth in the ceramic, resulting in residual pores at grain boundaries [15]. Second, due to the crystal structure of LiTaO3, it has significant crystallographic anisotropy in its coefficients of thermal expansion; this causes tremendous stress during cooling, thereby making it challenging to sinter a dense LiTaO3 ceramic [23]. Third, slight volatilization of Li2O at higher sintering temperatures (over 1300 °C) causes Li deficiency [24]. Therefore, preparing a dense LiTaO3-based ceramic requires an efficient sintering aid. Co is a multi-valence element that can improve the performance of many functional ceramics. Accordingly, researchers have shown that Co doping can improve the dielectric properties of ceramics [25,26,27,28]. In this paper, CoO was added to fabricate the LiTaO3-based ceramics through a pressureless sintering process using raw CoO and LiTaO3 powders. The LiTaO3-based ceramics were successfully fabricated herein by adding different amounts of CoO. The effects of the different components on the sinterability and dielectric properties of the LiTaO3-based ceramics were studied.
2. Experimental Details
LiTaO3-based ceramics with different amounts of CoO (mass fractions of 0, 1, 3, 5, and 7 wt.%, referred to as 0CLT, 1CLT, 3CLT, 5CLT, and 7CLT, respectively) were fabricated by a pressureless sintering method. Commercially available LiTaO3 powder (Fangxiang Industry Co. Ltd., Shanghai, China) and CoO powder (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) were used as the raw materials. On the basis of the mass ratio, the two powders were weighed and then ball-milled in alcohol with carnelian balls for 24 h, after which the slurry was stirred and dried to obtain CoO/LiTaO3 (CLT) composite powder. The composite powder was mixed with a 7 wt.% polyvinyl alcohol binder, pressed into discs with diameters of 16 mm and thicknesses of approximately 2 mm and calcinated at 800 °C for 2 h. Finally, each disc was sintered at a sintering temperature of 1250 °C for 3 h. The relative density of each sintered ceramic was calculated on the basis of the sample volume and mass; the theoretical density was approximately 7.45, 7.44, 7.42, 7.39, and 7.37 g/cm3 for 0, 1, 3, 5, and 7CLT, respectively. The crystalline structure was obtained using an X-ray diffractometer (X’Pert PRO; PANalytical, The Netherlands) using Cu Kα radiation. The surface element compositions and the chemical states of the samples were analyzed through X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Fisher, USA) with Al Ka radiation. The microstructure of the ceramic was characterized using a scanning electron microscope (SEM) (S-3400N; Hitachi, Japan). The samples were plated by silver painting on both sides of the polishing pellets and then kept warm at 850 °C for 10 min to characterize the dielectric properties. The frequency-dependent dielectric constant and the dielectric loss were obtained at room temperature from 100 Hz to 1 MHz using an impedance analyzer (Model Agilent E4990A, Central South University, Hunan, China). The temperature dependence of the dielectric constant and the dielectric loss was measured by an LCR meter (Model HP4284A, Agilent Technologies Ltd., Hyogo, Japan) at 10 kHz from room temperature to 750 °C.
3. Results and Discussion
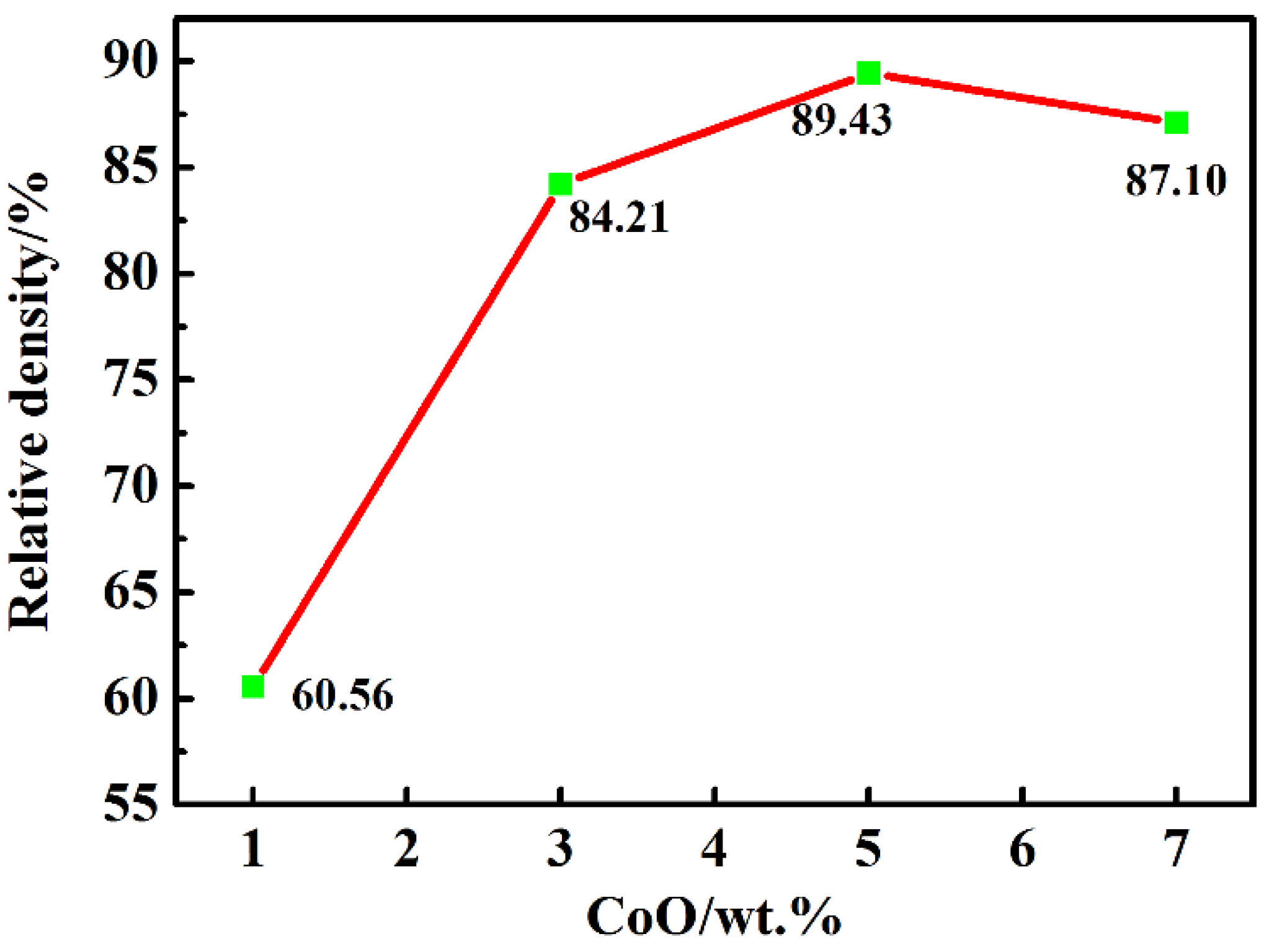
The relative densities of the LiTaO3-based ceramics of 1, 3, 5, and 7CLT sintered at 1250 °C are given in Figure 1. The relative density of all samples obviously increased at first and then decreased subsequently with an increasing amount of CoO; the maximum value (89.4%) was obtained at 5CLT. As a sintering aid, CoO can efficiently accelerate the sintering process of the LiTaO3-based ceramics and promote densification, but the addition of excessive CoO decreased the density of ceramics, which could be related to the inhomogeneity of the grain growth during the sintering process. Yao and Li et al. also reported similar results when studying the effect of MnO2 on the LiTaO3 and (Bi0.5Na0.5)0.94Ba0.06TiO3 ceramics [29,30].
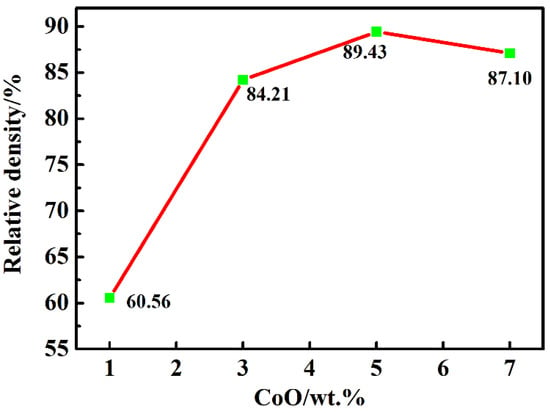
Figure 1.
Relative density of LiTaO3-based ceramics with different amounts of CoO.
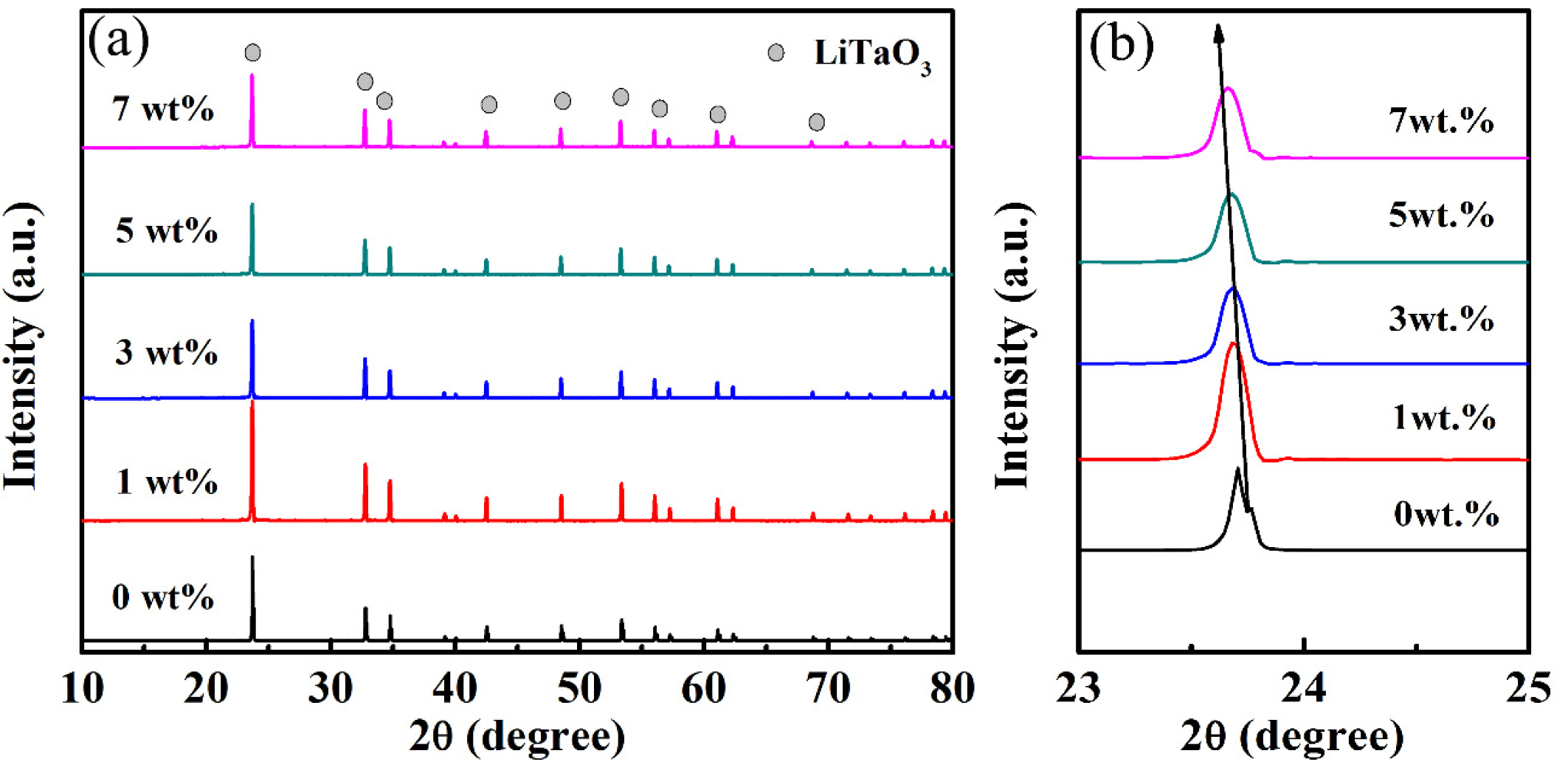
Figure 2 shows the XRD patterns of the LiTaO3-based ceramics with different amounts of CoO. It can be clearly seen that only one phase of LiTaO3 exists in the LiTaO3-based ceramics with different amounts of CoO added, and this was indexed well with a trigonal (rhombohedral) crystal structure (JCPDS card number: 29-0836). Furthermore, a secondary phase was not found in samples, which means that CoO probably diffused into the LiTaO3 lattice to form solid solutions, and the crystal structure of LiTaO3 was not significantly changed in the LiTaO3-based ceramics with the addition of CoO. The details of the XRD results can be observed in Figure 2b, which shows a partial main diffraction peak of the patterns of the LiTaO3-based ceramics. Figure 2b shows that the main diffraction peak moved towards the left (low angle direction) with an increase in the added amount of CoO. This result supports the prediction that the Co ions would diffuse into the LiTaO3 lattices, thereby leading to an expansion of the lattice. Since the radius of Co2+ is 0.065 nm (in low spin states) and 0.0735 nm (in high spin states), the radius of Co3+ is 0.0545 nm (in low spin states) and 0.061 nm (in high spin states), and that of Ta5+ is 0.064 nm [31]. The replacement of the smaller Ta5+ by Co2+ cations caused lattice expansion of LiTaO3 and the diffraction peak shifted.
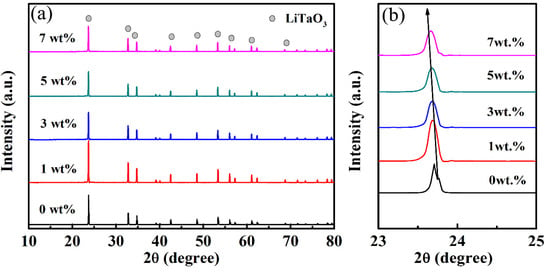
Figure 2.
XRD patterns (a) and partial enlarged patterns of CoO/LiTaO3-based ceramics (b).
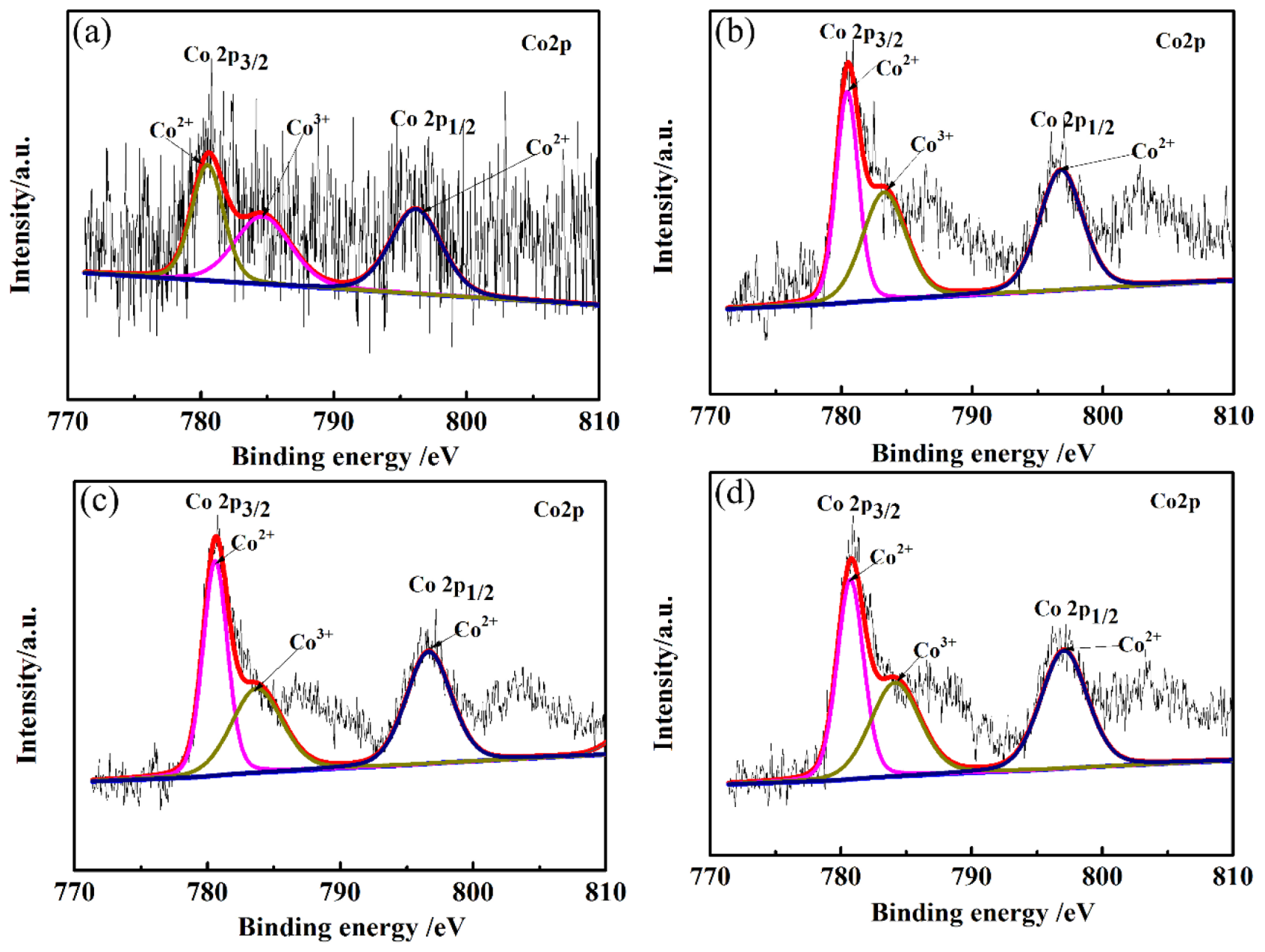
XPS studies were conducted in order to supplement the composition, electronic configuration, and surface state of the CLT ceramics. Co2p XPS spectra of LiTaO3-based ceramics with different amounts of CoO are shown in Figure 3. It can be seen from Figure 3 that the Co 2p spectrum consists of two wide peaks of Co 2p3/2 and Co 2p1/2, which are mainly ascribed to Co–O bonds. The different valence states of the doped Co ions were investigated by fitting overlapping Co 2p3/2 peaks. Mixed Co valence states (Co2+ and Co3+) in all samples were observed by XPS due to the oxidation of Co2+ ions. The peak of Co2+ ions appeared at a lower binding energy, while the peak of Co3+ ions appeared at a higher binding energy, revealing the co-existence of Co2+ and Co3+ ions. So, the Co ions mainly existed in two forms: Co2+ and Co3+. It can be seen that Co ions were mainly present in the form of Co2+ according to the fitting results of the Co 2p3/2 peak. The phase containing the Co element cannot be detected in XRD because of the small content in CLT ceramics.
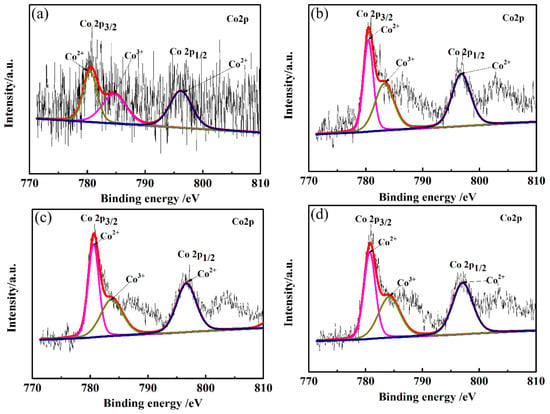
Figure 3.
Co2p XPS spectra of LiTaO3-based ceramics with different amounts of CoO. (a) 1 wt.% (b) 3 wt.% (c) 5 wt.% (d) 7 wt.%.
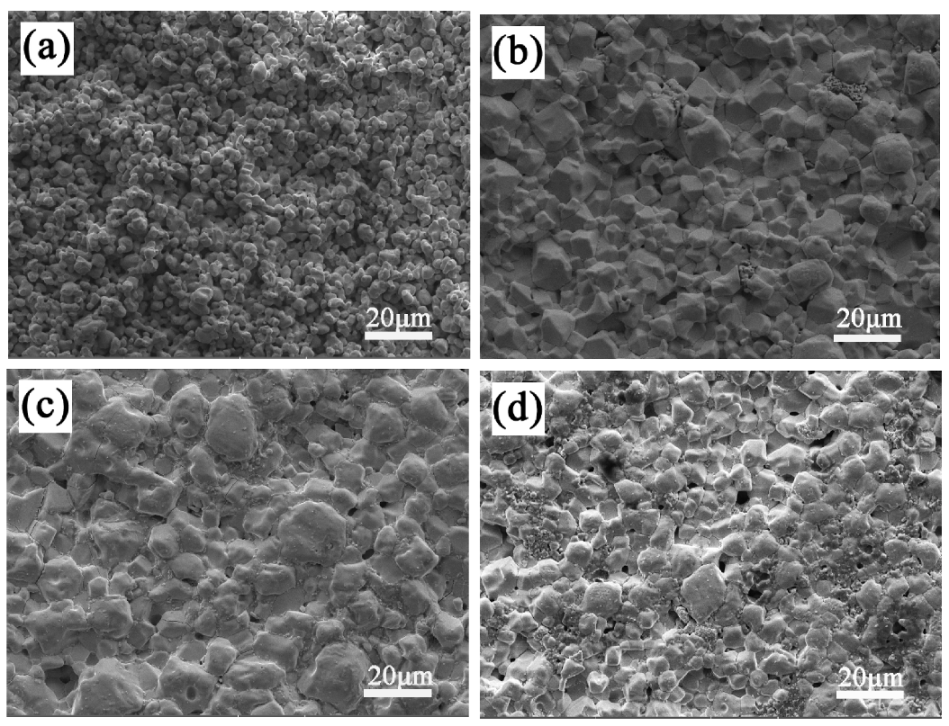
The SEM micrographs of the LiTaO3-based ceramics with different amounts of CoO are shown in Figure 4. In Figure 4, it can be seen that the porosity decreased at first and then increased as the added amount of CoO increased. The sinterability of the sample with 1 wt.% of added CoO was the worst; it was porous and not densified, which agrees with the investigation of relative density. The pores were achieved minimally when the added amount of CoO was 5 wt.%. The grain size of LiTaO3 was uniformly distributed in the ceramic 5CLT because adding Co cations into the LiTaO3-based ceramic would create a replacement of Ta5+ by Co2+/Co3+, leading to oxygen vacancies. Since the radius of O2− (0.140 nm) is much larger than the radii of other ions in LiTaO3-based ceramics, the sintering of LiTaO3-based ceramics is mainly restricted by the diffusion of oxygen ions [15]. Therefore, the occurrence of oxygen vacancies results in an easier grain boundary mobility, which is conducive to better sintering behavior. Combined with the XRD results, it can be seen that the second-phase particle morphology does not appear in LiTaO3-based ceramics with different amounts of CoO, which indicates that the Co ions may have dissolved completely into the LiTaO3 lattice. However, the addition of excessive CoO led to the worse sinterability for the LiTaO3-based ceramics.
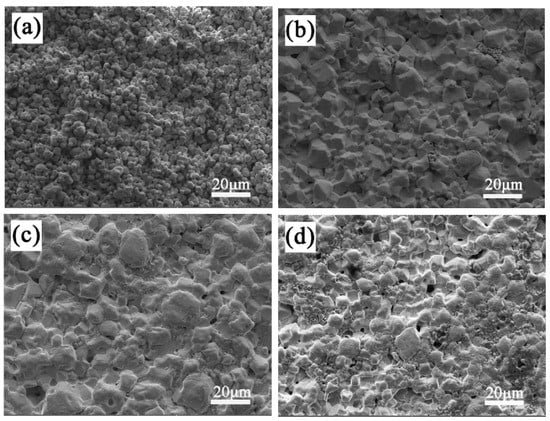
Figure 4.
SEM micrographs of CoO/LiTaO3-based ceramics. (a) 1 wt.% (b) 3 wt.% (c) 5 wt.% (d) 7 wt.%.
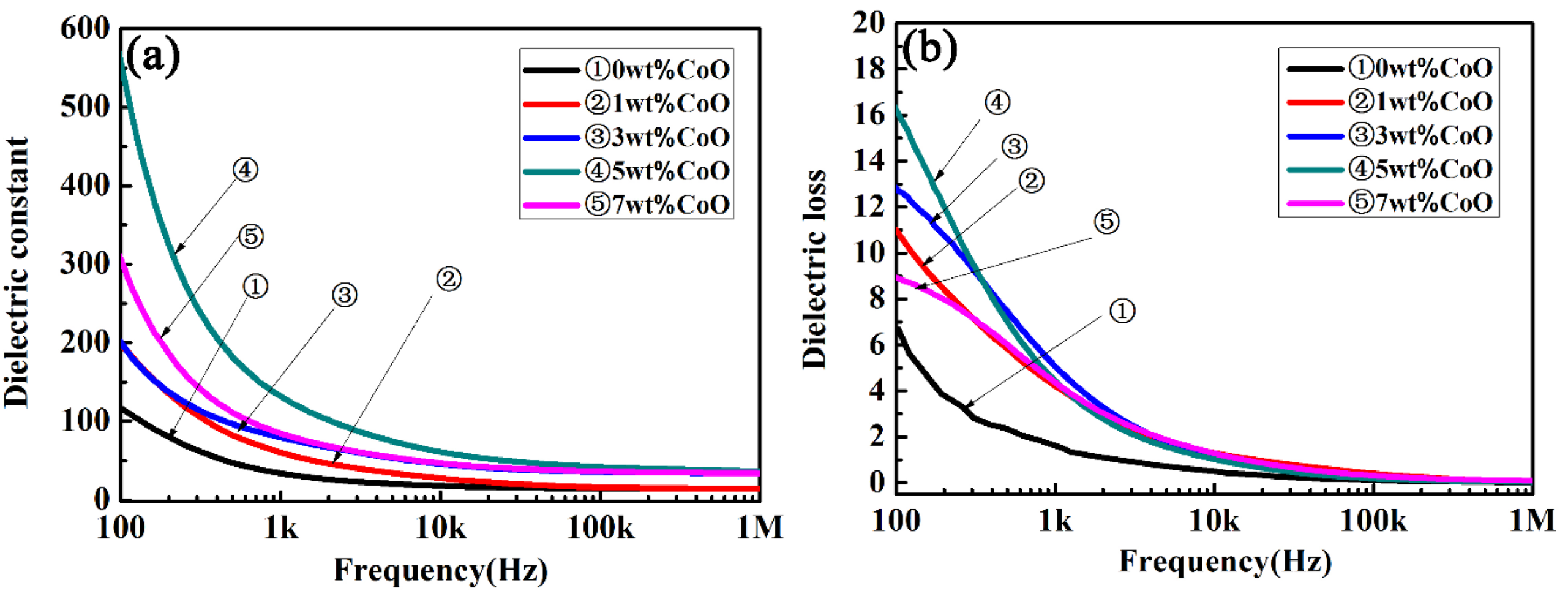
Figure 5 shows the frequency-dependent dielectric constant and the dielectric loss of CLT ceramics as a function of the frequency in the range from 100 Hz to 1 MHz at room temperature. A higher dielectric constant value was presented at a low frequency, and then the permittivity tended to have a stable value at frequencies over 10 kHz, as shown in Figure 5a. At the same frequency, the permittivity of CLT ceramics increased initially, and then decreased with an increase in the amount of CoO. Subsequently, a maximum value of about 50 was achieved when the CoO amount was 5 wt.%; this is similar to that of a single crystal LiTaO3. The dielectric loss of CLT ceramics with different amounts of CoO at room temperature are presented in Figure 5b. It can be seen that the dielectric loss of all samples decreased with an increase in the test frequency. The variation in the dielectric loss curves was contrary to the dielectric constant, i.e., it decreased firstly and then increased with an increase in the amount of CoO at the same frequency. The minimum value was obtained when the added amount of CoO was 5 wt.%. The largest dielectric constant and the smallest dielectric loss were gained when the addition of CoO was 5 wt.% in the LiTaO3-based ceramic. It is well known that many factors can affect the dielectric properties of ceramics, for example, porosity, second phase, and ionic polarizability [32]. The porosity gradually decreased when CoO was added, according to the SEM photographs and the curves of the relative density. A fine microstructure was gained when the addition of CoO was 3 or 5 wt.%, which resulted in improvement of the dielectric constant. The dielectric constant decreased and the dielectric loss increased when more than 5 wt.% CoO was added, due to the worsening sinterability.
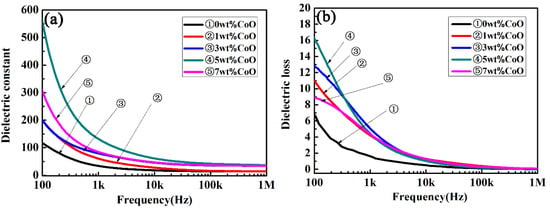
Figure 5.
The frequency-dependent dielectric constant (a) and dielectric loss (b) of the LiTaO3-based ceramics at room temperature.
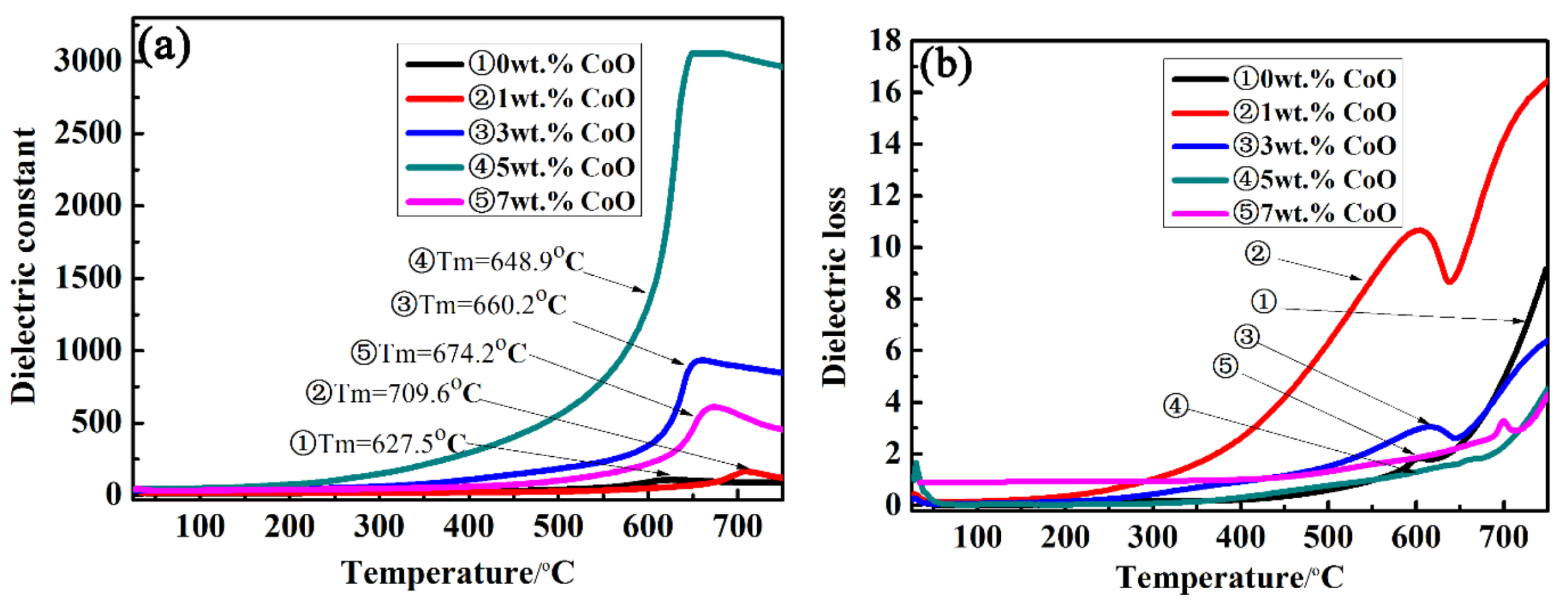
The temperature-dependent dielectric constant (εr) and dielectric loss (tanδ) of the LiTaO3-based ceramics with different amounts of CoO were measured at 10 kHz as a function of temperature from room temperature to 750 °C, and the results are shown in Figure 6. The maximum dielectric constant at 0CLT was 106; the maximum value of the dielectric constant increased by up to 3055 at 5CLT and then decreased as the added amount of CoO increased. It can be seen from Figure 6a that there was a peak value at the phase transition point (Curie point Tm) of ferroelectric to paraelectric in LiTaO3-based piezoelectric ceramics with an increase in temperature, followed by a slow decrease. The dielectric constant will be unusual when the dielectric changes in phase transition or other microstructures. The dielectric constant was lower when the temperature was lower than 300 °C because the relaxation of polarization takes a long time. With an increase in temperature, the polarization is established more fully, which results in the dielectric constant increasing gradually. Simultaneously, an increase in temperature prevents thermal movement that is against the mass. The regular motion of the mass point hinders an increase in polarization, and the corresponding dielectric constant decreases. This means that the motion of atoms in the system is intensified with an increase in temperature, which hinders the motion of particles participating in polarization and leads to a decrease in polarizability. Therefore, the dielectric constant peak value will appear at a certain temperature, i.e., at the Curie point. With an increase in the amount of CoO, the dielectric peak becomes wider, indicating that a large amount of CoO can induce phase transformation dispersion near the Curie point. This is mainly because the addition of CoO increases the disorder of cations and the volatility of the composition. In the LiTaO3 ceramic system, Li is a volatile element, so the more Co ions that are dissolved into the LiTaO3 lattice, the greater the disorder degree of the whole system and the faster the volatilization of Li ions. Figure 6b shows the temperature-dependent dielectric loss of LiTaO3-based piezoelectric ceramics with different amounts of CoO at 10 kHz. The dielectric loss of all samples increases with an increase in the temperature and a peak appears near the Curie temperature. A relatively small value of dielectric loss was obtained for the LiTaO3-based ceramics with the addition of 5 wt.%, as shown in Figure 6, which is related to the relative density of the LiTaO3-based ceramics.
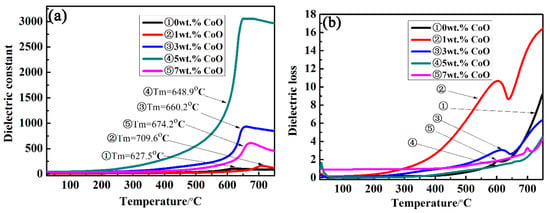
Figure 6.
Dielectric constant (a) and dielectric loss (b) of LiTaO3-based ceramics with different amounts of CoO doping as a function of temperature at 10 kHz.
For relaxor ferroelectrics, Uchino et al. proposed a modified Curie–Weiss law to describe the degree of phase transformation dispersion of relaxor ferroelectrics [33,34,35].
where εm is the maximum dielectric constant, T is the test temperature, Tm is the temperature of the εm, C is the Curie–Weiss constant, and γ is associated with the diffuseness degree. = 1 indicates that the normal phase transition satisfies the Curie–Weiss law; = 2 indicates the complete dispersion phase transition. The logarithm is taken on both sides of Formula (1-1) to get Formula (1-2):
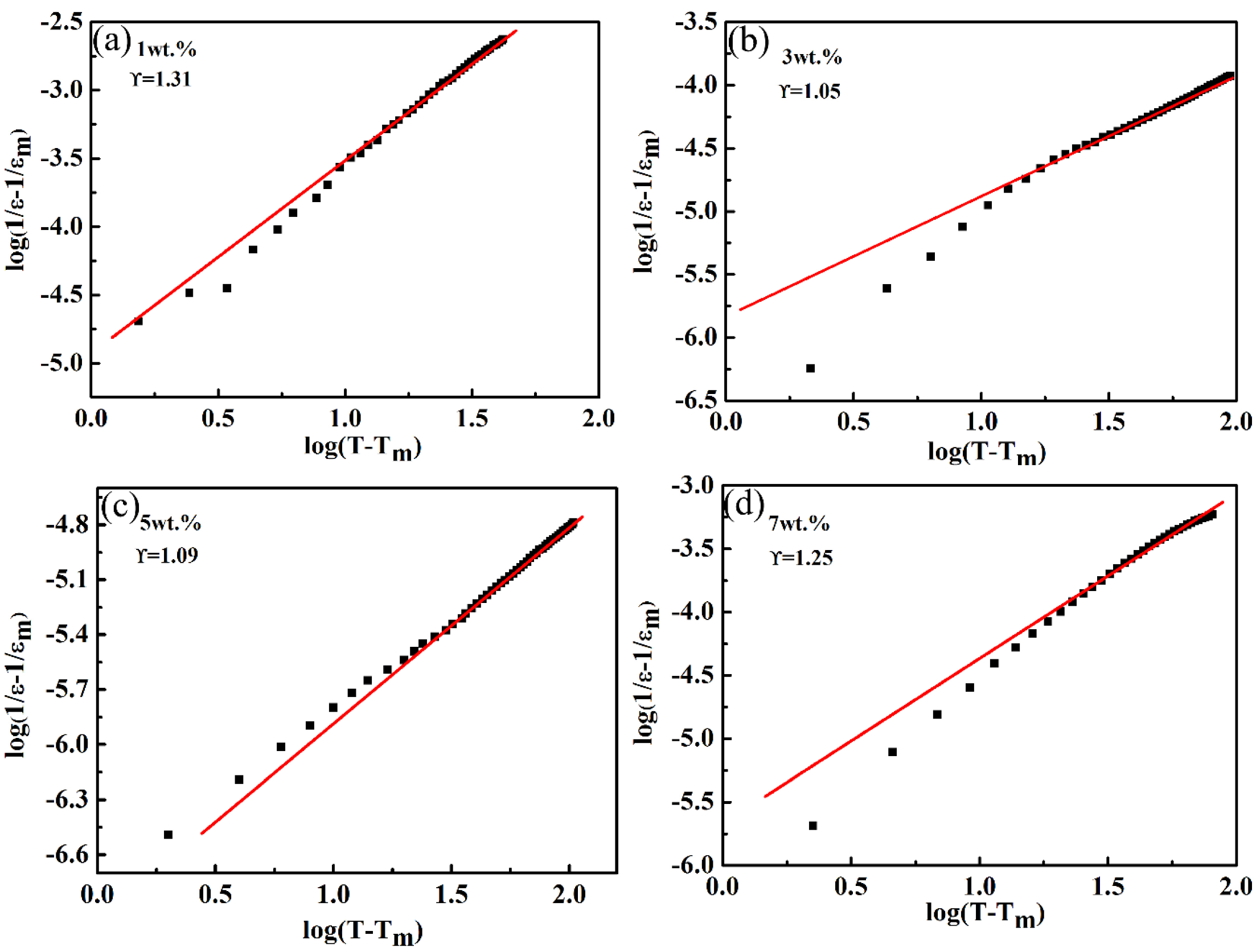
y = Ax + b was used for the simulation, and the value of was obtained by linear fitting of data points. Figure 7 shows the variation in with at different components and 10 kHz. It can be seen from Figure 7 that there was a linear relation, and the value of changed from 1.0 to 1.30, and it was close to 1.0 when the amount of CoO added was 3 wt.% or 5 wt.%, i.e., the ceramics presented normal ferroelectric behavior. Due to the different valence and ionic radii, ion substitutions of Co2+ or Co3+ for Ta5+ at B sites generated chemical and displacive disorder in the LiTaO3 lattice [36]. This would lead to a lattice with a deviated ferroelectric phase transition, but the diffuse phase transition phenomenon in LiTaO3 ceramics was not obvious. A similar influence of a new disordered structure was reported for an Mn-doped Pb-based ferroelectric ceramics system [37].
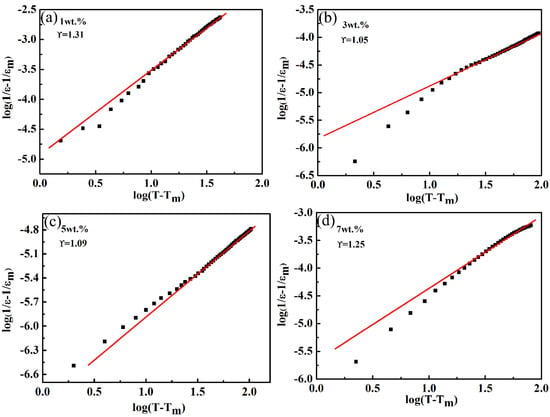
Figure 7.
The variation of log (1/ε-1/εm) versus log (T-Tm) for the LiTaO3-based ceramics with different compositions at 10 kHz. (a) 1 wt.% (b) 3 wt.% (c) 5 wt.% (d) 7 wt.%.
4. Conclusion
LiTaO3-based ceramics with different amounts of CoO were successfully fabricated by pressureless sintering at 1250 °C. The effects of different amounts of CoO on the sinterability, microstructures, and dielectric properties of the CLT ceramics were investigated. The sinterability of the LiTaO3-based ceramics was improved by increasing the amount of CoO added, and the relative densities of the LiTaO3-based ceramics were significantly enhanced. The LiTaO3-based ceramics achieved the highest relative density (89.4%) and obtained well-grained microstructures when the amount of CoO added was 5 wt.%. Only the LiTaO3 phase was observed in the ceramics, indicating that the Co ions diffused into the LiTaO3 lattices and mainly existed in two forms: Co2+ and Co3+. At room temperature, the dielectric constant of the LiTaO3-based ceramics increased first in the frequency range from 100 Hz to 1 MHz, and then decreased with an increase in the amount of CoO at the same frequency. It also achieved the maximum value of 50 when the CoO content was 5 wt.%. Similarly, the dielectric loss was the lowest when the added CoO was 5 wt.%. The maximum dielectric constant gradually increased up to 3055 at 5CLT and then decreased. The variation tendency of the dielectric loss versus the component was consistent with the dielectric constant in the temperature range of room temperature to 750 °C. Therefore, the highest relative density, a well-grained microstructure, and better dielectric properties were obtained with LiTaO3-based ceramics with 5 wt.% added CoO.
Author Contributions
Conceptualization, Y.Z. and Y.Y.; methodology, Y.Z.; software, Y.Y.; validation, Y.Z., S.H.; formal analysis, S.H.; investigation, Y.Z.; resources, Y.Z.; data curation, Y.Z.; writing—original draft preparation, Y.Z.; writing—review and editing, Y.Z.; project administration, Y.Z.; funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Nature Science Foundation of China under Grant Number 11604204.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liang, W.F.; Lu, Y.; Wu, J.; Gao, H.; Li, M. Application of LiTaO3 pyroelectric crystal for pulsed neutron detection. Nucl. Instrum. Methods A 2016, 827, 161–164. [Google Scholar] [CrossRef]
- Guo, E.J.; Xing, J.; Lu, H.B.; Jin, K.J.; Yang, G.Z. Ultraviolet fast-response photoelectric effects in LiTaO3 single crystal. J. Phys. D Appl. Phys. 2010, 43, 15402–15405. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Q.; Liu, T.; Guo, S.S.; Zhang, L.; Zhou, Y.F.; Wang, X.L. Visible and near-infrared waveguide properties in LiTaO3 crystal produced by swift Ar8+ ion irradiation. Appl. Phys. B 2012, 108, 675–681. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Du, J.; Yuan, L.; Qian, Z.; Zhang, Z.; Zhang, C. A lateral field excited (yxl) 88° LiTaO3 bulk acoustic wave sensor with interdigital electrodes. Ultrasonics 2013, 53, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Gruber, M.; Konetschnik, R.; Popov, M.; Spitaler, J.; Supancic, P.; Kiener, D.; Bermejo, R. Atomistic origins of the differences in anisotropic fracture behavior of LiTaO3 and LiNbO3 single crystals. Acta Mater. 2015, 150, 373–380. [Google Scholar] [CrossRef]
- Gruber, M.; Kraleva, I.; Supancic, P.; Bielen, J.; Kiener, D.; Berejo, R. Strength distribution and fracture analyses of LiNbO3 and LiTaO3 single crystals under biaxial loading. J. Eur. Ceram. Soc. 2017, 37, 4397–4406. [Google Scholar] [CrossRef]
- Sanna, S.; Neufeld, S.; Rüsing, M.; Berth, G.; Schmidt, W.G. Raman scattering efficiency in LiTaO3 and LiNbO3 crystals. Phys. Rev. B 2015, 91, 224302. [Google Scholar] [CrossRef]
- Kang, X.; Liang, L.; Song, W.; Wang, F.; Sang, Y.; Liu, H. Formation mechanism and elimination methods for anti-site defects in LiNbO3/LiTaO3 crystals. CrystEngComm 2016, 18, 8136–8146. [Google Scholar] [CrossRef]
- Chen, C.F.; Brennecka, G.L.; King, G.; Tegtmeier, E.L.; Holesinger, T.; Ivy, J.; Yang, P. Processing of crack-free high density polycrystalline LiTaO3 ceramics. J. Mater. Sci. Mater. Electron. 2017, 28, 3725–3732. [Google Scholar] [CrossRef]
- Zainuddin, L.W.; Kamarulzaman, N. Effect of sintering time on the purity and morphology of LiTaO3. Adv. Mater. Res. 2012, 501, 129–132. [Google Scholar] [CrossRef]
- Bomlai, P.; Sinsap, P.; Muensit, S.; Milne, S.J. Effect of MnO on the phase development, microstructures, and dielectric properties of 0.95Na0.5K0.5NbO3–0.05LiTaO3 ceramics. J. Am. Ceram. Soc. 2008, 91, 624–627. [Google Scholar] [CrossRef]
- Zhou, J.J.; Li, J.F.; Wang, K.; Zhang, X.W. Phase structure and electrical properties of (Li, Ta)-doped (K, Na)NbO3 lead-free piezoceramics in the vicinity of Na/K=50/50. J. Mater. Sci. 2011, 46, 5111–5116. [Google Scholar] [CrossRef]
- Yang, T.; Liu, Y.G.; Zhang, L.; Hu, M.L.; Yang, Q.; Huang, Z.H.; Fang, M.H. Powder synthesis and properties of LiTaO3 ceramics. Adv. Powder Technol. 2014, 25, 933–936. [Google Scholar] [CrossRef]
- Ye, Z.G.; Von Der Mühll, R.; Ravez, J. New oxyfluorides and highly densified ceramics related to LiNbO3. J. Phys. Chem. Solids 1989, 50, 809–812. [Google Scholar] [CrossRef]
- Shimada, S.; Kodaira, K.; Matsushita, T. Sintering LiTaO3 and KTaO3 with the aid of manganese oxide. J. Mater. Sci. 1984, 19, 1385–1390. [Google Scholar] [CrossRef]
- Huanosta, A.; Alvarez, E.; Villafuerte-Castrejón, M.E.; West, A.R. Electrical properties of Mg-doped LiTaO3 ceramics. Mater. Res. Bull. 2004, 39, 2229–2240. [Google Scholar] [CrossRef]
- Bamba, N.; Yokouchi, T.; Takaoka, J.; Elouadi, B.; Fukami, T. Effects of CaTiO3 on electrical properties in LiTaO3 ceramics. Ferroelectr 2004, 304, 135–138. [Google Scholar] [CrossRef]
- Tahiri, M.; Masaif, N.; Jennane, A.; Lemdek, E.M.; Benkhouja, K.; Lotfi, E.M. Experimental and theoretical study of the density of Cu-doped LiTaO3. Opt. Quant. Electorn. 2016, 48, 278. [Google Scholar] [CrossRef]
- Lin, P.J.; Bursill, L.A. High-resolution study of Li(1−x)AgxTaO3. Micron 1982, 13, 275–276. [Google Scholar]
- Ye, Z.G.; Von Der Mühll, R.; Ravez, J.; Hagenmuller, P. Dielectric, piezoelectric, and pyroelectric studies of LiTaO3-derived ceramics sintered at 900 °C following the addition of (LiF+MgF2). J. Mater. Res. 1988, 3, 112–115. [Google Scholar] [CrossRef]
- Rödel, J.; Jo, W.; Seifert, K.T.P.; Anton, E.M.; Granzow, T.; Damjanovic, D. Perspective on the development of lead-free piezoelectrics. J. Am. Ceram. Soc. 2009, 92, 1153–1177. [Google Scholar] [CrossRef]
- Gou, Q.; Wu, J.; Li, A.; Wu, B.; Xiao, D.; Zhu, J. Enhanced d33 value of Bi0.5Na0.5TiO3-(Ba0.85Ca0.15)(Ti0.90Zr0.10)O3 lead-free ceramics. J. Alloy. Compd. 2012, 521, 4–7. [Google Scholar] [CrossRef]
- Chen, C.F.; Llobet, A.; Brennecka, G.L.; Forsyth, R.T.; Guidry, D.R.; Papin, P.A.; McCabe, R.J. Powder synthesis and hot-pressing of a LiTaO3 ceramic. J. Am. Ceram. Soc. 2012, 95, 2820–2826. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Jia, D.; Li, H.; Meng, Q. Microstructure and mechanical properties of Al2O3 particle reinforced LiTaO3 piezoelectric ceramic matrix composites. Mater. Sci. Eng. A 2007, 448, 330–334. [Google Scholar] [CrossRef]
- Kulawik, J.; Szwagierczak, D. Dielectric properties of manganese and cobalt doped lead iron tantalate ceramics. J. Eur. Ceram. Soc. 2007, 27, 2281–2286. [Google Scholar] [CrossRef]
- Wang, F.L.; Li, Y.; Wang, N.; Zhu, L.; Jain, A.; Wang, Y.G.; Chen, F.G. Enhanced magnetic, ferroelectric and optical properties of Sr and Co co-doped BiFeO3 powders. J. Alloy. Compd. 2019, 810, 151941. [Google Scholar] [CrossRef]
- Dung, D.D.; Doan, N.B.; Dung, N.Q.; Bac, L.H.; Linh, N.H.; Thanh, L.T.H.; Thiet, D.V.; Trung, N.N.; Khang, N.C.; Trung, T.V.; et al. Role of Co dopants on the structural, optical and magnetic properties of lead-free ferroelectric Na0.5Bi0.5TiO3 materials. J. Sci. Adv. Mater. Devices 2019, 4, 584–590. [Google Scholar] [CrossRef]
- Gu, Y.; Zhao, J.; Zhang, W.; Liu, S.; Ge, S.; Chen, W.; Zhang, Y. Improved ferromagnetism and ferroelectricity of La and Co co-doped BiFeO3 ceramics with Fe vacancies. Ceram. Int. 2016, 42, 8863–8868. [Google Scholar] [CrossRef]
- Li, X.J.; Wang, Q.; Li, Q.L. Effects of MnO2 addition on microstructure and electrical properties of (Bi0.5Na0.5)0.94Ba0.06TiO3 ceramics. J. Electroceram. 2008, 20, 89–94. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, Y. Fabrication and dielectric properties of LiTaO3 matrix ceramics with added manganese dioxide. J. Ceram. Sci. Technol. 2019, 1–10. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- La Rosa-Toro, A.; Berenguer, R.; Quijada, C.; Montilla, F.; Morallón, E.; Vázquez, J.L. Preparation and Characterization of Copper-Doped Cobalt Oxide Electrodes. J. Phys. Chem. B 2006, 110, 24021–24029. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fang, C.; Lin, J.; Liu, C.; Luo, L.; Lin, M.; Zheng, X.; Lin, C. Tetragonal Er3+-doped (K0.48Na0.48Li0.04)(Nb0.96Bi0.04)O3: Lead-free ferroelectric transparent ceramics with electrical and optical multifunctional performances. Ceram. Int. 2017, 44, 4908–4914. [Google Scholar] [CrossRef]
- Liu, Y.; Du, Y.; Cheng, C.; Sun, X.; Jiang, N.; Wang, J.; Sun, X. Dielectric and impedance spectroscopy analysis of lead-free (1-x)(K0.44Na0.52Li0.04)(Nb0.86Ta0.10Sb0.04)O3-xBaTiO3 ceramics. Ceram. Int. 2019, 45, 13347–13353. [Google Scholar] [CrossRef]
- Hao, J.; Bai, W.; Li, W. Correlation between the microstructure and electrical properties in high-performance (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 Lead-free piezoelectric ceramics. J. Am. Ceram. Soc. 2012, 95, 1998–2006. [Google Scholar] [CrossRef]
- Bokov, A.A.; Ye, Z.G. Recent progress in relaxor ferroelectrics with perovskite structure. J. Mater. Sci. 2016, 41, 31–52. [Google Scholar] [CrossRef]
- Qi, X.; Sun, E.; Zhang, R.; Yang, B.; Li, S.; Cao, W. Effect of Mn-doping on dielectric relaxation behavior of Pb(In1/2Nb1/2)O3-Pb(Mg1/3Nb2/3)O3-PbTiO3 ferroelectric ceramics. Ceram. Int. 2017, 43, 16819–16826. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).