Diverse Surface Chemistry of Cobalt Ferrite Nanoparticles to Optimize Copper(II) Removal from Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of Primary Cobalt Ferrite MNPs@ODA
2.3. Surface Modifications of MNPs@ODA
2.3.1. PEI Modification onto MNPs@ODA
2.3.2. Direct Coupling of DTPA onto MNPs@ODA
2.3.3. Direct Coupling of DTPA onto MNPs@ODA@PEI
2.4. Ninhydrin Colorimetric Assay
2.5. Batch Adsorption Experiments and Copper(II) Detection
2.6. Copper Complex (Cux(DTPA)y)
2.6.1. Preparation of the Complex (Cux(DTPA)y)
2.6.2. Immobilization of (Cux(DTPA)y) onto MNPs@ODA@PEI
2.6.3. UV–Vis Studies of MNPs@ODA@PEI@(Cux(DTPA)y)
2.7. Characterization Techniques
3. Results and Discussion
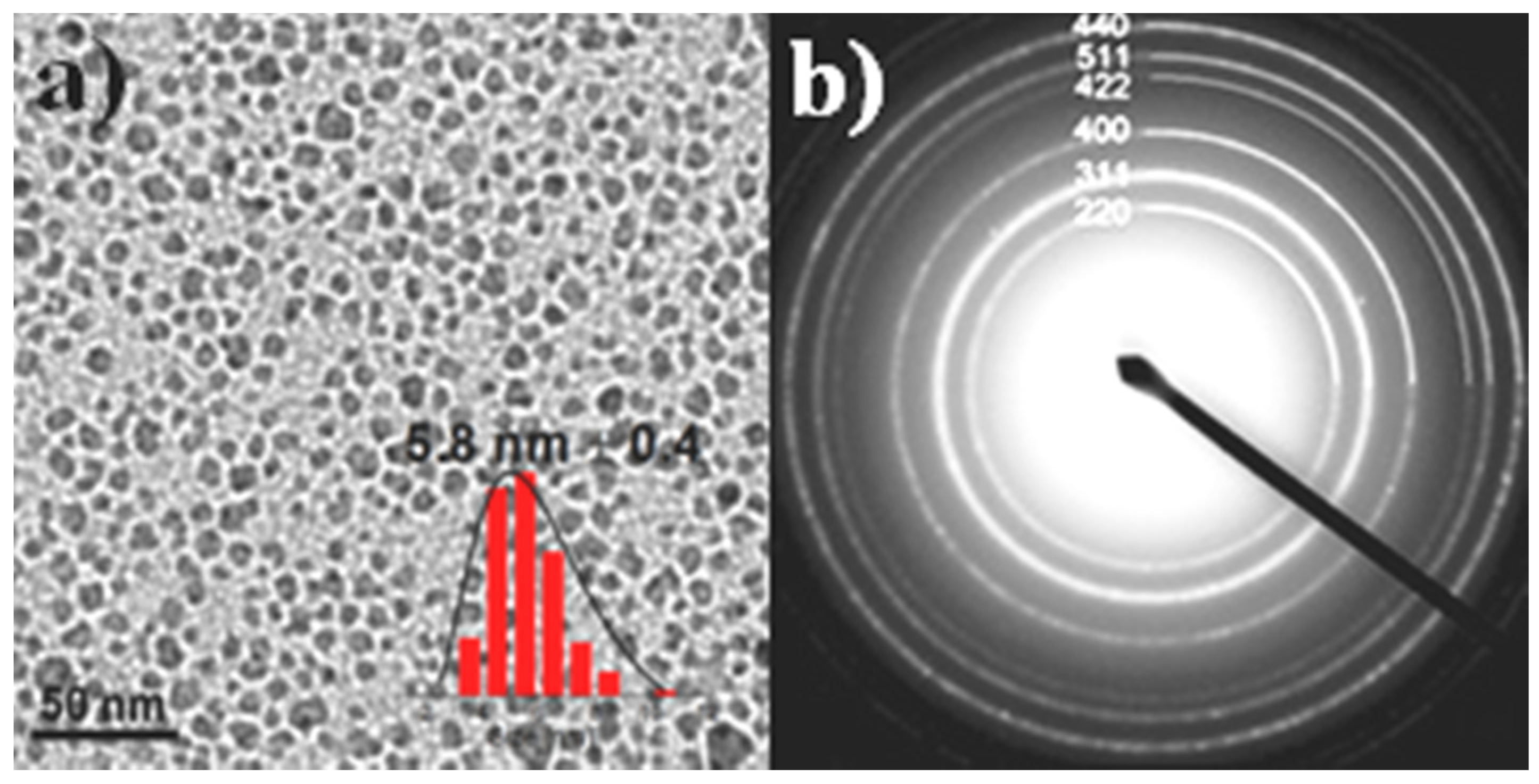
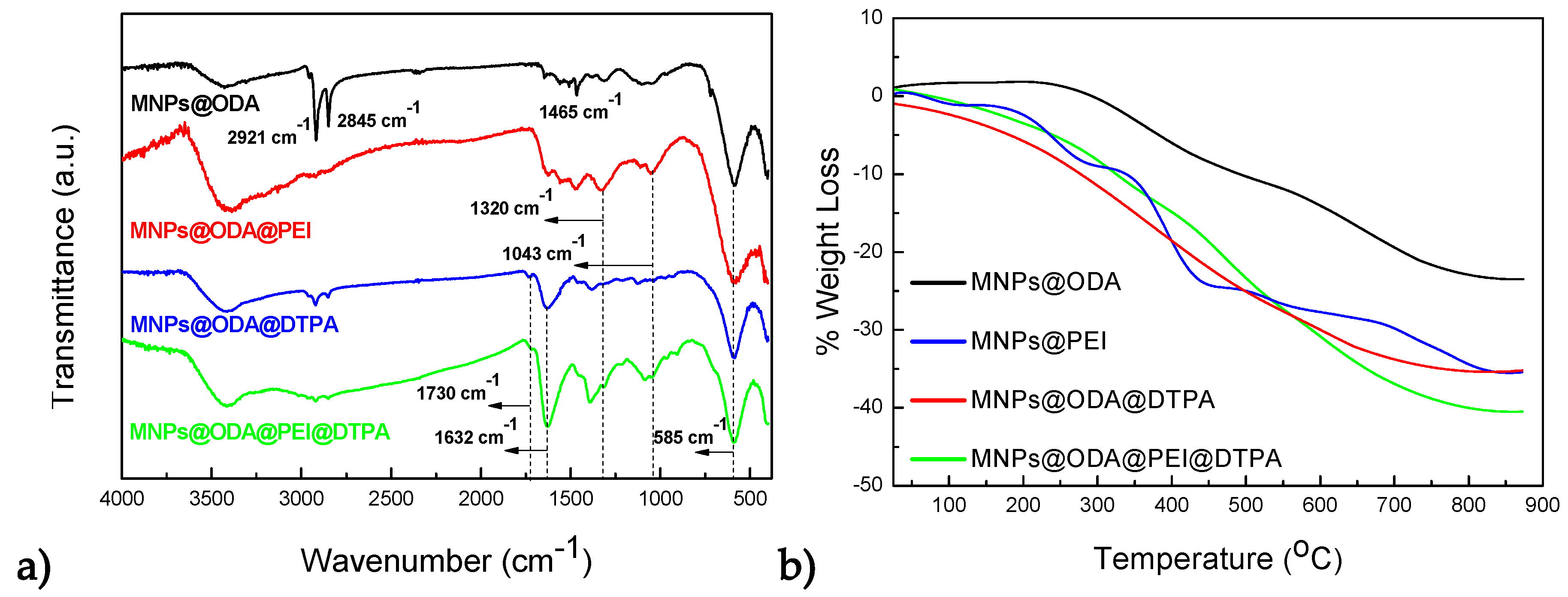
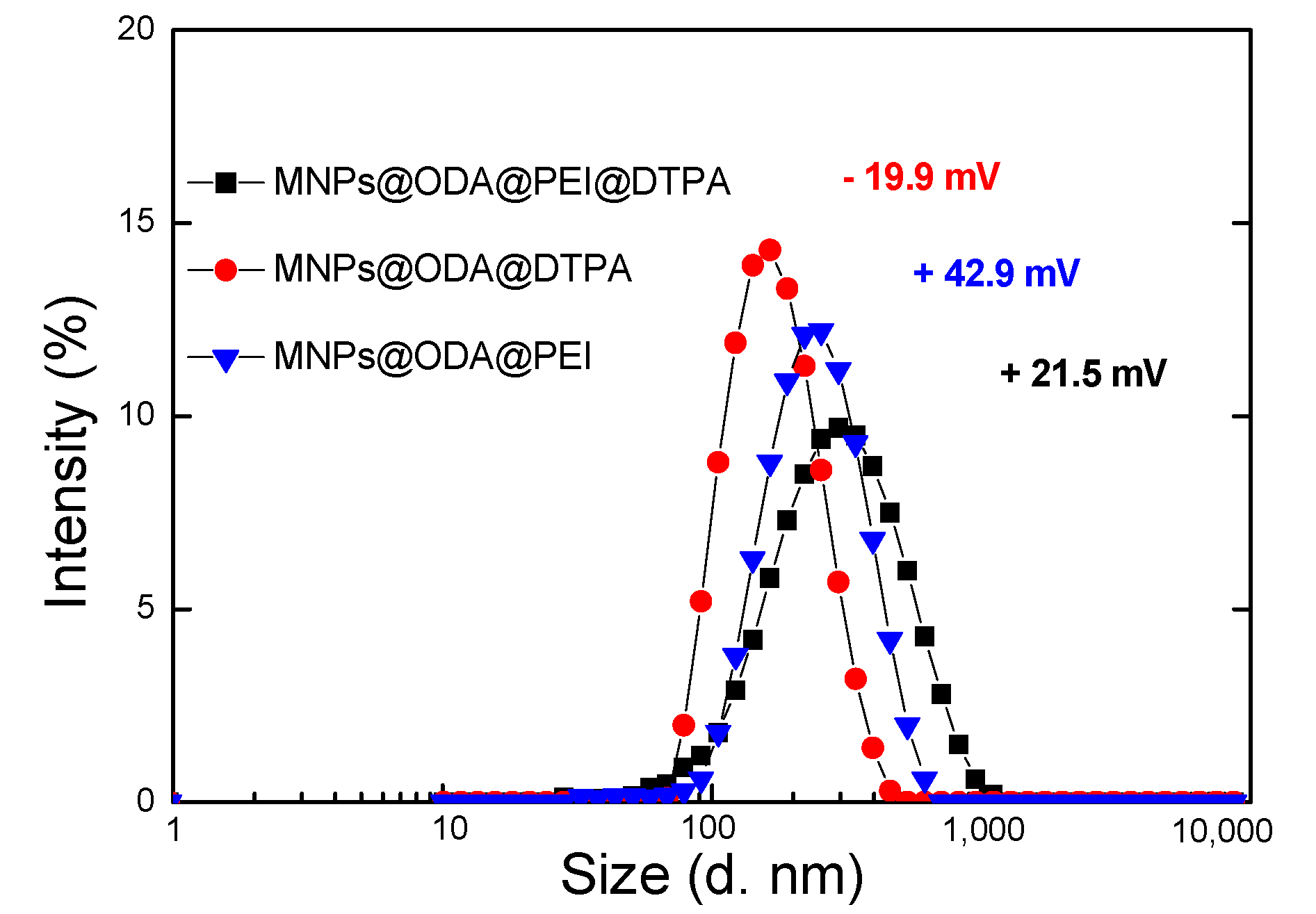
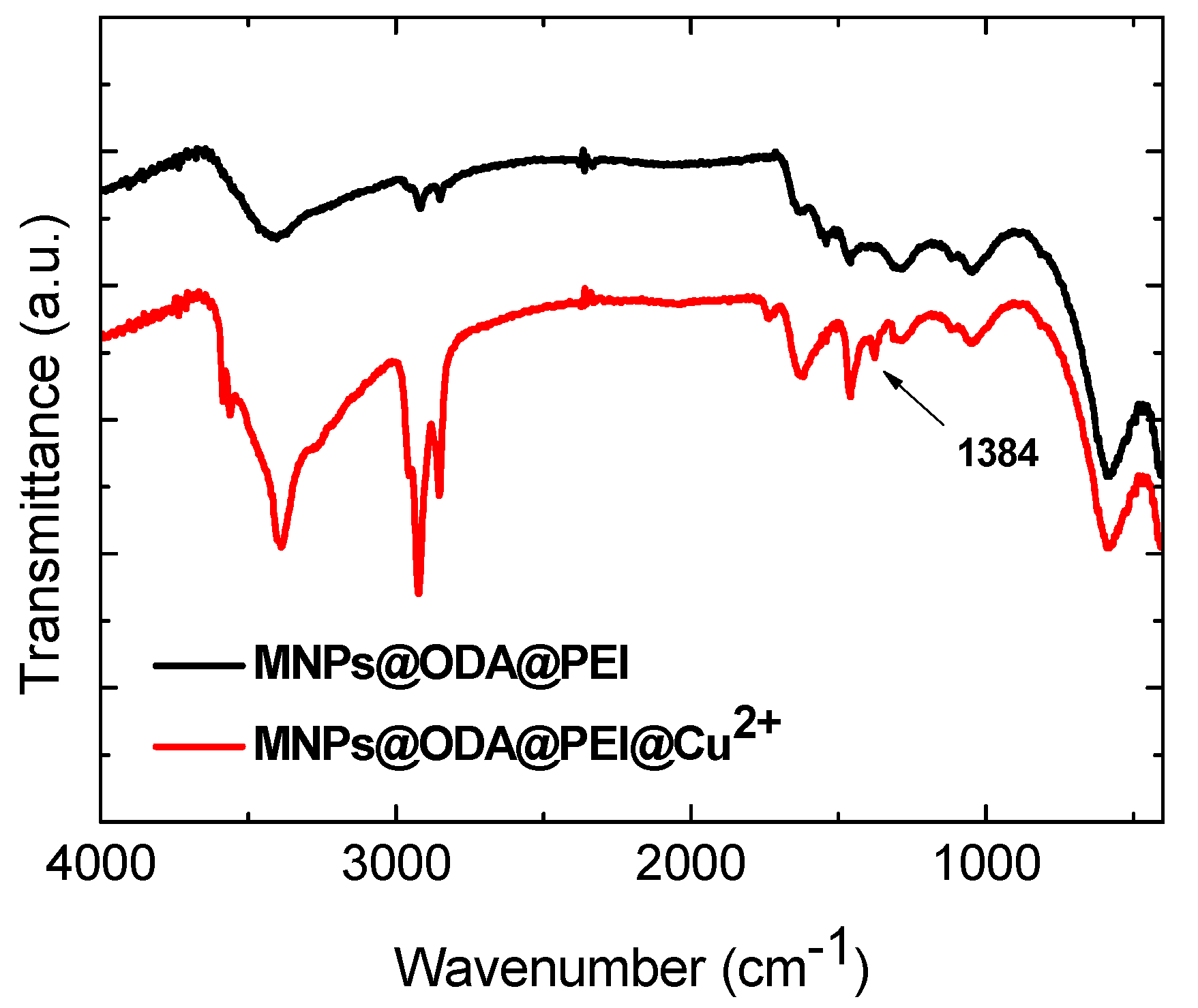
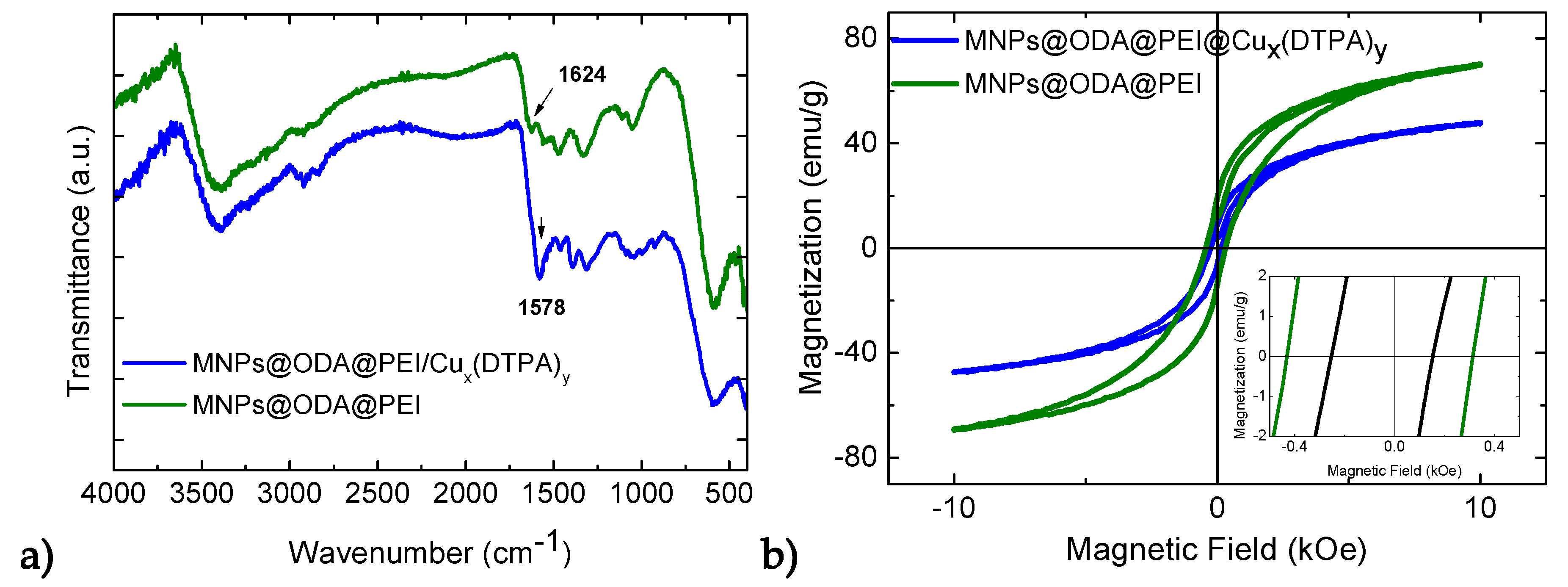
3.1. Structural and Surface Characterization
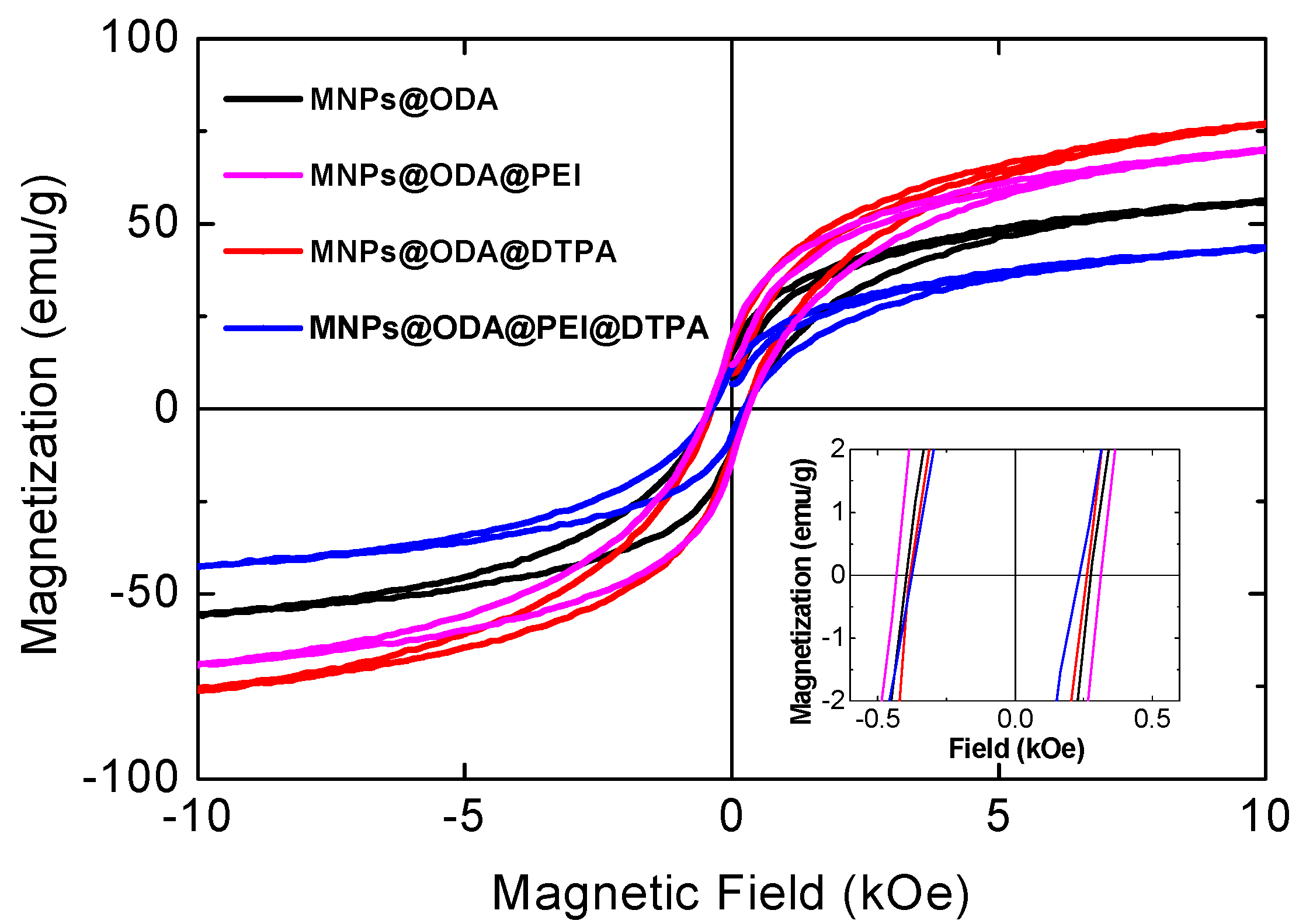
3.2. Magnetic Studies
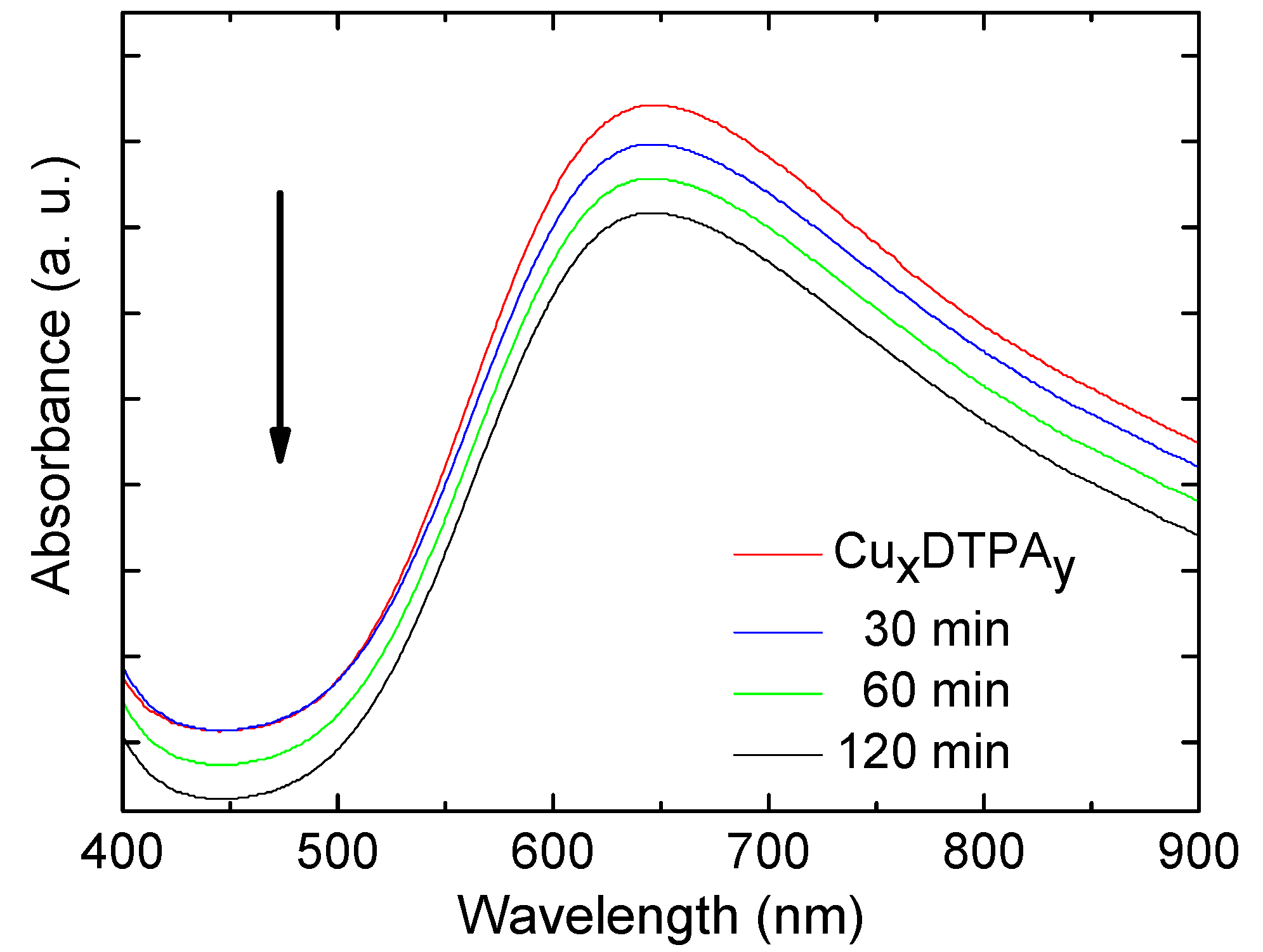
3.3. Absorption Spectroscopy Studies
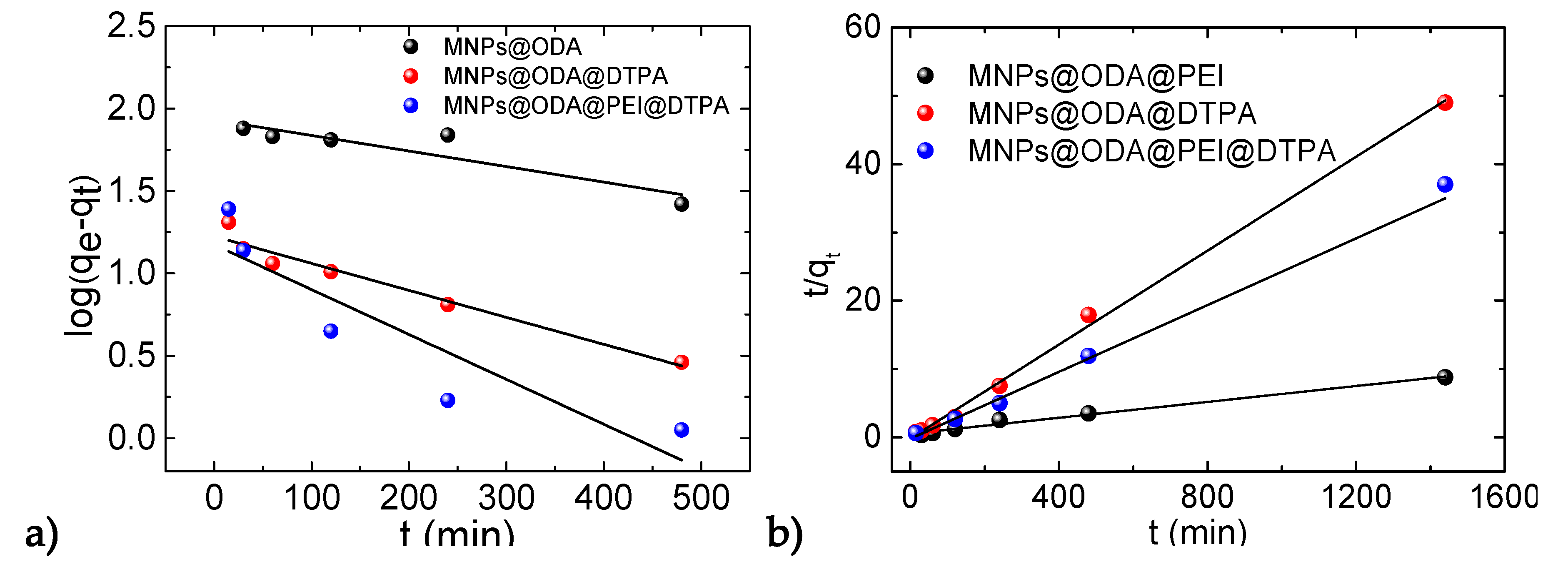
3.4. Adsorption Kinetics
3.5. Immobilization of the (Cux(DTPA)y) onto MNPs’ Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nriagu, J.O.; Pacyna, J.M. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 1988, 333, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.S.; Chen, D.H. Fast removal of copper ions by gum arabic modified magnetic nano-adsorbent. J. Hazard. Mater. 2007, 147, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Copper Homeostasis and Neurodegenerative Disorders (Alzheimer’s, Prion, and Parkinson’s Diseases and Amyotrophic Lateral Sclerosis). Chem. Rev. 2006, 106, 1995–2044. [Google Scholar] [CrossRef]
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998, 67, 952S–959S. [Google Scholar] [CrossRef]
- Waggoner, D.J.; Bartnikas, T.B.; Gitlin, J.D. The role of copper in neurodegenerative disease. Neurobiol. Dis. 1999, 6, 221–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengaraj, S.; Kim, Y.; Joo, C.K.; Yi, J. Removal of copper from aqueous solution by aminated and protonated mesoporous aluminas: Kinetics and equilibrium. J. Colloid Interface Sci. 2004, 273, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Flemming, C.A.; Trevors, J.T. Copper toxicity and chemistry in the environment: A review. Water Air Soil Pollut. 1989, 44, 143–158. [Google Scholar] [CrossRef]
- Valentine, J.S.; Hart, P.J. Misfolded CuZnSOD and amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2003, 100, 3617–3622. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Wang, H.; Lan, H.; Liu, H.; Qu, J. Adsorption of Cu(II)–EDTA chelates on tri-ammonium-functionalized mesoporous silica from aqueous solution. Water Res. 2015, 87, 378–384. [Google Scholar] [CrossRef]
- Filice, S.; D’Angelo, D.; Scarangella, A.; Iannazzo, D.; Compagnini, G.; Scalese, S. Highly effective and reusable sulfonated pentablock copolymer nanocomposites for water purification applications. RSC Adv. 2017, 7, 45521–45534. [Google Scholar] [CrossRef] [Green Version]
- Molinari, R.; Poerio, T.; Argurio, P. Selective separation of copper(II) and nickel(II) from aqueous media using the complexation–ultrafiltration process. Chemosphere 2008, 70, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Azarudeen, R.S.; Ahamed, M.A.R.; Subha, R.; Burkanudeen, A.R. Heavy and toxic metal ion removal by a novel polymeric ion-exchanger: Synthesis, characterization, kinetics and equilibrium studies. J. Chem. Technol. Biotechnol. 2014, 90, 2170–2179. [Google Scholar] [CrossRef]
- Wu, J.; Ma, L.; Chen, Y.; Cheng, Y.; Liu, Y.; Zha, X. Catalytic ozonation of organic pollutants from bio-treated dyeing and finishing wastewater using recycled waste iron shavings as a catalyst: Removal and pathways. Water Res. 2016, 92, 140–148. [Google Scholar] [CrossRef]
- Yang, X.; He, Y.; Zeng, G.Y.; Zhan, Y.Q.; Pan, Y.; Shi, H.; Chen, Q. Novel hydrophilic PVDF ultrafiltration membranes based on a ZrO2-multiwalled carbon nanotube hybrid for oil/water separation. J. Mater. Sci. 2016, 51, 8965–8976. [Google Scholar] [CrossRef]
- Yuvaraja, G.; Subbaiah, M.V.; Krishnaiah, A. Caesalpinia bonducella Leaf Powder as Biosorbent for Cu(II) Removal from Aqueous Environment: Kinetics and Isotherms. Ind. Eng. Chem. Res. 2012, 51, 11218–11225. [Google Scholar] [CrossRef]
- Dean, J.G.; Bosqui, F.L.; Lanouette, K.L. Removing heavy metals from wastewater. Environ. Sci. Technol. 1997, 6, 518–524. [Google Scholar] [CrossRef]
- Dönmez, G.; Aksu, Z. The effect of copper(II) ions on the growth and bioaccumulation properties of some yeasts. Process Biochem. 1999, 35, 135–142. [Google Scholar] [CrossRef]
- Ali, I. The Quest for Active Carbon Adsorbent Substitutes: Inexpensive Adsorbents for Toxic Metal Ions Removal from Wastewater. Sep. Purif. Rev. 2010, 39, 95–171. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low Cost Adsorbents for Removal of Organic Pollutants from Wastewater. J. Environ. Manage. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Huang, C.; Hu, B. Silica-coated magnetic nanoparticles modified with γ-mercaptopropyltrimethoxysilane for fast and selective solid phase extraction of trace amounts of Cd, Cu, Hg, and Pb in environmental and biological samples prior to their determination by inductively coupled plasma mass spectrometry. Spectrochim. Acta B 2008, 63, 437–444. [Google Scholar]
- Tan, Y.; Chen, M.; Hao, Y. High efficient removal of Pb (II) by amino-functionalized Fe3O4 magnetic nano-particles. Chem. Eng. J. 2012, 191, 104–111. [Google Scholar] [CrossRef]
- Tran, H.V.; Tran, L.D.; Nguyen, T.N. Preparation of chitosan/magnetite composite beads and their application for removal of Pb(II) and Ni(II) from aqueous solution. Mater. Sci. Eng. C 2010, 30, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Yang, P.; Yu, H.; Yan, L.; Wei, Q.; Du, B. Development of a novel water-soluble magnetic fluorescent nanoparticle for the selective detection and removal of Cu2+. Nanotechnology 2013, 24, 495502–495510. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, C.T.; Prakash, A.; Mayo, J.T.; Colvin, V.L. Magnetic separations: From steel plants to biotechnology. Chem. Eng. Sci. 2009, 64, 2510–2521. [Google Scholar] [CrossRef]
- Anbia, M.; Kargosha, K.; Khoshbooei, S. Heavy metal ions removal from aqueous media by modified magnetic mesoporous silica MCM-48. Chem. Eng. Res. Des. 2015, 93, 779–788. [Google Scholar] [CrossRef]
- Hai, B.; Wu, J.; Chen, X.; Protasiewicz, J.D.; Scherson, D.A. Metal-Ion Adsorption on Carboxyl-Bearing Self-Assembled Monolayers Covalently Bound to Magnetic Nanoparticles. Langmuir 2005, 21, 3104–3105. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.M.C.; Chen, G. Performance and mechanism of chromate (VI) adsorption by δ-FeOOH-coated maghemite (γ-Fe2O3) nanoparticles. Sep. Purif. Technol. 2007, 58, 76–82. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, D.H. Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of Cu(II) ions. J. Colloid Interface Sci. 2005, 283, 446–451. [Google Scholar] [CrossRef]
- Viltuznik, B.; Kosak, A.; Zub, Y.L.; Lobnik, A. Removal of Pb(II) ions from aqueous systems using thiol-functionalized cobalt–ferrite magnetic nanoparticles. J. Sol. Gel. Sci. Technol. 2013, 68, 365–373. [Google Scholar] [CrossRef]
- Viltuznik, B.; Lobnik, A.; Kosak, A. The removal of Hg(II) ions from aqueous solutions by using thiol-functionalized cobalt ferrite magnetic nanoparticles. J. Sol. Gel. Sci. Technol. 2015, 74, 199–207. [Google Scholar] [CrossRef]
- Ren, C.; Ding, X.; Fu, H.; Li, W.; Wu, H.; Yang, H. Core–shell superparamagnetic monodisperse nanospheres based on amino-functionalized CoFe2O4@SiO2 for removal of heavy metals from aqueous solutions. RSC Adv. 2017, 7, 6911–6921. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Ding, X.; Fu, H.; Meng, C.; Li, W.; Yang, H. Preparation of amino-functionalized CoFe2O4@SiO2 magnetic nanocomposites for potential application in absorbing heavy metal ions. RSC Adv. 2016, 6, 72479–72486. [Google Scholar] [CrossRef]
- Zhao, F.; Zou, Y.; Lv, X.; Liang, H.; Jia, Q.; Ning, W. Synthesis of CoFe2O4-Zeolite Materials and Application to the Adsorption of Gallium and Indium. J. Chem. Eng. Data 2015, 60, 1338–1344. [Google Scholar] [CrossRef]
- Sailor, M.J.; Lee, E.J. Surface chemistry of Luminescent Silicon Nanocrystallites. Adv. Mater. 1997, 9, 783–793. [Google Scholar] [CrossRef]
- Buriak, J.M. Organometallic Chemistry on Silicon and Germanium Surfaces. Chem. Rev. 2002, 102, 1271–1308. [Google Scholar] [CrossRef]
- Ciampi, S.; Harper, J.B.; Gooding, J.J. Wet chemical routes to the assembly of organic monolayers on silicon surfaces via the formation of Si–C bonds: Surface preparation, passivation and functionalization. Chem. Soc. Rev. 2010, 39, 2158–2183. [Google Scholar] [CrossRef] [Green Version]
- Georgiadou, V.; Makris, G.; Papagiannopoulou, D.; Vourlias, G.; Dendrinou-Samara, C. Octadecylamine-Mediated Versatile Coating of CoFe2O4 NPs for the Sustained Release of Anti-Inflammatory Drug Naproxen and in Vivo Target Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 9345–9360. [Google Scholar] [CrossRef]
- Belagali, S.L.; Himaja, M. Synthesis and evaluation of anti-inflammatory activity of 2-(4-isobutylpbenyl) propionyl derivatives of amino acids and peptides. Indian J. Chem. 1999, 38, 505–507. [Google Scholar]
- Georgiadou, V.; Dendrinou-Samara, C. Impact of the Presence of Octadecylamine on the Properties of Hydrothermally Prepared CoFe2O4 Nanoparticles. Eur. J. Inorg. Chem. 2014, 23, 3645–3656. [Google Scholar] [CrossRef]
- Wen, T.; Qu, F.; Li, N.B.; Luo, H.Q. A facile, sensitive, and rapid spectrophotometric method for copper(II) ion detection in aqueous media using polyethyleneimine. Arab. J. Chem. 2013, 10, 1680–1685. [Google Scholar] [CrossRef]
- Zhou, B.; Zheng, L.; Peng, C.; Li, D.; Li, J.; Wen, S.; Shen, M.; Zhang, G.; Shi, X. Synthesis and characterization of PEGylated polyethylenimine-entrapped gold nanoparticles for blood pool and tumor CT imaging. ACS Appl. Mater. Interfaces 2014, 6, 17190–17199. [Google Scholar] [CrossRef] [PubMed]
- Appelhans, D.; Komber, H.; Quadir, M.A.; Richter, S.; Schwarz, S.; Vlist, J.; Aigner, A.; Muller, M.; Loos, K.; Seidel, J.; et al. Hyperbranched PEI with Various Oligosaccharide Architectures: Synthesis, Characterization, ATP Complexation, and Cellular Uptake Properties. Biomacromolecules 2009, 10, 1114–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.H.; Chen, D.H. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 2009, 163, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Wei, Q.; Yang, J.; Yan, L.; Feng, R.; Chen, G.; Du, B.; Li, H. Highly efficient removal of heavy metal ions by amine-functionalized mesoporous Fe3O4 nanoparticles. Chem. Eng. J. 2012, 184, 132–140. [Google Scholar] [CrossRef]
- Zhang, J.M.; Zhai, S.R.; Li, S.; Xiao, Z.Y.; Song, Y.; An, Q.D.; Tian, G. Removal of Pb(II) and Zn(II) from aqueous solution by ceramisite prepared by sintering bentonite, iron powder and activated carbon. Chem. Eng. J. 2013, 215, 461–471. [Google Scholar] [CrossRef]
- Fellenz, N.; Martin, P.; Marchetti, S.; Bengoa, F. Aminopropyl-modified mesoporous silica nanospheres for the adsorption of Cr(VI) from water. J. Porous Mater. 2015, 22, 729–738. [Google Scholar] [CrossRef]
- Sillanpää, M.; Orama, M.; Rämö, J.; Oikari, A. The importance of ligand speciation in environmental research: A case study. Sci. Total Environ. 2001, 267, 23–31. [Google Scholar] [CrossRef]
- Koehler, F.M.; Rossier, M.; Waelle, M.; Athanassiou, E.K.; Limbach, L.K.; Grass, R.N.; Gunther, D.; Stark, W.J. Magnetic EDTA: Coupling heavy metal chelators to metal nanomagnets for rapid removal of cadmium, lead and copper from contaminated water. Chem. Commun. 2009, 32, 4862–4864. [Google Scholar] [CrossRef]
- Nowack, B.; VanBriesen, J.M. Biogeochemistry of Chelating Agents, 1st ed.; ACS Symposium Series: Washington, DC, USA, 2005. [Google Scholar]
- Byegard, J.; Skarnemark, G.; Skalberg, M. The stability of some metal EDTA, DTPA and DOTA complexes: Application as tracers in groundwater studies. J. Radioanal. Nucl. Chem. 1999, 241, 281–290. [Google Scholar] [CrossRef]
- Allen, H.E.; Huang, C.P.; Bailey, G.W.; Bowers, A.R. Metal Speciation and Contamination of Soil, 1st ed.; Lewis Publishers: Boca Raton, FL, USA, 1995. [Google Scholar]
- Waldron, R.D. Infrared Spectra of Ferrites. Phys. Rev. 1955, 99, 1727–1735. [Google Scholar] [CrossRef]
- Cordente, N.; Respaud, M.; Senocq, F.; Casanove, M.J.; Amiens, C.; Chaudret, B. Synthesis and Magnetic Properties of Nickel Nanorods. Nano Lett. 2001, 1, 565–568. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Liu, Y.; Jiang, L.; Song, B.; Li, M.; Zeng, G.; Tan, X.; Cai, X.; Ding, Y. Adsorption of Cu(II), Pb(II), and Cd(II) Ions from Acidic Aqueous Solutions by Diethylenetriaminepentaacetic Acid-Modified Magnetic Graphene Oxide. J. Chem. Eng. Data 2017, 62, 407–416. [Google Scholar] [CrossRef]
- Miller, F.A.; Wilkins, C.H. Infrared Spectra and Characteristic Frequencies of Inorganic Ions. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Farhan, A.M.; Salem, N.M.; Al-Dujaili, A.H.; Awwad, A.M. Biosorption Studies of Cr(VI) Ions from Electroplating Wastewater by Walnut Shell Powder. Am. J. Environ. Eng. 2012, 2, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Matouq, M.; Jildeh, N.; Qtaishat, M.; Hindiyeh, M.; Al Syouf, M.Q. The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. J. Environ. Chem. Eng. 2015, 3, 775–784. [Google Scholar] [CrossRef]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef] [PubMed]
- Pechurova, N.I.; Kapitanova, R.P.; Varlamova, G.L.; Martynenko, L.I.; Spitsyn, V.I. IR-Spectroscopic Study of Diethylaminepentaacetic Acid and it’s Potassium Salts. Russ. Chem. Bull. 1972, 21, 1448–1450. [Google Scholar] [CrossRef]
- Silva, V.L.; Carvalho, R.; Freitas, M.P.; Tormena, C.F.; Melo, W.C. Spectrometric and Theoretical Investigation of the Structures of Cu and Pb/DTPA Complexes. Struct. Chem. 2007, 18, 605–609. [Google Scholar] [CrossRef]
- Vestal, C.R.; Zhang, Z.J. Effects of Surface Coordination Chemistry on the Magnetic Properties of MnFe2O4 Spinel Ferrite Nanoparticles. J. Am. Chem. Soc. 2003, 125, 9828–9833. [Google Scholar] [CrossRef]


| Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|
| Sample | qe(cal) (mg/g) | k1 (1/min) | R2 | qe(cal) (mg/g) | k2 (mg/gmin) | R2 | qe(exp) (mg/g) |
| MNPs@ODA@PEI | 85.1 | 0.245 | 0.774 | 171.8 | 0.011 | 0.984 | 164.2 |
| MNPs@ODA@DTPA | 16.6 | 0.144 | 0.948 | 29.1 | 0.007 | 0.997 | 29.4 |
| MNPs@ODA@PEI@DTPA | 14.9 | 0.115 | 0.772 | 40.9 | 0.003 | 0.984 | 40.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamvakidis, K.; Kostitsi, T.-M.; Makridis, A.; Dendrinou-Samara, C. Diverse Surface Chemistry of Cobalt Ferrite Nanoparticles to Optimize Copper(II) Removal from Aqueous Media. Materials 2020, 13, 1537. https://doi.org/10.3390/ma13071537
Vamvakidis K, Kostitsi T-M, Makridis A, Dendrinou-Samara C. Diverse Surface Chemistry of Cobalt Ferrite Nanoparticles to Optimize Copper(II) Removal from Aqueous Media. Materials. 2020; 13(7):1537. https://doi.org/10.3390/ma13071537
Chicago/Turabian StyleVamvakidis, Kosmas, Theodora-Marianna Kostitsi, Antonis Makridis, and Catherine Dendrinou-Samara. 2020. "Diverse Surface Chemistry of Cobalt Ferrite Nanoparticles to Optimize Copper(II) Removal from Aqueous Media" Materials 13, no. 7: 1537. https://doi.org/10.3390/ma13071537






