1. Introduction
Diaphyseal fractures in dogs and cats are common small animal orthopedic injuries that can be treated by using interlocking nails. Interlocking nails were developed to increase treatment abilities, especially in canine femur, tibia, and humerus fractures [
1]. However, the removal of interlocking nails is still required due to complications in long-term implantation [
2]. Titanium and stainless steel are the biomaterials commonly used in interlocking nails. According to the bioinert properties of these materials, biological support to the living cells is not activated, and bone regeneration does not occur in the interlocking nail cavity [
3]. Therefore, the interlocking nail will leave a blank cavity inside the healed bone. Tissue engineering has become necessary for bone treatment to eliminate the limitations of the natural healing process using several materials and fabrication processes [
4,
5,
6]. Fused filament fabrication (FFF) is a well-known 3D printing method and an additive manufacturing (AM) technology that is widely used in several fields [
7]. It provides several benefits, such as product customization, cost-effectiveness, and the possibility to create complex structures, making it ideal for patient-specific devices in biomedical applications [
8,
9]. To implement FFF for implantable biomedical devices, meltable biomaterial filaments are required [
10,
11]. Generally, meltable polymers are key materials for FFF 3D printing. Thus, the load carrying and mechanical properties of the polymers interlocking nails should be focused on. Although polymers provide low mechanical properties, they are still able to carry the mechanical load on small animal bones, such as feline and canine femurs [
12]. Thus, we aimed to use an FFF 3D-printed interlocking nail for the treatment of canine orthopedic injuries. However, each individual biomaterial has its own disadvantages for tissue regeneration. Thus, copolymers or blended composite materials are required to improve the properties of the fabricated materials [
13]. Currently, biomaterial filaments, such as polylactic acid (PLA) and poly-caprolactone (PCL), are commercially available for FFF 3D printing. PLA is widely used in biomedical applications because the compound released during its degradation (lactic acid) is a non-cytotoxic compound found in the mammalian body [
14,
15]. However, PLA is brittle and rapidly degraded in body fluids [
16]. Therefore, blending PLA with other biodegradable polymers was performed to decrease the substance’s limitations [
17,
18]. PCL is a biodegradable polymer blended with PLA to decrease the brittleness problem and prolong the degradation period [
18]. Furthermore, PLA and PCL are combined into a copolymer that provides the required abilities for tissue engineering applications, such as being environmentally friendly, non-toxic, biocompatible, and biodegradable [
13,
19]. To increase osteoconductivity and induce bone growth, hydroxyapatite (HA), the main mineral component in mammalian bone, has been used in several studies [
20,
21,
22,
23]. Moreover, a biomaterial with good osteoconductivity and osteoinductivity is silk fibroin (SF), which is commonly used in biomedical applications [
24,
25,
26]. Thus, PLA, PCL, HA, and SF were selected for use in this study. The composite biomaterial filaments were locally extruded from three combination ratios between PLA, PCL, and HA. The filaments were then FFF 3D-printed into four design specimens for the different tests. Finally, SF was coated on the printed specimens using the lyophilization technique for cytotoxicity and cell proliferation tests. The mechanical and biological properties of the specimens were used to investigate the possibility to fabricate an interlocking nail using FFF 3D-printed PLA/PCL/HA composite filaments and coatings using local silk fibroin for the canine bone fracture treatment.
2. Materials and Methods
Commercial PLA and PCL granules were used for filament extrusion. PLA is mainly composed of a semicrystalline poly-lactide resin (Ingeo 3D850, Natureworks LLC, Blair, Nebraska, USA) [
27]. The PCL (number-average molecular weight (Mw) = 60,000–80,000 Da) was provided by Daigang Biomaterials (Shandong, China). PCL is a semicrystalline biodegradable hydrophobic polyester that is widely used in 3D printing [
28].
HA was synthesized from bovine bone. After cutting into small pieces and soaked in H
2O
2 for 2 days to remove the tissues, the small pieces of bones were boiled in water to eliminate organic substances. Then, the boiled bones were dried in a hot air oven at 120 °C for 7 h to reduce their moisture. The dried bones were calcinated to approximately 850 °C for 3 h and ground to less than 20 microns by a high ball-milling machine [
5,
29]. HA powder was added to a silane coupling solution at a ratio of 1:2 w/v. Finally, the slurry HA was dried at 100–120 °C [
30].
Local Thai
Bombyx mori (Luang Saraburi) silk cocoons were used as raw materials for SF extraction. Each cocoon was cut small and degummed in 100 °C Na
2CO
3 solution for 30 min and rinsed with warm deionized (DI) water. The degummed silk fibers were dried overnight in a 37 °C hot air oven and then dissolved at 70 °C for 6 h in a ternary solvent (CaC
l2/CH
3CH
2OH/H
2O; 1:2:8 in mole ratio) [
31,
32]. The silk-ternary solution was dialyzed using a cellulose membrane with 4 °C DI water for 3 days, and the DI water was changed every day. The dialyzed SF solution was filtered centrifuged and frozen at −80 °C. Finally, the frozen SF was lyophilized to obtain SF sponges [
6].
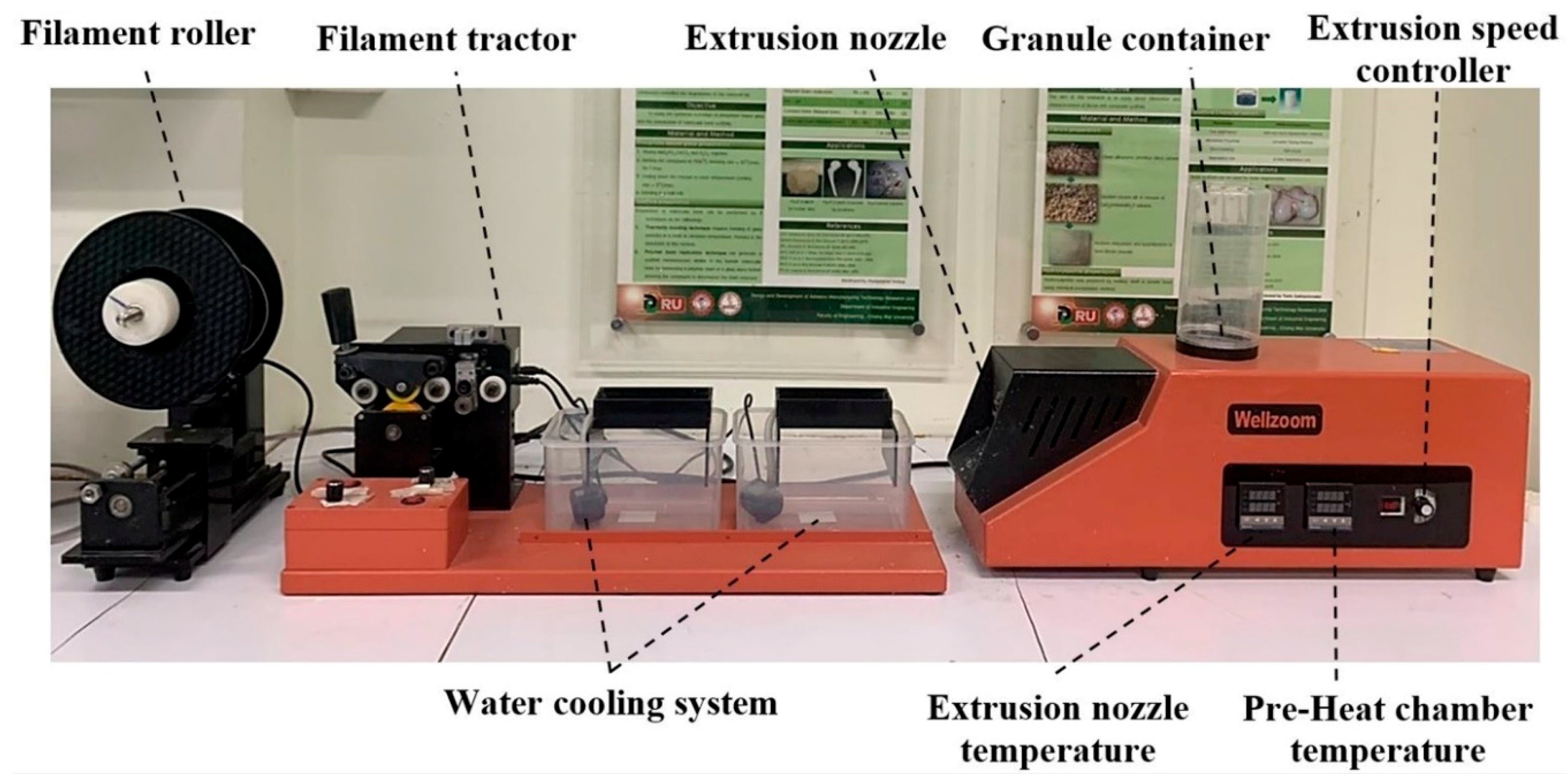
The mixed materials were extruded to be a single filament using a desktop single-screw extruder (Wellzoom Desktop Extruder Line II, Shenzhen, China) with a filament cooling system, as shown in
Figure 1. The mixing ratio between the PLA and PCL was fixed at 70:30 [
33]. HA powder was added to the prepared PLA/PCL mixed granules at 0%, 5%, and 15% w/w. From our preliminary experiments, the maximum percentage of HA powder in the PLA/PCL mixed granules was limited by the ability of the extruder and the extruded filament conditions. Moreover, the maximum HA percentage from previous studies was presented at 15% [
34,
35,
36]. The mixing ratios were labeled and are described in
Table 1. The moisture in the PLA granules was reduced in a 60 °C oven for 4 h prior to mixing [
37]. Then, PLA, PCL, and HA were homogenously mixed using a homemade ball milling machine (1440 rpm). The controlled extrusion parameters consisted of the extrusion rotational speed, the temperature of the pre-heated chamber, and the temperature of the extrusion nozzle, as presented in
Table 2. The first run filament was tracked to the cooling system manually until the tip of the filaments reached the filament tractor. Then, the tractor speed was adjusted until the extruded filament diameter was 1.75 ± 0.05 mm and ran smoothly [
38,
39]. The filament was rolled into a filament spool using a filament roller, which adjusted the rolling speed based on the filament’s tractor speed.
The 200 mm long extruded filaments were clamped with tensile grips in a universal testing machine (UTM: Instron 5566, Ithaca, NY, USA). A 500 N loading cell was used with a displacement rate of 5 mm/min [
40].
All specimens were printed using a homemade FFF 3D-printer in our laboratory, as shown in
Figure 2. A 0.4 mm printing nozzle was used. The printing parameters were implemented via a computer program for 3D-printing. The melting temperature of the printing nozzle was 200 °C, the extrusion speed was 50 mm/s, and the printing layer height was 0.2 mm [
41]. The printed specimens were designed based on mechanical standard testing and previous publications.
Cylindrical specimens 10 mm in diameter and 10 mm in thickness were printed for the compression test. Each specimen was placed in the compressing station of an Instron 5566 UTM with a 5 mm/min crosshead motion rate and a 10 kN loading cell [
42]. The specimens for tensile and flexural testing were printed using the recommended dimensions of the American Society for Testing and Materials (ASTM) standard. The tensile specimen was printed using the recommended dimensions of the tensile testing standard (ASTM D638). The specimen was set in the tensile jig fixture of the UTM. The crosshead motion of the UTM was 5 mm/min with a 100 N loading cell [
37]. The ASTM D790 standard for flexural testing was implemented on the rectangular-shaped 3D-printed specimen. Each specimen was placed in a 3-point bending testing set of the UTM. The crosshead motion with a 5 kN loading cell was 10 mm/min [
43].
For Fourier transform infrared spectroscopy (FTIR: Thermo Fisher Scientific, Waltham, MA, USA), a Thermo Fisher FTIR spectrometer equipped with an ATR (attenuated total reflectance) accessory was used to obtain the functional groups of the molecules in the SF sample. Sixty-four repetitive scans from 500 to 2500 cm−1 were averaged and presented.
For X-ray diffraction (XRD: Rigaku, Tokyo, Japan), the phase composit ion of the extracted HA powder and the HA within the composite PLA/PCL/HA filaments were characterized by XRD. The XRD chart was acquired with CuKα radiation (λ = 1.5418 Å) in the 2θ range of 20°–60°.
For differential scanning calorimetry (DSC: Mettler Toledo DSC1 Star System, Columbus, OH, USA), to evaluate the effect of HA particles on the glass transition temperature and melting temperature of the PLA/PCL, the extruded composite filaments were examined at temperatures ranging from 25 to 200 °C. To check the consistency of the tests, the DSC experiments were run at least three times.
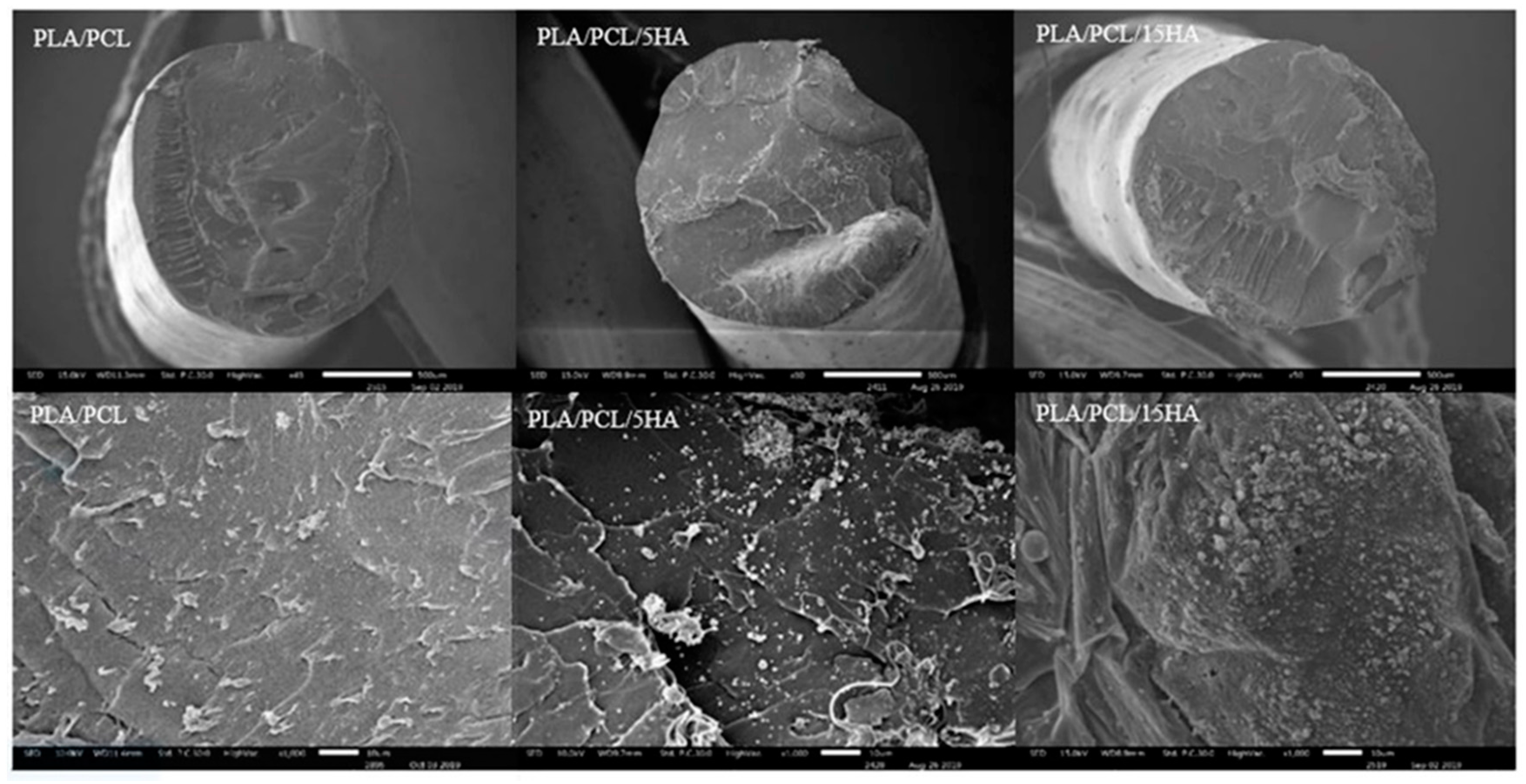
The morphology of the extruded filaments and printed specimens were then observed under a scanning electron microscope (SEM: JEOL JSM 6400, Tokyo, Japan).
For biodegradability testing, the specimens were printed in a square of 8 mm × 8 mm with a thickness of 2 mm. This design was printed for the SF coating, as well. The initial weight of each specimen was measured and recorded as
wi. A phosphate-buffered saline (PBS) solution containing 1.6 µg/mL of lysozyme was prepared. The specimens were prepared for a 10 pieces/ratio/degradation period (90 pieces, total). The prepared specimens were placed in 24 well-plates with 1 mL of PBS containing lysozyme and kept in a 37 °C CO
2 incubator for 7, 15, and 30 degradation days. At the end of days 7, 15, and 30 the specimens were rinsed with DI water and frozen in a −80°C freezer overnight. The frozen specimens were lyophilized for 48 h to obtain dried specimens. The weight of dried residual specimens was recorded as
wf, and the percentage of weight loss was calculated using Equation (1) [
32,
44].
For silk fibroin coating on the 3D-printed specimens, the printed specimens of all material ratios were placed in a 10 mm × 10 mm rectangular mold with a thickness of 3 mm. The SF sponge was mixed with the PBS solution (0.05 g/mL). Glutaraldehyde was used as a crosslinking agent for 0.0025% in the SF solution. Then, 200 µL of the preparing solution was dropped on each specimen in the rectangular mold. The specimens were completely covered by the solution. The mold containing the specimens with the SF solution was frozen in a −80 °C freezer overnight and lyophilized for 48 h. The specimens coated with SF were used for the biocompatibility test [
45].
For biocompatibility testing, a human fetal osteoblast cell line (hFOB1.19, CRL NO.11372) was purchased from ATCC and expanded in DMEM/Ham’s F-12 medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin (basal media) until reaching cell confluence. Cells were seeded in 24-well plates at a density of 2 × 10
5 cells/well and incubated at 37 °C with 5% CO
2 for 24 h. The specimens consisted of PLA/PCL, PLA/PCL/5HA, and PLA/PCL/15HA and were divided into 2 groups: without an SF coating and with an SF coating. Each group was placed into the wells of the plates and incubated for 2 incubation periods (1 day and 3 days). At the indicated time of treatment, Alamar blue dye (10%
ν/ν in PBS) was added to each well, and the plates were incubated at 37 °C for 4 h. Then, the condition media in each well was harvested, and the absorbances at 540 nm (test wavelength) and 620 nm (reference wavelength) were measured using a Titertek Multiskan M340 multiplate reader (ICN Flow, Costa Mesa, CA, USA) [
44]. Each treatment was tested in triplicate. The formula for calculating the difference in the reduction percentage is shown in Equation (2):