Vitamin Derived Nitrogen Doped Carbon Nanotubes for Efficient Oxygen Reduction Reaction and Arsenic Removal from Contaminated Water
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of Nitrogen Doped Carbon Nanotubes from Niacin
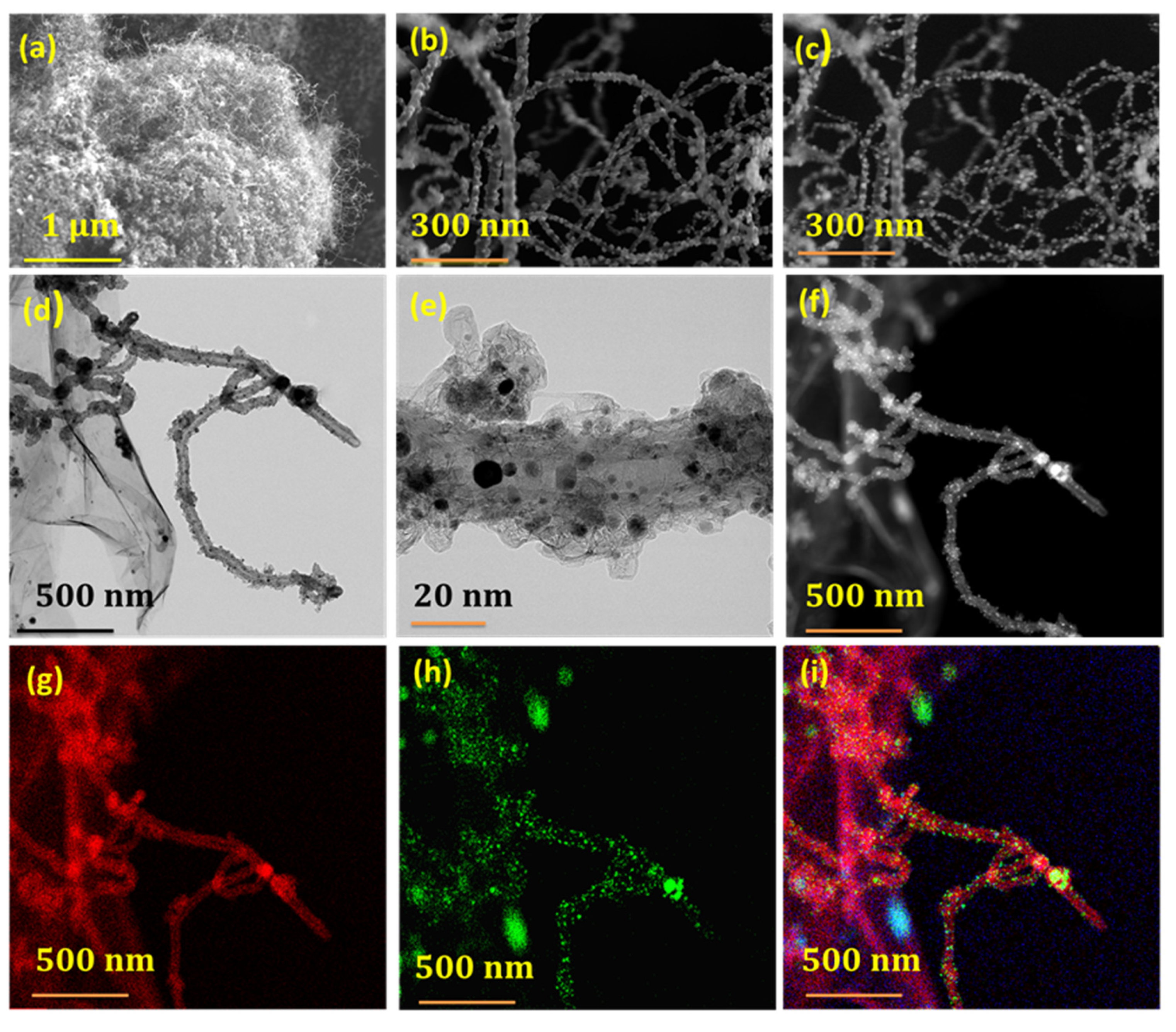
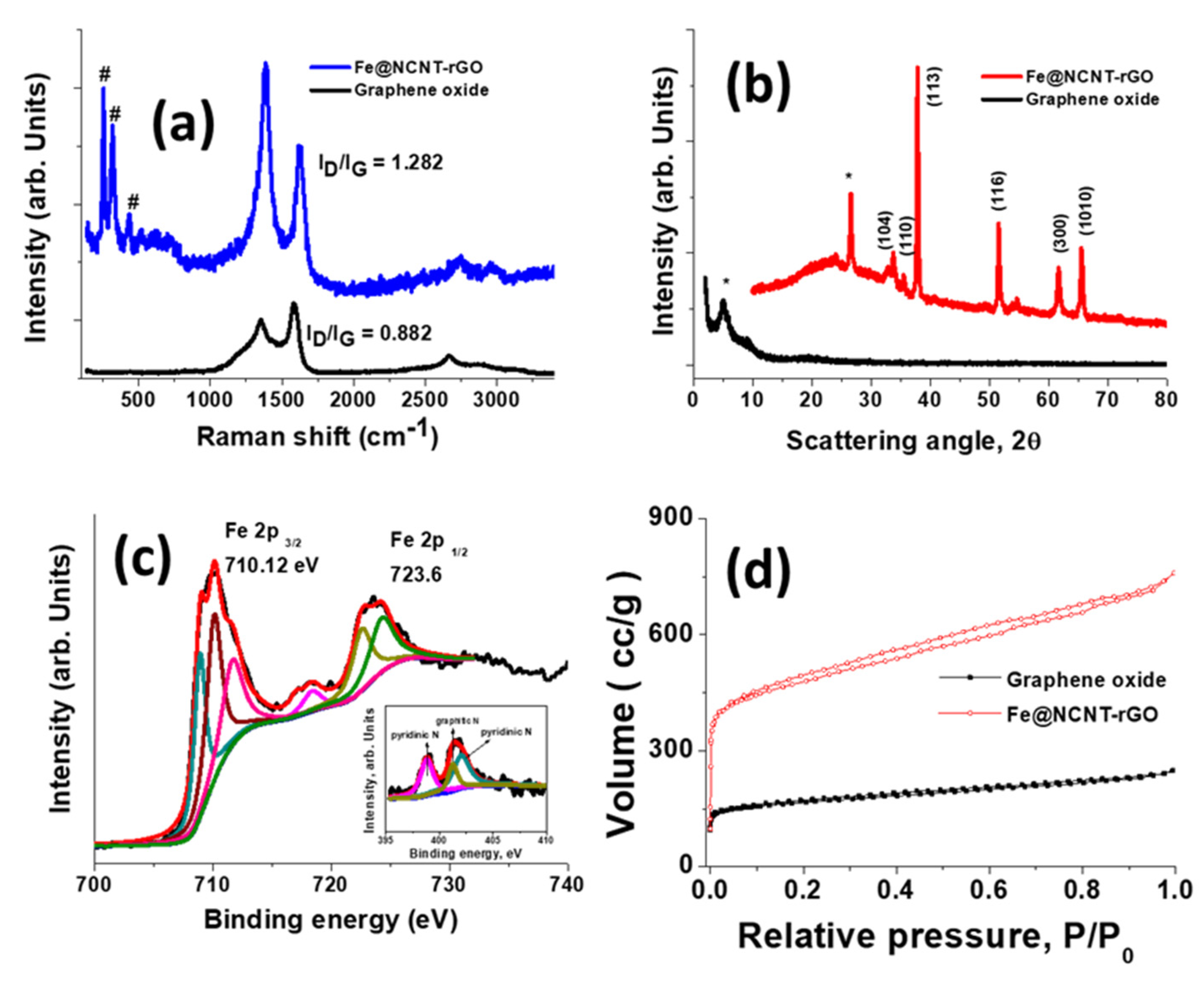
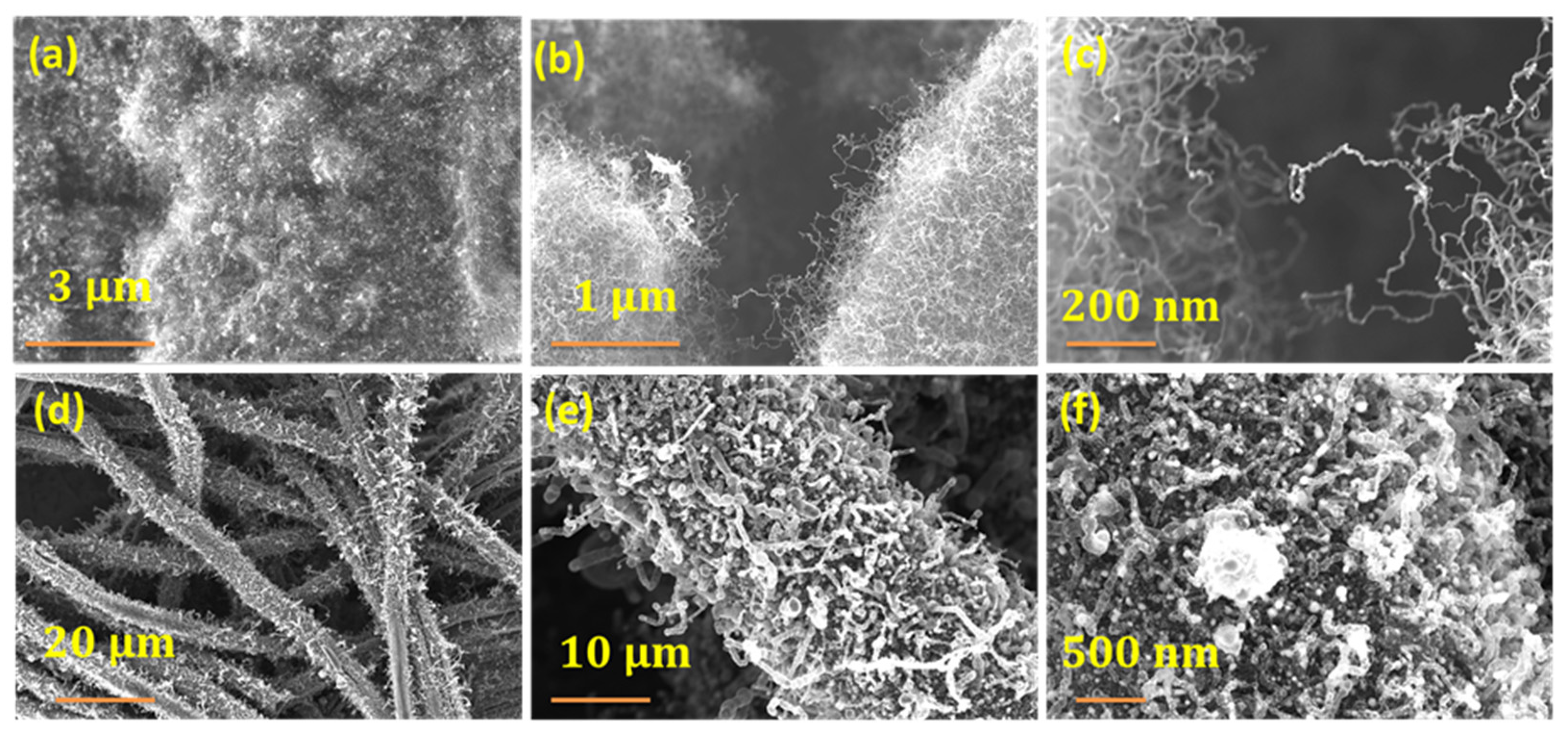
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, W.; Sun, L.; Hu, R.; Liao, W.; Li, Z.; Li, Y.; Guo, C. Surface Modification of Multi-Walled Carbon Nanotubes via Hemoglobin-Derived Iron and Nitrogen-Rich Carbon Nanolayers for the Electrocatalysis of Oxygen Reduction. Materials 2017, 10, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhu, M.; Wang, G.; Dai, B.; Yu, F.; Tian, Z.; Guo, X. Enhanced Oxygen Reduction Reaction by In Situ Anchoring Fe2N Nanoparticles on Nitrogen-Doped Pomelo Peel-Derived Carbon. Nanomaterials 2017, 7, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Liang, C. High-Density Cobalt Nanoparticles Encapsulated with Nitrogen-Doped Carbon Nanoshells as a Bifunctional Catalyst for Rechargeable Zinc-Air Battery. Materials 2019, 12, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Ma, J.; Yang, H.; Lu, G.; Yang, S.; Chang, Z. Nitrogen and Cobalt Co-Doped Carbon Materials Derived from Biomass Chitin as High-Performance Electrocatalyst for Aluminum-Air Batteries. Catalysts 2019, 9, 954. [Google Scholar] [CrossRef] [Green Version]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.-G.; Yoon, T.; Kim, K.S. Nickel-Based Electrocatalysts for Energy-Related Applications: Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reactions. ACS Catalysis 2017, 7, 7196. [Google Scholar] [CrossRef]
- Goubert-Renaudin, S.N.S.; Wieckowski, A. Ni and/or Co nanoparticles as catalysts for oxygen reduction reaction (ORR) at room temperature. J. Electroanal. Chem. 2011, 652, 44. [Google Scholar] [CrossRef]
- Karunagaran, R.; Coghlan, C.; Shearer, C.; Tran, D.; Gulati, K.; Tung, T.T.; Doonan, C.; Losic, D. Green Synthesis of Three-Dimensional Hybrid N-Doped ORR Electro-Catalysts Derived from Apricot Sap. Materials 2018, 11, 205. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, H.; Ji, S.; Lv, W.; Wang, R. Harvesting a 3D N-Doped Carbon Network from Waste Bean Dregs by Ionothermal Carbonization as an Electrocatalyst for an Oxygen Reduction Reaction. Materials 2017, 10, 1366. [Google Scholar] [CrossRef] [Green Version]
- Alegre, C.; Sebastián, D.; Gálvez, M.E.; Baquedano, E.; Moliner, R.; Aricò, A.S.; Baglio, V.; Lázaro, M.J. N-Doped Carbon Xerogels as Pt Support for the Electro-Reduction of Oxygen. Materials 2017, 10, 1092. [Google Scholar] [CrossRef]
- Huo, P.; Zhao, P.; Wang, Y.; Liu, B.; Yin, G.; Dong, M. A Roadmap for Achieving Sustainable Energy Conversion and Storage: Graphene-Based Composites Used Both as an Electrocatalyst for Oxygen Reduction Reactions and an Electrode Material for a Supercapacitor. Energies 2018, 11, 167. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yin, J.; Zhao, L.; Tian, C.; Yu, P.; Wang, J. Ion-exchanged route synthesis of Fe2N–N-doped graphitic nanocarbons composite as advanced oxygen reduction electrocatalyst. Chem. Commun. 2013, 49, 3022–3024. [Google Scholar] [CrossRef] [PubMed]
- Mamtani, K.; Jain, D.; Dogu, D.; Gustin, V.; Gunduz, S.; Co, A.C. Insights into oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) active sites for nitrogen-doped carbon nanostructures (CNx) in acidic media. Appl. Catal., B. 2018, 220, 88–97. [Google Scholar] [CrossRef]
- Mostazo-López, M.J.; Salinas-Torres, D.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Nitrogen-Doped Superporous Activated Carbons as Electrocatalysts for the Oxygen Reduction Reaction. Materials 2019, 12, 1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shui, J.; Wang, M.; Du, F.; Dai, L. N-doped carbon nanomaterials are durable catalysts for oxygen reduction reaction in acidic fuel cells. Sci. Adv. 2015, 1, e1400129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Zhou, T.; Xing, L.; Xu, K.; Tong, Y.; Xie, H. Atomically Dispersed Iron-Nitrogen Species as Electrocatalysts for Bifunctional Oxygen Evolution and Reduction Reactions. Angew. Chem., Int. Ed. 2016, 56, 610–614. [Google Scholar] [CrossRef]
- Zhang, H.; Hwang, S.; Wang, M.; Feng, Z.; Karakalos, S.; Luo, L. Single Atomic Iron Catalysts for Oxygen Reduction in Acidic Media: Particle Size Control and Thermal Activation. J. Am. Chem. Soc. 2017, 139, 14143–14149. [Google Scholar] [CrossRef]
- Begum, H.; Kim, Y.-B. Improvement of Catalytic Activity of Platinum Nanoparticles Decorated Carbon Graphene Composite on Oxygen Electroreduction for Fuel Cells. Processes 2019, 7, 586. [Google Scholar] [CrossRef] [Green Version]
- Jafri, R.I.; Rajalakshmi, N.; Ramaprabhu, S. Nitrogen-Doped Multi-Walled Carbon Nanocoils as Catalyst Support for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cell. J. Power Sources. 2010, 195, 8080–8083. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Y.; Yan, B.; Wei, T.; Lv, Y.; Chen, L.; Yu, F.; Guo, X. Flocculant-Assisted Synthesis of Graphene-Like Carbon Nanosheets for Oxygen Reduction Reaction and Supercapacitor. Nanomaterials 2019, 9, 1135. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zeng, G.; Chen, J.; Bi, L.; Dai, L.; Wen, Z. N-doped porous carbon nanosheets as pH-universal ORR electrocatalyst in various fuel cell devices. Nano Energy 2018, 49, 393–402. [Google Scholar] [CrossRef]
- Odedairo, T.; Yan, X.; Ma, J.; Jiao, Y.; Yao, X.; Du, A. Nanosheets Co3O4 Interleaved with Graphene for Highly Efficient Oxygen Reduction. ACS Appl. Mater. Interfaces 2015, 7, 21373–21380. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, S.; Chudoba, T.; Wojnarowicz, J.; Łojkowski, W. Current Trends in the Development of Microwave Reactors for the Synthesis of Nanomaterials in Laboratories and Industries: A Review. Crystals 2018, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- Horikoshi, S.; Schiffmann, R.F.; Fukushima, J.; Serpone, N. Microwave Chemical and Materials Processing; Springer: Singapore, 2018. [Google Scholar]
- Cintas, P.; Veronesi, P.; Leonelli, C.; Keglevich, G.; Mucsi, Z.; Radoiu, M.; de la Hoz, A.; Prieto, P.; Wada, Y.; Mochizuki, D.; et al. Microwave Chemistry; Cravotto, G., Carnaroglio, D., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2017. [Google Scholar]
- Sridhar, V.; Lee, I.; Chun, H.-H.; Park, H. Microwave synthesis of nitrogen-doped carbon nanotubes anchored on graphene substrates. Carbon 2015, 87, 186–192. [Google Scholar] [CrossRef]
- Gangaraju, D.; Sridhar, V.; Lee, I.; Park, H. Graphene–carbon nanotube–Mn3O4 mesoporous nano-alloys as high capacity anodes for lithium-ion batteries. J. Alloys Compd. 2017, 699, 106–111. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, H. Zeolitic imidazolate frameworks as novel precursors for microwave synthesis of carbon nanotubes. J. Alloys Compd. 2019, 781, 166–173. [Google Scholar] [CrossRef]
- Wu, C.; Tu, J.; Tian, C.; Geng, J.; Lin, Z.; Dang, Z. Defective magnesium ferrite nano-platelets for the adsorption of As(V): The role of surface hydroxyl groups. Environ. Pollut. 2018, 235, 11–19. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Lundberg, M.; Kroll, T.; DeBeer, S.; Bergmann, U.; Wilson, S.A.; Glatzel, P. Metal–Ligand Covalency of Iron Complexes from High-Resolution Resonant Inelastic X-ray Scattering. J. Am. Chem. Soc. 2013, 135, 17121–17134. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Gao, X.; Wei, X.; Wang, X.; Li, Y.; Wu, T.; Guo, J.; Gu, Q.; Wu, W.D.; Chen, X.D. Directly anchoring Fe3 C nanoclusters and FeNx sites in ordered mesoporous nitrogen-doped graphitic carbons to boost electrocatalytic oxygen reduction. Carbon 2017, 121, 143–153. [Google Scholar] [CrossRef]
- Hung, T.-F.; Tu, M.-H.; Tsai, C.-W.; Chen, C.-J.; Liu, R.-S.; Liu, W.-R.; Lo, M.-Y. Influence of pyrolysis temperature on oxygen reduction reaction activity of carbon-incorporating iron nitride/nitrogen-doped graphene nanosheets catalyst. Int. J. Hydrog. Energy. 2013, 38, 3956–3962. [Google Scholar] [CrossRef]
- Rohith Vinod, K.; Saravanan, P.; Sakar, M.; Balakumar, S. Insights into the nitridation of zero-valent iron nanoparticles for the facile synthesis of iron nitride nanoparticles. RSC Adv. 2016, 6, 45850–45857. [Google Scholar] [CrossRef]
- Panda, R.N.; Gajbhiye, N.S. Magnetic properties of single domain ε-Fe3N synthesized by borohydride reduction route. J Appl Phys. 1997, 81, 335–339. [Google Scholar] [CrossRef]
- Sosinsky, B.A.; Norem, N.; Shelly, J. Spectroscopic study of a series of iron carbido clusters. Inorg. Chem. 1982, 21, 348–356. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Yuan, M.; Sun, G.; Liao, Q.; Zhang, Y. Solid and macroporous Fe3C/N-C nanofibers with enhanced electromagnetic wave absorbability. Sci. Rep. 2018, 8, 16832. [Google Scholar] [CrossRef]
- Tang, F.; Lei, H.; Wang, S.; Wang, H.; Jin, Z. A novel Fe–N–C catalyst for efficient oxygen reduction reaction based on polydopamine nanotubes. Nanoscale 2017, 9, 17364–17370. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.C.-K.; Dahn, J.R. Fe-N-C oxygen reduction catalysts supported on vertically aligned carbon nanotubes. Appl. Catal., A 2008, 347, 43–49. [Google Scholar] [CrossRef]
- Du, P.; Ma, F.-X.; Lyu, F.; He, K.; Li, Z.; Lu, J.; Li, Y.Y. Bottom-up synthesis of iron and nitrogen dual-doped porous carbon nanosheets for efficient oxygen reduction. Chem. Commun. 2019, 55, 5789–5792. [Google Scholar] [CrossRef]
- Kishi, H.; Sakamoto, T.; Asazawa, K.; Yamaguchi, S.; Kato, T.; Zulevi, B.; Serov, A.; Artyushkova, K.; Atanassov, P.; Matsumura, D.; et al. Structure of Active Sites of Fe-N-C Nano-Catalysts for Alkaline Exchange Membrane Fuel Cells. Nanomaterials 2018, 8, 965. [Google Scholar] [CrossRef] [Green Version]
- Contreras, E.; Dominguez, D.; Tiznado, H.; Guerrero-Sanchez, J.; Takeuchi, N.; Alonso-Nunez, G.; Contreras, O.E.; Oropeza-Guzmán, M.T.; Romo-Herrera, J.M. N-Doped carbon nanotubes enriched with graphitic nitrogen in a buckypaper configuration as efficient 3D electrodes for oxygen reduction to H2O2. Nanoscale 2019, 11, 2829–2839. [Google Scholar] [CrossRef]
- Vadahanambi, S.; Lee, S.-H.; Kim, W.-J.; Oh, I.-K. Arsenic Removal from Contaminated Water Using Three-Dimensional Graphene-Carbon Nanotube-Iron Oxide Nanostructures. Environ. Sci. Technol. 2013, 47, 10510–10517. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Chen, J.P. Preparation and evaluation of a magnetite-doped activated carbon fiber for enhanced arsenic removal. Carbon. 2010, 48, 60–67. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sridhar, V.; Jung, K.H.; Park, H. Vitamin Derived Nitrogen Doped Carbon Nanotubes for Efficient Oxygen Reduction Reaction and Arsenic Removal from Contaminated Water. Materials 2020, 13, 1686. https://doi.org/10.3390/ma13071686
Sridhar V, Jung KH, Park H. Vitamin Derived Nitrogen Doped Carbon Nanotubes for Efficient Oxygen Reduction Reaction and Arsenic Removal from Contaminated Water. Materials. 2020; 13(7):1686. https://doi.org/10.3390/ma13071686
Chicago/Turabian StyleSridhar, Vadahanambi, Kwang Hyo Jung, and Hyun Park. 2020. "Vitamin Derived Nitrogen Doped Carbon Nanotubes for Efficient Oxygen Reduction Reaction and Arsenic Removal from Contaminated Water" Materials 13, no. 7: 1686. https://doi.org/10.3390/ma13071686





