3.1. TGA of the Major Pyrolysis Properties of Flame Retardant Cables
The pyrolysis properties required for flame retardant cables composed of multiple materials, i.e., the reference temperature and reference rate, were obtained by TGA.
Figure 3,
Figure 4,
Figure 5,
Figure 6 and
Figure 7 present the TGA results for the five cables considered in this study as well as the mass fraction and reaction rate as functions of temperature. As mentioned in
Section 2.2, TGA was performed in two stages. Firstly, the single materials composing each cable were analyzed separately. Secondly, the multiple materials corresponding to the mass fractions shown in
Figure 2 were analyzed simultaneously. Based on this analysis approach, the dominant reference temperatures and rates of the pyrolysis reactions of flame retardant cables composed of multiple materials were determined.
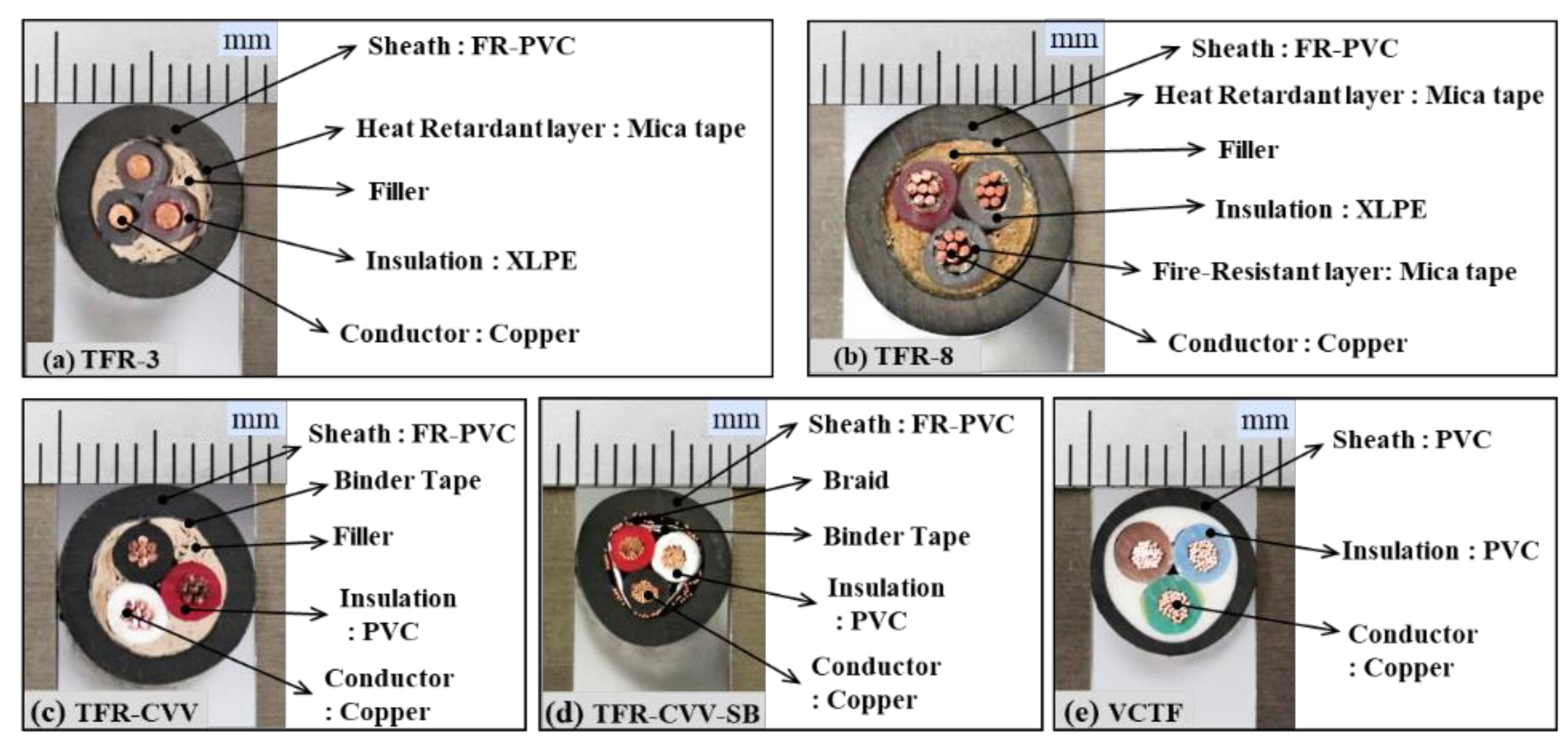
Figure 3 shows the results of the TGA of TFR-3, which was the tray heat resistant control and signal cable for fire service.
Figure 3a,b show the mass fractions and reaction rates as functions of temperature for the sheath, mica tape, filler, and insulation. The sheath with the highest mass fraction exhibits a two-stage mass reduction, i.e., a two-step reaction, depending on the temperature change. Although the sheath is known to consist primarily of PVC, it can be interpreted as containing two components, assuming that each component undergoes a single-step pyrolysis reaction [
23]. Quantitatively, the reference temperatures at which the pyrolysis reaction of the sheath occurs the most rapidly are 288 °C and 475 °C, and the maximum reaction rates at these temperatures (i.e., the reference rates) are 8.17 × 10
−3 s
−1 and 1.12 × 10
−3 s
−1, respectively. PVC is well known to produce hydrogen chloride via a pyrolysis reaction in the temperature range 250-300 °C in air or nitrogen [
25], corresponding to the first-order reaction in
Figure 3a. The reference temperature of the mica tape is 349 °C, which is between the reference temperatures of the two components of the sheath. The reference rate of the mica tape is considerably lower than that of the sheath. For the filler and insulation, the reference temperatures are 457 °C and 475 °C, with reference rates of 1.06 × 10
−2 s
−1 and 2.00 × 10
−2 s
−1, respectively. These reference temperatures are similar to the temperature related to the second-order reaction of the sheath, and the reaction rates are higher than those of the sheath. It should be noted that XLPE insulation is generally used for fire protection cables, and the initial pyrolysis reaction occurs at a significantly higher temperature than that of the sheath consisting mainly of PVC.
Figure 3c shows the results of the simultaneous analysis of the four materials comprising TFR-3 according to their mass fractions. In the figure, TFR-3 exhibits a two-step pyrolysis reaction, with reference temperatures of 284 °C and 464 °C. Comparison of the individual results for each material (
Figure 3a,b) clearly indicates that the first-order reaction of TFR-3 is caused by the first-order reaction of the sheath. The second-order reaction takes place over a wider temperature range than the first-order reaction and occurs in the temperature range in which the second-order reaction of the sheath and the reactions of the filler and insulation occur. In other words, the second-order reaction of TFR-3 is presumed to coincide with the effects of the sheath, filler, and insulation. The mica tape does not significantly affect the overall pyrolysis reaction of TFR-3 because of its very low mass fraction. In summary, the pyrolysis reaction of TFR-3 can be expressed as a first-order reaction by a specific component of the sheath and a second-order reaction by multiple materials, including the sheath, filler, and insulation.
Figure 4a,b show the mass fractions and reaction rates with respect to temperature for the individual materials (the sheath, mica tape, filler, and insulation) constituting TFR-8, which is a tray flame retardant power cable for fire service. The changes in the mass fraction and reaction rate with temperature are quite similar to those of TFR-3, except that the reference temperature of the mica tape is similar to the temperature at which the second-order reaction of the sheath takes place. In addition, the reference temperature of the filler is approximately 350 °C, which is lower than that of 457 °C for TFR-3.
Figure 4c shows the results of the simultaneous analysis of the four materials comprising TFR-8 according to the mass fraction. As in TFR-3, the first-order reaction corresponds to the pyrolysis reaction exhibited by a specific component of the sheath. However, the second-order reaction is caused by multiple materials, i.e., the sheath, mica tape, and insulation. In addition, the filler in TFR-8 causes a fine inflection point between the temperature at which the first and second reactions occur, unlike the effect of the filler that contributed to the second-order reaction in TFR-3. These results indicate that these two types of fire protection cables (TFR-3 and TFR-8) undergo two-step pyrolysis reactions. That is, the first-order reaction is that of a specific component of the sheath, and the second-order reaction occurs as a result of simultaneous pyrolysis of multiple materials. The second-order reaction is that of multiple materials, including the sheath and insulation, regardless of the cable type. This reaction is also associated with the mica tape and filler, which depend on the cable type. The mass fractions of these two materials are very low, as shown in
Figure 2; thus, it seems reasonable to disregard the effects of the tape and filler on the major pyrolysis reactions of the cables.
Figure 5 and
Figure 6 show the TGA results of TFR-CVV and TFR-CVV-SB, which are the tray flame retardant control cables with PVC sheaths. Note that because the TFR-CVV-SB cable does not contain filler, it is not considered in
Figure 6. As in the fire protection cables, the pyrolysis of the control cables occurs via a two-step reaction. However, the insulation of the control cables undergoes a two-step pyrolysis reaction unlike that of the fire protection cables, because the main component of the insulation surrounding the control cables is PVC, which is similar to that of the sheath. In other words, in the fire protection cables, only the sheath undergoes the first-order reaction, but in the control cables, this reaction is caused by both the sheath and interior insulation material. The second-order reaction of the TFR-CVV cable occurs as a result of the simultaneous pyrolysis of multiple materials (the sheath, binder tape, filler, and insulation). On the other hand, in the case of the TFR-CVV-SB cable, multiple materials (the sheath, binder tape, and insulation) participate in the pyrolysis reaction.
Figure 7 shows the mass fractions and reaction rates as functions of temperature of the sheath and insulation of the VCTF cable. This cable also exhibits a two-step pyrolysis reaction, similar to the fire protection and control cables. Because both of these materials have PVC as their main components, the reference temperatures for the individual materials (
Figure 7) and for multiple materials with their corresponding mass fractions (
Figure 7b) are quite similar. These results confirm that all of the cables considered in this study undergo two-step pyrolysis reactions. In addition, each pyrolysis reaction can involve single or multiple materials depending on the cable type and reaction order. However, assuming that each material (or specific component) undergoes a single-step reaction [
23], the composition of each cable can be simplified as two artificial materials, even when there are multiple components.
Table 2 summarizes the TGA results of the five cables considered in this study, showing the reference temperatures and rates in the two-step pyrolysis reaction, which are required as the input parameters for the fire spread simulation. All of the data are presented as the mean values of three repeated experiments. To facilitate understanding of these quantitative values,
Figure 8 shows the reference temperatures and rates of the two dominant pyrolysis reactions (R1 and R2) of each cable. The structures, compositions, and insulation performances of the five cables considered in this study differ considerably, except for the conductor specifications. Nevertheless, the reference temperatures of the two-step pyrolysis reaction as presented in
Figure 8a are quite similar regardless of the cable type. Quantitatively, the mean reference temperatures for R1 and R2 are 283 °C and 464 °C, respectively, and the standard deviations are identical at ±7 °C. That is, the standard deviations are approximately ±2% and ±3% of the mean values. On the other hand, the reference rates presented in
Figure 8b vary considerably depending on the cable type. The standard deviations for R1 and R2 are ±22% and ±56%, respectively, of the mean values. In terms of the reference rate of the first-order reaction caused by the sheath or insulation, TFR-CVV-SB and VCTF have higher values than the other cables. In all of the cables, the sheath and insulation have a common primary component, PVC. However, as shown in
Figure 2, the sums of the sheath and insulation mass fractions for TFR-CVV-SB and VCTF are larger than those of the other cables, resulting in higher reference rates. On the other hand, in the second-order reaction, TFR-CVV-SB and VCTF have lower reference rates than the other cables. This difference is evident because the effects of additional materials, except for the sheath and insulation, do not apply to TFR-CVV-SB and VCTF, considering that the second-order reaction is caused by multiple materials almost simultaneously. In summary, it can be concluded that, regardless of the cable composition, the mean values in this study can be used as the reference temperature required to analyze the pyrolysis reactions of flame retardant cables. However, the mean value of the reference rate, which varies significantly according to the cable type, may have to be used with great caution. To this end, sensitivity analysis was performed on the effect of the changes in the reference temperatures and rates on the mass loss of combustibles due to the pyrolysis reaction.
3.2. Sensitivity Analysis of Major Pyrolysis Properties using Pyrolysis Simulation
As discussed in
Section 3.1, sensitivity analysis was performed to examine the effects of the reference temperature and reference rate on the pyrolysis reaction by quantitative difference according to the cable type. For this purpose, the 1D pyrolysis model included in the FDS was used, and the PVC material with the highest mass fraction among all of the cables was assumed to be combustible.
Figure 9 shows the change in mass of the PVC material when the reference temperature and rate corresponding to the mean value of all of the cables were applied. To understand the two-step pyrolysis reaction, the mean reference temperatures and rates of R1 and R2 were considered both separately and simultaneously. Based on a square specimen with side lengths of 0.1 m, the initial masses of the PVC and foam glass were 65.8 g and 12.0 g, respectively. Assuming that a material undergoes a single-step reaction [
23], when R1 and R2 were considered simultaneously, the initial masses of PVC for R1 and R2 were set to 48.8 g and 17.0 g, respectively. The mass distributions were calculated from the average mass reductions of all of the cables resulting from the two-step reaction. If R1 and R2 are considered separately, it can be seen that the change in mass over time varies significantly depending on the reference temperature and rate of each reaction. When R1 and R2 are considered simultaneously, however, the mass gradually decreases until time
t1 due to R1, and additional pyrolysis subsequently occurs up until
t2 due to R2, which has a higher reference temperature. Note that the mass that remaining after completion of the reaction corresponds to the mass of the foam glass (12.0 g) used as insulation on the rear side of the specimen. The results in
Figure 9 show that the effects of the quantitative differences between the reference temperatures and rates of the different cable types on the pyrolysis reaction or mass reduction can be directly assessed by comparing the changes in
t1 and
t2 associated with R1 and R2, respectively.
Figure 10 shows the results of the sensitivity analysis of the effects of changes in the reference temperatures of the two pyrolysis reactions on the mass changes. Here it should be noted that, as shown in
Figure 8, the mean reference temperatures (
Tp,1 and
Tp,2) of the different cable types were 283 °C and 464 °C for R1 and R2, respectively.
Figure 10a represents the mass when
Tp,1 is changed from ±3% to ±50%, while
rp,1/
Ys,1 of R1 and
T p,2 and
rp,2/
Ys,2 of R2 remain constant. In this figure, a significant change in
t1 at the end of the first-order reaction due to the change in
Tp,1 can be clearly identified. In
Figure 10b, where
Tp,2 is changed, a significant change in
t2 corresponding to the end of the second-order reaction is observable when
Tp,2 is decreased to −50%. Furthermore, when
Tp,2 is increased to +30%, a large increase in
t2 is observable. In addition, when
Tp,2 is increased to +50%, the second-order reaction does not occur within 700 s. Thus, it can be concluded that the reference temperature has a considerable influence on the pyrolysis reaction rate. However, as shown in
Figure 8a, it should be noted that the standard deviations of the reference temperatures for R1 and R2 are only ±3% and ±2%, respectively. Therefore, the quantitative differences between the reference temperatures of the flame retardant cables examined in this study are expected to have little effect on the pyrolysis rate.
Figure 11 presents the results of the sensitivity analysis of the effects of the reference rates of the two pyrolysis reactions (
rp,1/
Ys,1 and
rp,2/
Ys,2) on the mass changes. Even if
rp,1/
Ys,1 for R1 is varied over a wide range, the mass hardly changes because of the first-order pyrolysis reaction. Furthermore, the second-order reaction due to the change of
rp,2/
Ys,2 also has little effect on the mass. As shown in
Figure 8, the reference rates of the flame retardant cables have significantly larger standard deviations than the reference temperatures. However, the large quantitative differences in the reference rates have very small effects on the actual pyrolysis rates.
These results indicate that the mean values of the reference temperature and reference rate obtained via TGA can be applied to simulate the pyrolysis reactions of flame retardant cables. However, they also seem to suggest that the heat transfer and pyrolysis reaction path associated with the multi-layered cable structure may be more important for accurate consideration of the ignition and fire spread characteristics due to the differences in the cable structure, composition, and insulation performance.