Low Ppm Atom Transfer Radical Polymerization in (Mini)Emulsion Systems
Abstract
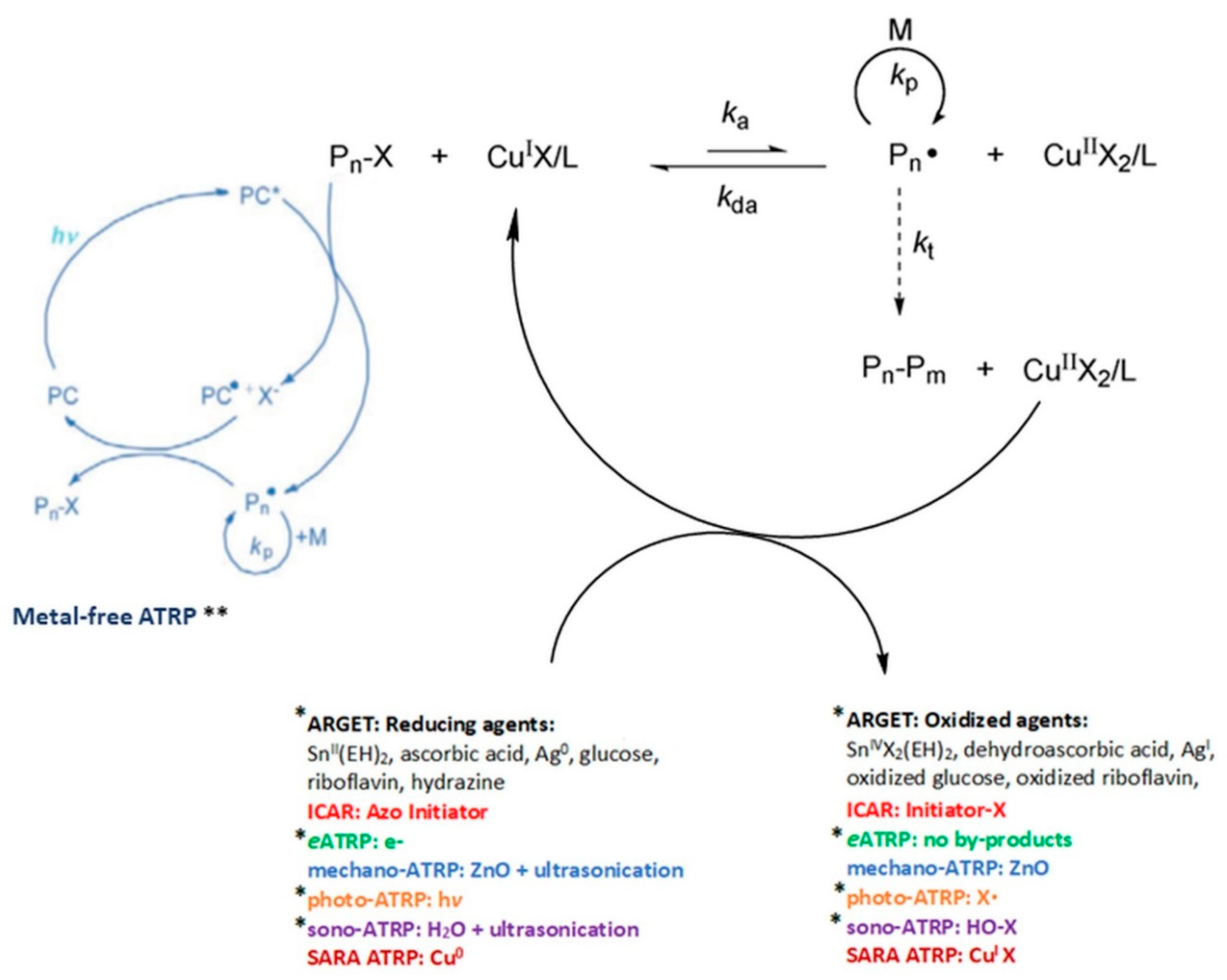
1. Introduction
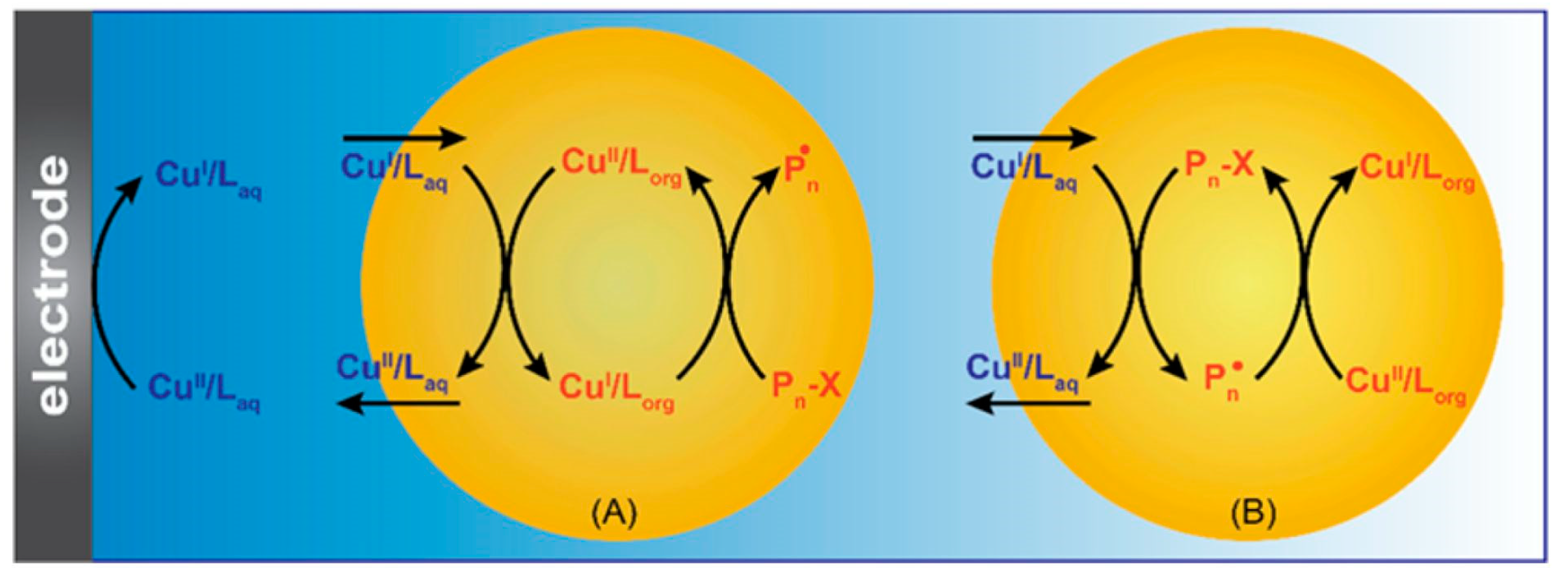
2. Electrochemically Mediated Atom Transfer Radical Polymerization (eATRP)
3. Activators Regenerated by Electron Transfer Atom Transfer Radical Polymerization (ARGET ATRP)
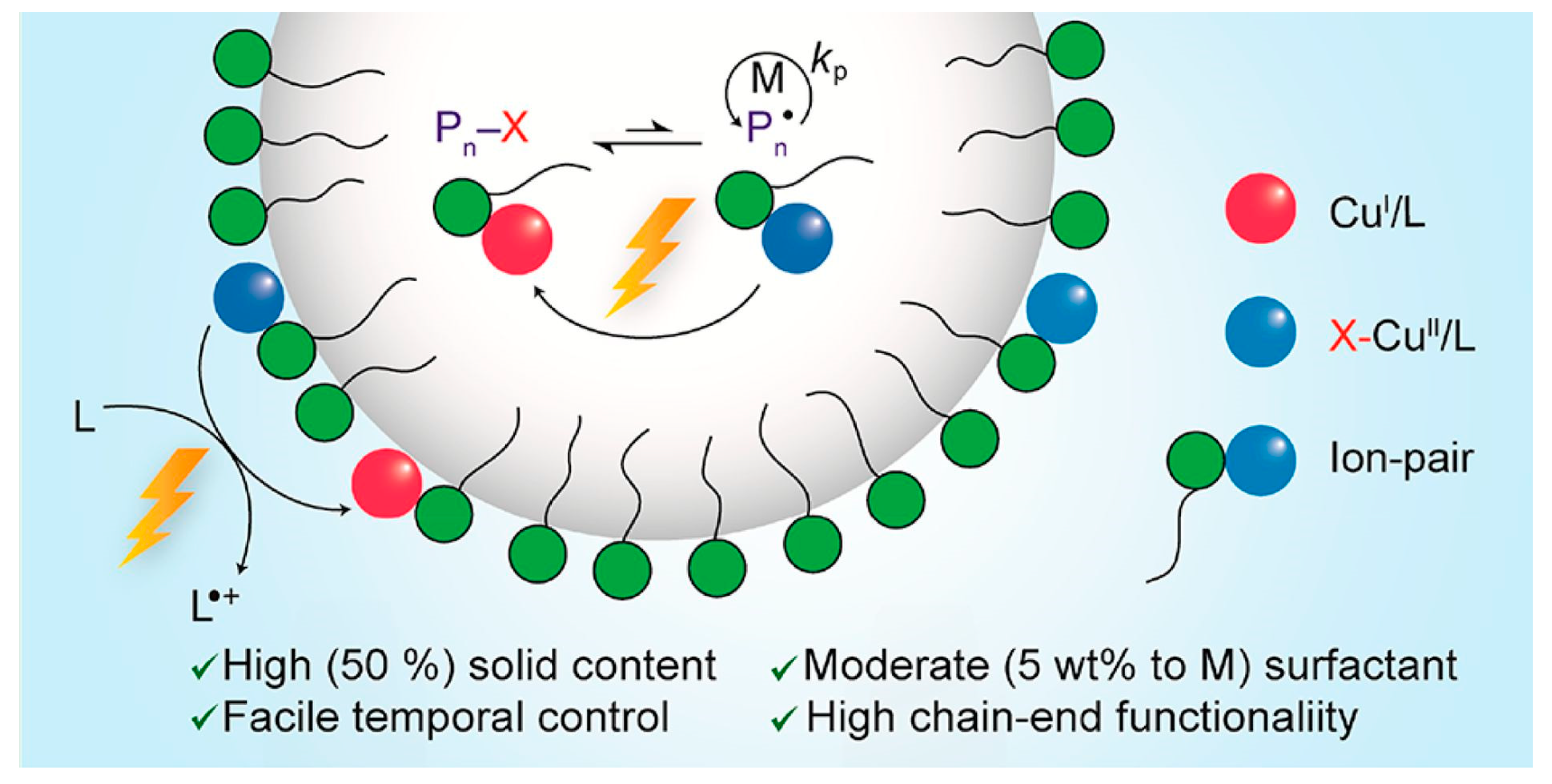
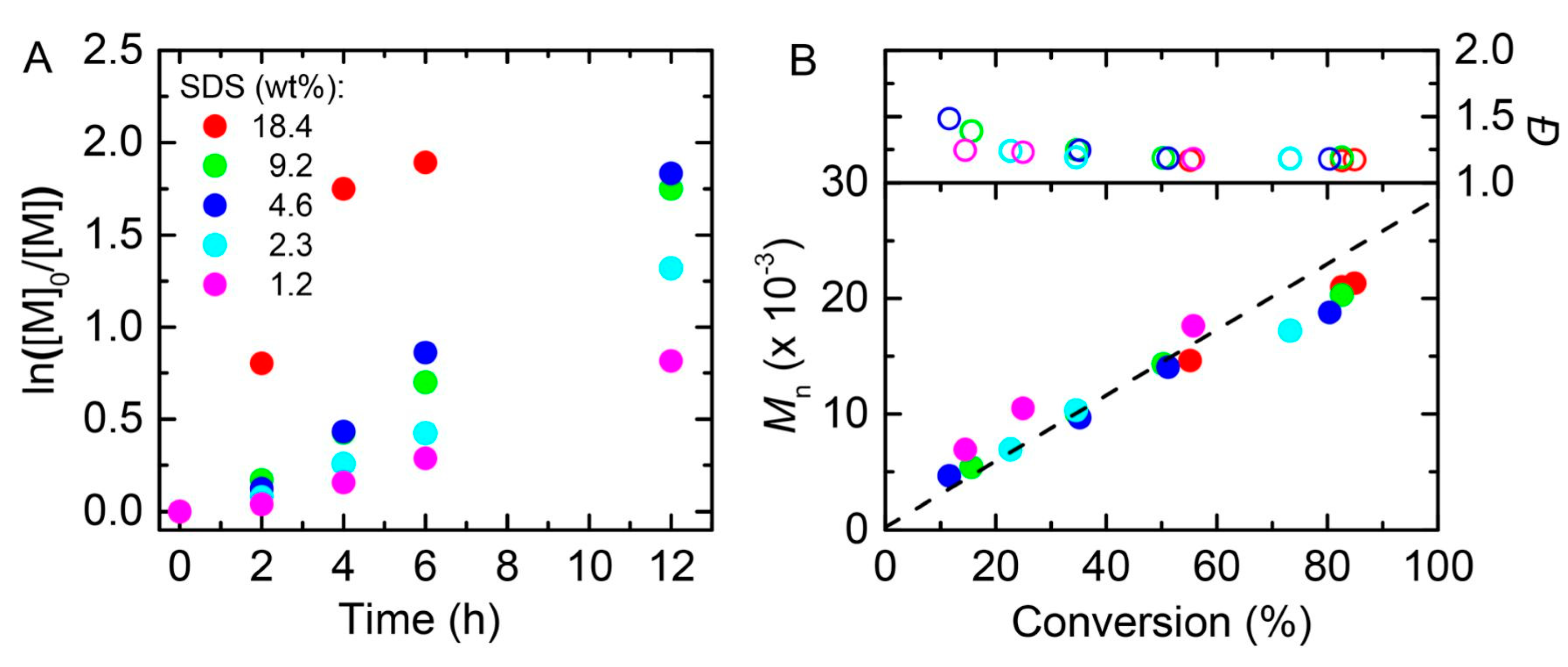
4. Photoinduced Atom Transfer Radical Polymerization (photo-ATRP)
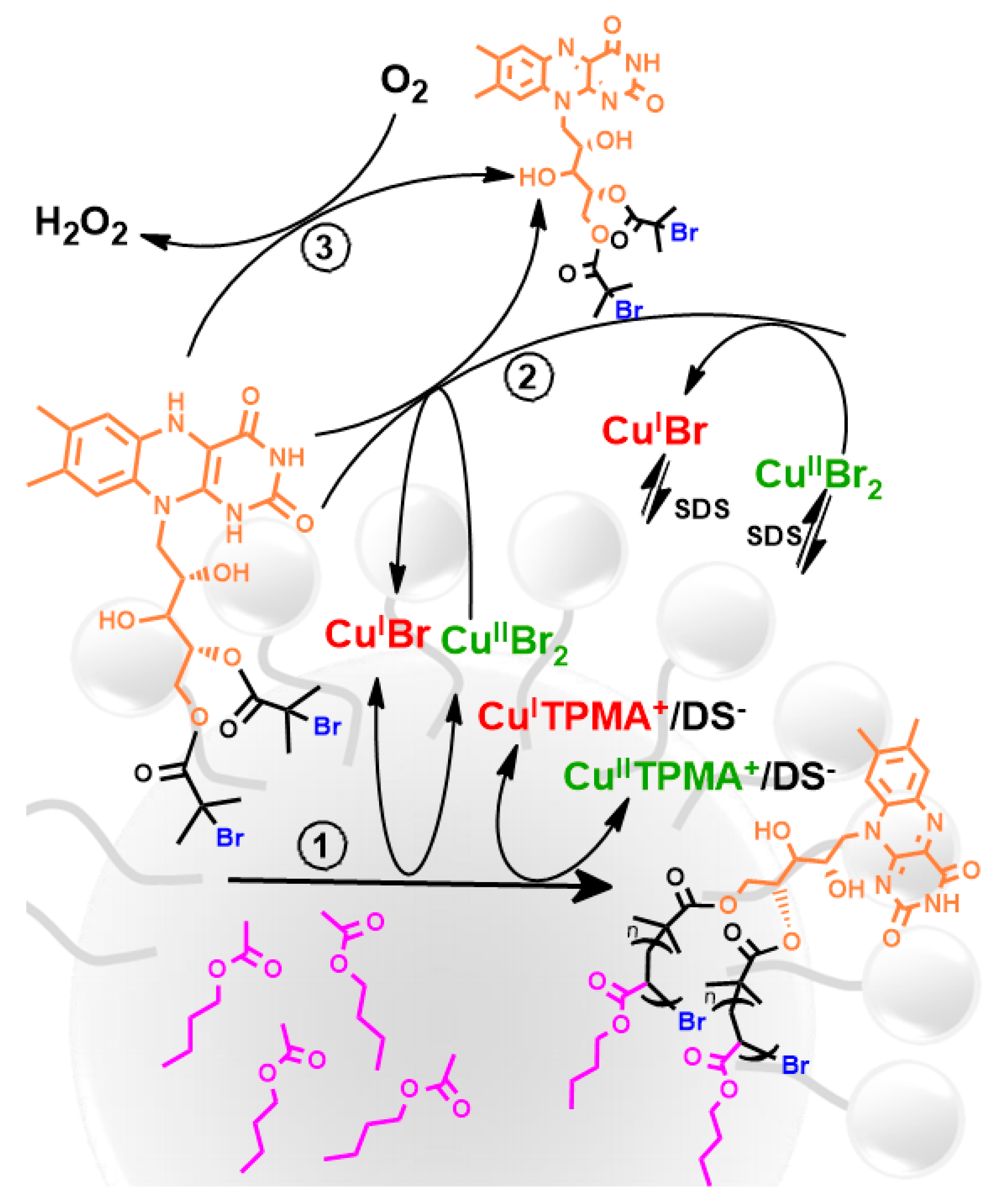
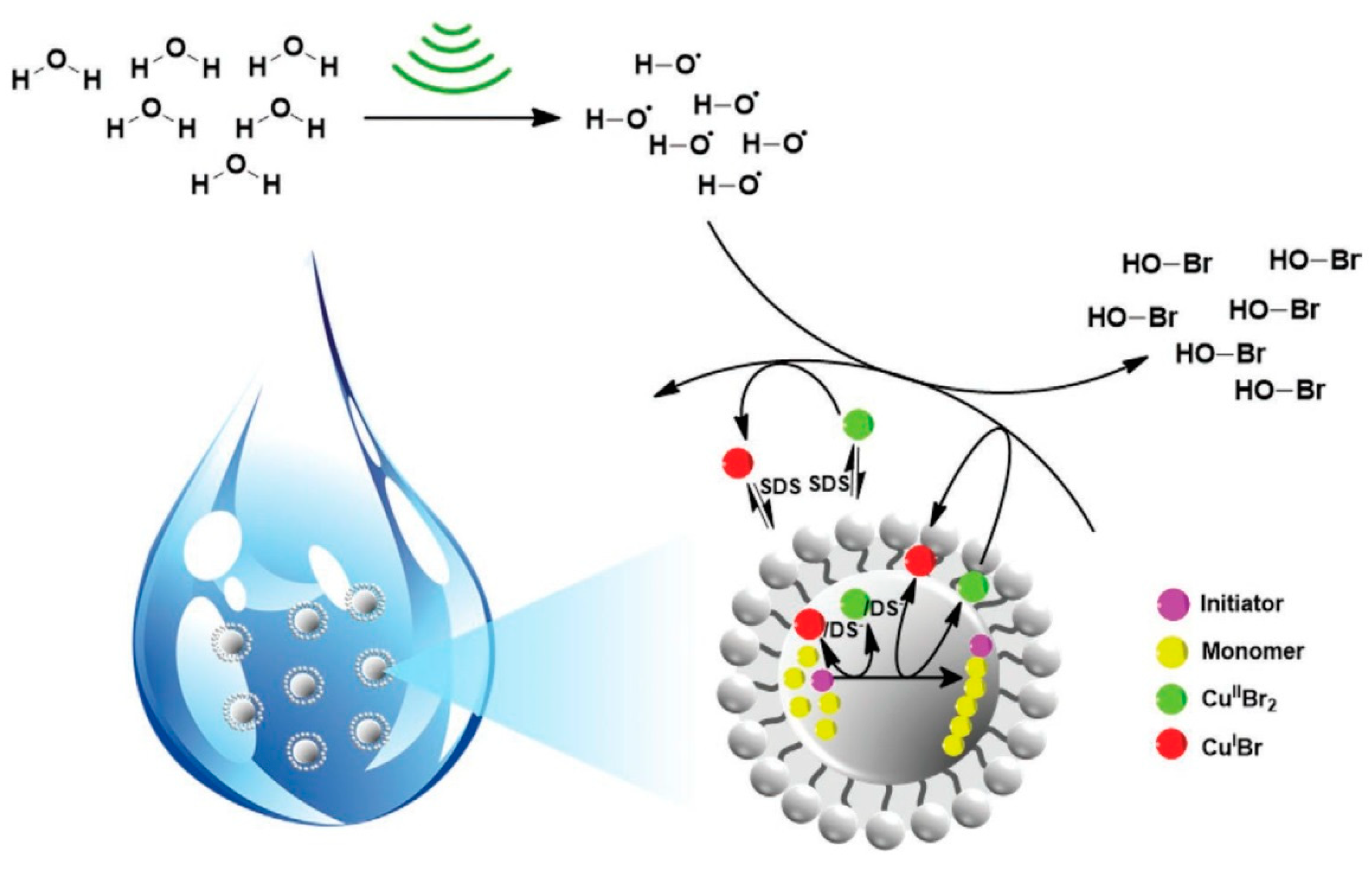
5. Ultrasound-Mediated Atom Transfer Radical Polymerization (sono-ATRP)
6. Conclusion and Future Prospective
Funding
Acknowledgments
Conflicts of Interest
Abbreviations (alphabetical order)
| ARGET ATRP | activators regenerated by electron transfer atom transfer radical polymerization |
| AsAc | ascorbic acid |
| ATRP | atom transfer radical polymerization |
| nBA | n-butyl acrylate |
| nBMA | n-butyl methacrylate |
| BPMEA | N,N-bis(2-pyridylmethyl)-2-hydroxyethylamine |
| BPMODA | bis(2-pyridylmethyl)octadecylamine |
| BPMODA* | bis[2-(4-methoxy-3,5-dimethyl)pyridylmethyl]octadecylamine |
| BPY | 2,2′-bipyridine |
| Brij-98 | polyoxyethylene(20) oleyl ether |
| DOD-BPED* | N,N-dioctadecyl-N,N-bis[2-(4-methoxy-3,5-dimethyl)pyridylmethyl]ethane-1,2-diamine |
| eATRP | electrochemically mediated atom transfer radical polymerization |
| EBiB | ethyl α-bromoisobutyrate |
| EBPA | ethyl α-bromophenylacetate |
| EMA | ethyl methacrylate |
| Epc | cathodic peak potential |
| ICAR ATRP | initiators for continuous activator regeneration atom transfer radical polymerization |
| LMA | lauryl methacrylate |
| Me6TREN | tris(2-(dimethylamino)ethyl)-amine |
| mechano-ATRP | mechanically induced atom transfer radical polymerization |
| MMA | methyl methacrylate |
| MWD | molecular weight distribution |
| PnBA | poly(n-butyl acrylate) |
| PnBMA | poly(n-butyl methacrylate) |
| PtBMA | poly(t-butyl methacrylate) |
| PEO2K-BiB | poly(ethlene glycol) 2-bromoisobutyrate |
| PEO2K-BPA | poly(ethylene glycol) 2-bromophenylacetate |
| photo-ATRP | photoinduced atom transfer radical polymerization |
| PMMA | poly(methyl methacrylate) |
| Rib-Br2 | brominated riboflavin |
| SARA ATRP | supplemental activator and reducing agent atom transfer radical polymerization |
| SDA | sodium dodecanoate |
| SDBS | sodium dodecylbenzenesulfonate |
| SDS | sodium dodecyl sulfate |
| Sn(EH)2 | tin(II) 2-ethylhexanoate |
| sono-ATRP | ultrasound-mediated atom transfer radical polymerization |
| tBA | tert-butyl acrylate |
| TEA | triethylamine |
| TPMA | tris(2-pyridylmethyl)amine |
| TPMA*2 | 1-(4methoxy-3,5-dimethylpyridin-2-yl)-N-((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)-N-(pyridin-2-ylmethyl)methanamine |
References
- Min, K.; Matyjaszewski, K. Atom transfer radical polymerization in aqueous dispersed media. Cent. Eur. J. Chem. 2009, 7, 657–674. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Tsarevsky, N.V. Macromolecular engineering by atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 6513–6533. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K. Advanced materials by atom transfer radical polymerization. Adv. Mater. 2018, 30, 1706441. [Google Scholar] [CrossRef] [PubMed]
- Zaborniak, I.; Chmielarz, P.; Matyjaszewski, K. Modification of wood-based materials by atom transfer radical polymerization methods. Eur. Polym. J. 2019, 120, 109253. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P. Ultrasound-mediated atom transfer radical polymerization (ATRP). Materials 2019, 12, 3600. [Google Scholar] [CrossRef] [PubMed]
- Patten, T.E.; Matyjaszewski, K. Atom transfer radical polymerization and the synthesis of polymeric materials. Adv. Mater. 1998, 10, 901–915. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Wei, M.L.; Xia, J.H.; McDermott, N.E. Controlled/”living” radical polymerization of styrene and methyl methacrylate catalyzed by iron complexes. Macromolecules 1997, 30, 8161–8164. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J.H. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Park, S.; Chmielarz, P.; Gennaro, A.; Matyjaszewski, K. Simplified electrochemically mediated atom transfer radical polymerization using a sacrificial anode. Angew. Chem. Int. Ed. 2015, 54, 2388–2392. [Google Scholar] [CrossRef]
- Baker, S.L.; Kaupbayeva, B.; Lathwal, S.; Das, S.R.; Russell, A.J.; Matyjaszewski, K. Atom transfer radical polymerization for biorelated hybrid materials. Biomacromolecules 2019, 20, 4272–4298. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, P.; Park, S.; Sobkowiak, A.; Matyjaszewski, K. Synthesis of β-cyclodextrin-based star polymers via a simplified electrochemically mediated ATRP. Polymer 2016, 88, 36–42. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of α-D-glucose-based star polymers through simplified electrochemically mediated ATRP. Polymer 2016, 102, 192–198. [Google Scholar] [CrossRef]
- Chmielarz, P.; Sobkowiak, A. Ultralow ppm seATRP synthesis of PEO-b-PBA copolymers. J. Polym. Res. 2017, 24, 77. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of inositol-based star polymers through low ppm ATRP methods. Polym. Adv. Technol. 2017, 28, 1804–1812. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Yan, J.J.; Liu, T.; Wei, Q.B.; Li, S.P.; Olszewski, M.; Wu, J.N.; Sobieski, J.L.; Fantin, M.; Bockstaller, M.R.; et al. Control of dispersity and grafting density of particle brushes by variation of ATRP catalyst concentration. ACS Macro Lett. 2019, 8, 859–864. [Google Scholar] [CrossRef]
- Jakubowski, W.; Matyjaszewski, K. Activators regenerated by electron transfer for atom-transfer radical polymerization of (meth) acrylates and related block copolymers. Angew. Chem. Int. Ed. 2006, 45, 4482–4486. [Google Scholar] [CrossRef]
- Król, P.; Chmielarz, P. Synthesis of PMMA-b-PU-b-PMMA tri-block copolymers through ARGET ATRP in the presence of air. Express Polym. Lett. 2013, 7, 249–260. [Google Scholar] [CrossRef]
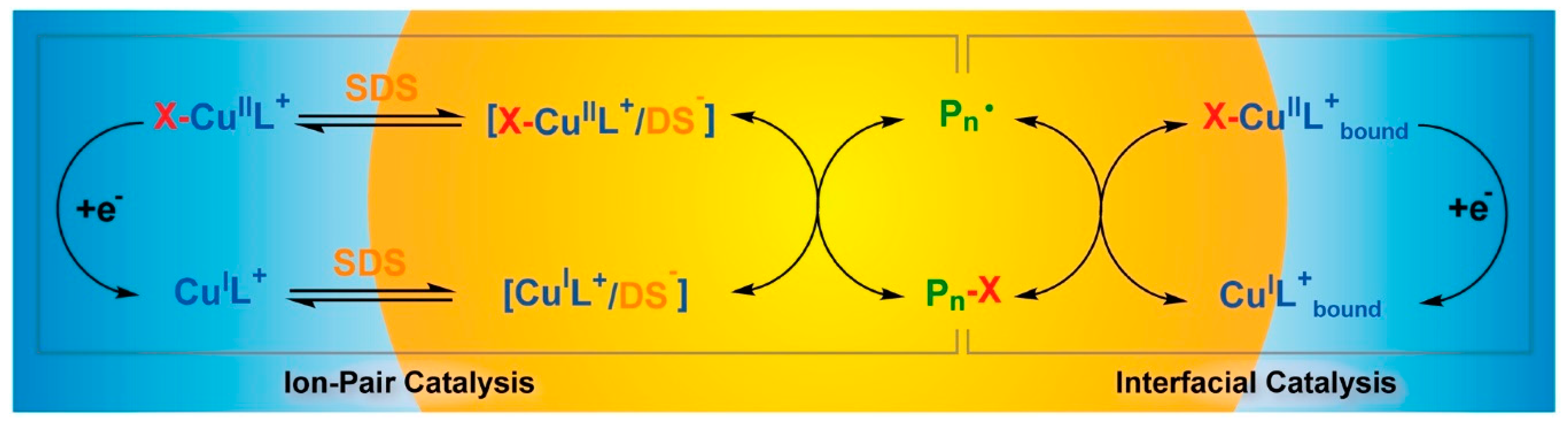
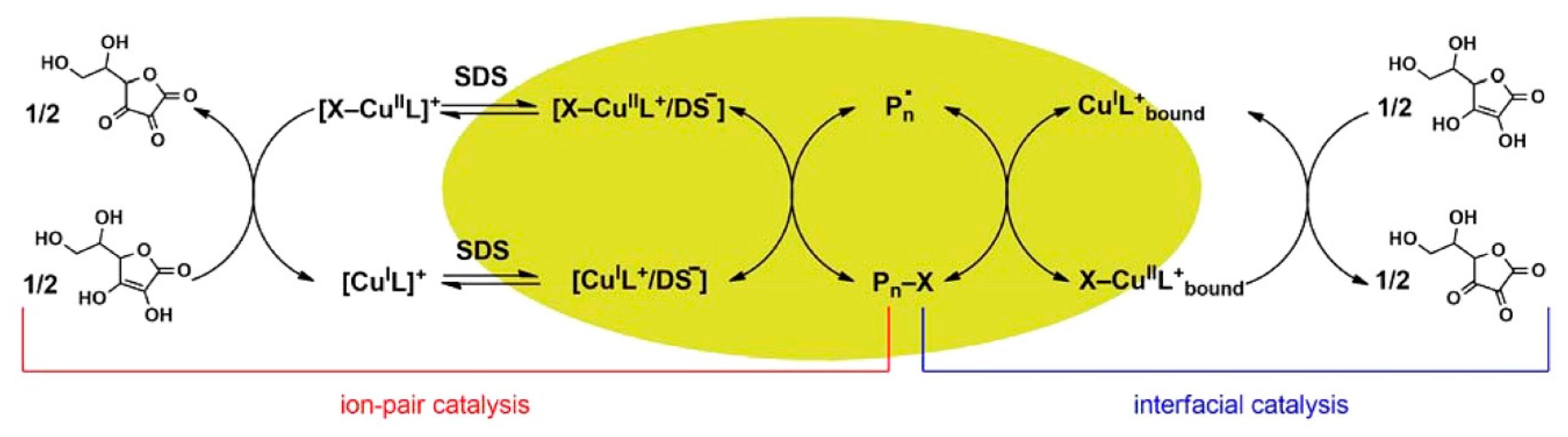
- Wang, Y.; Lorandi, F.; Fantin, M.; Chmielarz, P.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Miniemulsion ARGET ATRP via interfacial and ion-pair catalysis: from ppm to ppb of residual copper. Macromolecules 2017, 50, 8417–8425. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P. Dually-functional riboflavin macromolecule as a supramolecular initiator and reducing agent in temporally-controlled low ppm ATRP. Express Polym. Lett. 2020, 14, 235–247. [Google Scholar] [CrossRef]
- Pintauer, T.; Matyjaszewski, K. Atom transfer radical addition and polymerization reactions catalyzed by ppm amounts of copper complexes. Chem. Soc. Rev. 2008, 37, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Schmitt, M.; Wang, Z.Y.; Lee, B.; Pan, X.C.; Fu, L.Y.; Yan, J.J.; Li, S.P.; Xie, G.J.; Bockstaller, M.R.; et al. Polymerization-induced self-assembly (PISA) using ICAR ATRP at low catalyst concentration. Macromolecules 2016, 49, 8605–8615. [Google Scholar] [CrossRef]
- Fierens, S.K.; Van Steenberge, P.H.M.; Reyniers, M.F.; Marin, G.B.; D’Hooge, D.R. How penultimate monomer unit effects and initiator influence ICAR ATRP of n-butyl acrylate and methyl methacrylate. AIChE J. 2017, 63, 4971–4986. [Google Scholar] [CrossRef]
- Wang, W.Q.; Julaiti, P.; Ye, G.; Huo, X.; Chen, J. Controlled architecture of macrocyclic ligand functionalized polymer brushes from glass fibers using surface-initiated ICAR ATRP technique for adsorptive separation of lithium isotopes. Chem. Eng. J. 2018, 336, 669–678. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Wang, Y.; Zhong, M.J.; Krys, P.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Reversible-deactivation radical polymerization in the presence of metallic copper. A critical assessment of the SARA ATRP and SET-LRP mechanisms. Macromolecules 2013, 46, 8749–8772. [Google Scholar] [CrossRef]
- Mendonca, P.V.; Konkolewicz, D.; Averick, S.E.; Serra, A.C.; Popov, A.V.; Guliashvili, T.; Matyjaszewski, K.; Coelho, J.F.J. Synthesis of cationic poly((3-acrylamidopropyl)-trimethylammonium chloride) by SARA ATRP in ecofriendly solvent mixtures. Polym. Chem. 2014, 5, 5829–5836. [Google Scholar] [CrossRef]
- Chmielarz, P.; Krys, P.; Park, S.; Matyjaszewski, K. PEO-b-PNIPAM copolymers via SARA ATRP and eATRP in aqueous media. Polymer 2015, 71, 143–147. [Google Scholar] [CrossRef]
- Abreu, C.M.R.; Fu, L.Y.; Carmali, S.; Serra, A.C.; Matyjaszewski, K.; Coelho, J.F.J. Aqueous SARA ATRP using inorganic sulfites. Polym. Chem. 2017, 8, 375–387. [Google Scholar] [CrossRef]
- Oliveira, A.S.R.; Mendonca, P.V.; Serra, A.C.; Coelho, J.F.J. Self-degassing SARA ATRP mediated by Na2S2O4 with no external additives. J. Polym. Sci. Pol. Chem. 2020, 58, 145–153. [Google Scholar]
- Wang, Z.H.; Pan, X.C.; Li, L.C.; Fantin, M.; Yan, J.J.; Wang, Z.Y.; Wang, Z.H.; Xia, H.S.; Matyjaszewski, K. Enhancing mechanically induced ATRP by promoting interfacial electron transfer from piezoelectric nanoparticles to Cu catalysts. Macromolecules 2017, 50, 7940–7948. [Google Scholar] [CrossRef]
- Wang, Z.H.; Lorandi, F.; Fantin, M.; Wang, Z.Y.; Yan, J.J.; Wang, Z.H.; Xia, H.S.; Matyjaszewski, K. Atom transfer radical polymerization enabled by sonochemically labile Cu-carbonate species. ACS Macro Lett. 2019, 8, 161–165. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Uygun, M.; Yagci, Y. Photoinduced controlled radical polymerization. Macromol. Rapid Commun. 2011, 32, 58–62. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Schroder, K.; Buback, J.; Bernhard, S.; Matyjaszewski, K. Visible light and sunlight photoinduced ATRP with ppm of Cu catalyst. ACS Macro Lett. 2012, 1, 1219–1223. [Google Scholar] [CrossRef]
- Wang, Y.; Dadashi-Silab, S.; Matyjaszewski, K. Photoinduced miniemulsion atom transfer radical polymerization. ACS Macro Lett. 2018, 7, 720–725. [Google Scholar] [CrossRef]
- Wang, Y.; Dadashi-Silab, S.; Lorandi, F.; Matyjaszewski, K. Photoinduced atom transfer radical polymerization in ab initio emulsion. Polymer 2019, 165, 163–167. [Google Scholar] [CrossRef]
- Magenau, A.J.D.; Strandwitz, N.C.; Gennaro, A.; Matyjaszewski, K. Electrochemically mediated atom transfer radical polymerization. Science 2011, 332, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Lorandi, F.; Fantin, M.; Isse, A.A.; Gennaro, A. Electrochemically mediated atom transfer radical polymerization of n-butyl acrylate on non-platinum cathodes. Polym. Chem. 2016, 7, 5357–5365. [Google Scholar] [CrossRef]
- Fantin, M.; Chmielarz, P.; Wang, Y.; Lorandi, F.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Harnessing the interaction between surfactant and hydrophilic catalyst to control eATRP in miniemulsion. Macromolecules 2017, 50, 3726–3732. [Google Scholar] [CrossRef]
- Chmielarz, P.; Fantin, M.; Park, S.; Isse, A.A.; Gennaro, A.; Magenau, A.J.D.; Sobkowiak, A.; Matyjaszewski, K. Electrochemically mediated atom transfer radical polymerization (eATRP). Prog. Polym. Sci. 2017, 69, 47–78. [Google Scholar] [CrossRef]
- Chmielarz, P.; Krys, P.; Wang, Z.Y.; Wang, Y.; Matyjaszewski, K. Synthesis of well-defined polymer brushes from silicon wafers via surface-initiated seATRP. Macromol. Chem. Phys. 2017, 218, 1700106. [Google Scholar] [CrossRef]
- Li, M.M.; Guo, Z.Z.; Zheng, X.K.; Yang, H.X.; Feng, W.S.; Kong, J.M. An electrochemical aptasensor based on eATRP amplification for the detection of bisphenol A. Analyst 2019, 144, 5691–5699. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Yang, H.X.; Zheng, X.K.; Li, J.G.; Jian, L.H.; Feng, W.S.; Kong, J.M. Dual signal amplification by polysaccharide and eATRP for ultrasensitive detection of CYFRA 21-1 DNA. Biosens. Bioelectron. 2020, 150, 111895. [Google Scholar] [CrossRef] [PubMed]
- Zaborniak, I.; Chmielarz, P. Temporally controlled ultrasonication-mediated atom transfer radical polymerization in miniemulsion. Macromol. Chem. Phys. 2019, 220, 1900285. [Google Scholar] [CrossRef]
- Treat, N.J.; Sprafke, H.; Kramer, J.W.; Clark, P.G.; Barton, B.E.; de Alaniz, J.R.; Fors, B.P.; Hawker, C.J. Metal-free atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 16096–16101. [Google Scholar] [CrossRef]
- Discekici, E.H.; Anastasaki, A.; de Alaniz, J.R.; Hawker, C.J. Evolution and future directions of metal-free atom transfer radical polymerization. Macromolecules 2018, 51, 7421–7434. [Google Scholar] [CrossRef]
- Wang, G.L.; Xi, M.Z.; Bai, L.J.; Liang, Y.; Yang, L.X.; Wang, W.X.; Chen, H.; Yang, H.W. Pickering emulsion of metal-free photoinduced electron transfer-ATRP stabilized by cellulose nanocrystals. Cellulose 2019, 26, 5947–5957. [Google Scholar] [CrossRef]
- Faivre, J.; Shrestha, B.R.; Burdynska, J.; Xie, G.; Moldovan, F.; Delair, T.; Benayoun, S.; David, L.; Matyjaszewski, K.; Banquy, X. Wear protection without surface modification using a synergistic mixture of molecular brushes and linear polymers. ACS Nano 2017, 11, 1762–1769. [Google Scholar] [CrossRef]
- Chmielarz, P.; Yan, J.; Krys, P.; Wang, Y.; Wang, Z.; Bockstaller, M.R.; Matyjaszewski, K. Synthesis of nanoparticle copolymer brushes via surface-initiated seATRP. Macromolecules 2017, 50, 4151–4159. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Martinez, M.R.; Wolski, K.; Wang, Z.; Matyjaszewski, K. Synthesis of high molecular weight poly(n-butyl acrylate) macromolecules via seATRP: from polymer stars to molecular bottlebrushes. Eur. Polym. J. 2020, 126, 109566. [Google Scholar] [CrossRef]
- Qiu, J.; Charleux, B.; Matyjaszewski, K. Controlled/living radical polymerization in aqueous media: homogeneous and heterogeneous systems. Prog. Polym. Sci. 2001, 26, 2083–2134. [Google Scholar] [CrossRef]
- Ding, S.J.; Radosz, M.; Shen, Y.Q. Ionic liquid catalyst for biphasic atom transfer radical polymerization of methyl methacrylate. Macromolecules 2005, 38, 5921–5928. [Google Scholar] [CrossRef]
- Oliveira, M. RAFT inverse microemulsion polymerization: effects of monomer solubility and different types of initiators. Macromol. React. Eng. 2017, 11, 1600066. [Google Scholar] [CrossRef]
- Jenjob, R.; Phakkeeree, T.; Seidi, F.; Theerasilp, M.; Crespy, D. Emulsion techniques for the production of pharmacological nanoparticles. Macromol. Biosci. 2019, 19, e1900063. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, P.; Park, S.; Simakova, A.; Matyjaszewski, K. Electrochemically mediated ATRP of acrylamides in water. Polymer 2015, 60, 302–307. [Google Scholar] [CrossRef]
- Wang, X.F.; Graff, R.W.; Shi, Y.; Gao, H.F. One-pot synthesis of hyperstar polymers via sequential ATRP of inimers and functional monomers in aqueous dispersed media. Polym. Chem. 2015, 6, 6739–6745. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Matyjaszewski, K. Synthesis of riboflavin-based macromolecules through low ppm ATRP in aqueous media. Macromol. Chem. Phys. 2020, 221, 1900496. [Google Scholar] [CrossRef]
- Dai, F.Y.; Sun, P.; Liu, Y.J.; Liu, W.G. Redox-cleavable star cationic PDMAEMA by arm-first approach of ATRP as a nonviral vector for gene delivery. Biomaterials 2010, 31, 559–569. [Google Scholar] [CrossRef]
- Fang, L.J.; Chen, S.J.; Guo, X.Z.; Zhang, Y.; Zhang, H.Q. Azobenzene-containing molecularly imprinted polymer microspheres with photo- and thermoresponsive template binding properties in pure aqueous media by atom transfer radical polymerization. Langmuir 2012, 28, 9767–9777. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Li, Y.J.; Lu, G.L.; Huang, X.Y. A novel poly(N-vinylcaprolactam)-based well-defined amphiphilic graft copolymer synthesized by successive RAFT and ATRP. Polym. Chem. 2013, 4, 1402–1411. [Google Scholar] [CrossRef]
- Xie, L.Q.; Lan, F.; Li, W.L.; Liu, Z.Y.; Ma, S.H.; Yang, Q.; Wu, Y.; Gu, Z.W. Polyacrylic acid brushes grafted from P(St-AA)/Fe3O4 composite microspheres via ARGET-ATRP in aqueous solution for protein immobilization. Colloids Surf. B 2014, 123, 413–418. [Google Scholar] [CrossRef]
- Airaud, C.; Ibarboure, E.; Gaillard, C.; Heroguez, V. Nanostructured polymer composite nanoparticles synthesized in a single step via simultaneous ROMP and ATRP under microemulsion conditions. J. Polym. Sci. Pol. Chem. 2009, 47, 4014–4027. [Google Scholar] [CrossRef]
- Ciftci, M.; Tasdelen, M.A.; Li, W.W.; Matyjaszewski, K.; Yagci, Y. Photoinitiated ATRP in inverse microemulsion. Macromolecules 2013, 46, 9537–9543. [Google Scholar] [CrossRef]
- Cuneo, T.; Graff, R.W.; Wang, X.F.; Gao, H.F. Synthesis of highly branched copolymers in microemulsion. Macromol. Chem. Phys. 2019, 220, 1800546. [Google Scholar] [CrossRef]
- Min, K.; Yu, S.; Lee, H.I.; Mueller, L.; Sheiko, S.S.; Matyjaszewski, K. High yield synthesis of molecular brushes via ATRP in miniemulsion. Macromolecules 2007, 40, 6557–6563. [Google Scholar] [CrossRef]
- Teo, V.L.; Davis, B.J.; Tsarevsky, N.V.; Zetterlund, P.B. Successful miniemulsion ATRP using an anionic surfactant: minimization of deactivator loss by addition of a halide salt. Macromolecules 2014, 47, 6230–6237. [Google Scholar] [CrossRef]
- Zaborniak, I.; Surmacz, K.; Flejszar, M.; Chmielarz, P. Triple-functional riboflavin-based molecule for efficient atom transfer radical polymerization in miniemulsion media. J. Appl. Polym. Sci. 2020, e49275. [Google Scholar] [CrossRef]
- Li, W.W.; Matyjaszewski, K. Cationic surface-active monomers as reactive surfactants for AGET emulsion ATRP of n-butyl methacrylate. Macromolecules 2011, 44, 5578–5585. [Google Scholar] [CrossRef]
- Rusen, E.; Diacon, A.; Mocanu, A.; Culita, D.C.; Dinescu, A.; Zecheru, T. “A real” emulsion polymerization using simple ATRP reaction in the presence of an oligo-initiator with a dual activity of emulsifier and initiator. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 1–7. [Google Scholar] [CrossRef]
- Lorandi, F.; Wang, Y.; Fantin, M.; Matyjaszewski, K. Ab initio emulsion atom-transfer radical polymerization. Angew. Chem. Int. Ed. 2018, 57, 8270–8274. [Google Scholar] [CrossRef]
- Cui, J.Y.; Xie, A.T.; Zhou, S.; Liu, S.W.; Wang, Q.Q.; Wu, Y.L.; Meng, M.J.; Lang, J.H.; Zhou, Z.P.; Yan, Y.S. Development of composite membranes with irregular rod-like structure via atom transfer radical polymerization for efficient oil-water emulsion separation. J. Colloid Interface Sci. 2019, 533, 278–286. [Google Scholar] [CrossRef]
- Gharieh, A.; Khoee, S.; Mandavian, A.R. Emulsion and miniemulsion techniques in preparation of polymer nanoparticles with versatile characteristics. Adv. Colloid Interface Sci. 2019, 269, 152–186. [Google Scholar] [CrossRef] [PubMed]
- Castor, C.A.; Pontier, A.; Durand, J.; Pinto, J.C.; Prat, L. Real time monitoring of the quiescent suspension polymerization of methyl methacrylate in microreactors-part 1. A kinetic study by Raman spectroscopy and evolution of droplet size. Chem. Eng. Sci. 2015, 131, 340–352. [Google Scholar] [CrossRef][Green Version]
- Asua, J.M. Challenges and opportunities in continuous production of emulsion polymers: a review. Macromol. React. Eng. 2016, 10, 311–323. [Google Scholar] [CrossRef]
- Gao, H.F. A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures. Chin. Chem. Lett. 2019, 30, 1996–2002. [Google Scholar] [CrossRef]
- Landfester, K.; Bechthold, N.; Tiarks, F.; Antonietti, M. Miniemulsion polymerization with cationic and nonionic surfactants: a very efficient use of surfactants for heterophase polymerization. Macromolecules 1999, 32, 2679–2683. [Google Scholar] [CrossRef]
- Fantin, M.; Park, S.; Wang, Y.; Matyjaszewski, K. Electrochemical atom transfer radical polymerization in miniemulsion with a dual catalytic system. Macromolecules 2016, 49, 8838–8847. [Google Scholar] [CrossRef]
- Fantin, M.; Lorandi, F.; Isse, A.A.; Gennaro, A. Sustainable electrochemically-mediated atom transfer radical polymerization with inexpensive non-platinum electrodes. Macromol. Rapid Commun. 2016, 37, 1318–1322. [Google Scholar] [CrossRef]
- Chmielarz, P. Cellulose-based graft copolymers prepared by simplified electrochemically mediated ATRP. Express Polym. Lett. 2017, 11, 140–151. [Google Scholar] [CrossRef]
- Magenau, A.J.D.; Bortolamei, N.; Frick, E.; Park, S.; Gennaro, A.; Matyjaszewski, K. Investigation of electrochemically mediated atom transfer radical polymerization. Macromolecules 2013, 46, 4346–4353. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of naringin-based polymer brushes via seATRP. Polym. Adv. Technol. 2018, 29, 470–480. [Google Scholar] [CrossRef]
- Asua, J.M. Challenges for industrialization of miniemulsion polymerization. Prog. Polym. Sci. 2014, 39, 1797–1826. [Google Scholar] [CrossRef]
- Simms, R.W.; Cunningham, M.F. High molecular weight poly(butyl methacrylate) by reverse atom transfer radical polymerization in miniemulsion initiated by a redox system. Macromolecules 2007, 40, 860–866. [Google Scholar] [CrossRef]
- Kitayama, Y.; Yorizane, M.; Kagawa, Y.; Minami, H.; Zetterlund, P.B.; Okubo, M. Preparation of onion-like multilayered particles comprising mainly poly-(iso-butyl methacrylate)-block-polystyrene by two-step AGET ATRP. Polymer 2009, 50, 3182–3187. [Google Scholar] [CrossRef]
- Kagawa, Y.; Kawasaki, M.; Zetterlund, P.B.; Minami, H.; Okubo, M. Atom transfer radical polymerization of iso-butyl methacrylate in microemulsion with cationic and non-ionic emulsifiers. Macromol. Rapid Commun. 2007, 28, 2354–2360. [Google Scholar] [CrossRef]
- Oh, J.K. Recent advances in emulsion and in controlled/living radical polymerization dispersion. J. Polym. Sci. Pol. Chem. 2008, 46, 6983–7001. [Google Scholar] [CrossRef]
- Elsen, A.M.; Burdynska, J.; Park, S.; Matyjaszewski, K. Active ligand for low ppm miniemulsion atom transfer radical polymerization. Macromolecules 2012, 45, 7356–7363. [Google Scholar] [CrossRef]
- Elsen, A.M.; Burdynska, J.; Park, S.; Matyjaszewski, K. Activators regenerated by electron transfer atom transfer radical polymerization in miniemulsion with 50 ppm of copper catalyst. ACS Macro Lett. 2013, 2, 822–825. [Google Scholar] [CrossRef]
- Min, K.; Gao, H.F.; Matyjaszewski, K. Preparation of homopolymers and block copolymers in miniemulsion by ATRP using activators generated by electron transfer (AGET). J. Am. Chem. Soc. 2005, 127, 3825–3830. [Google Scholar] [CrossRef]
- Leibfarth, F.A.; Mattson, K.M.; Fors, B.P.; Collins, H.A.; Hawker, C.J. External regulation of controlled polymerizations. Angew. Chem. Int. Ed. 2013, 52, 199–210. [Google Scholar] [CrossRef]
- Shanmugam, S.; Boyer, C. Stereo-, temporal and chemical control through photoactivation of living radical polymerization: Synthesis of block and gradient copolymers. J. Am. Chem. Soc. 2015, 137, 9988–9999. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, X.; Yan, J.; Dadashi-Silab, S.; Xie, G.; Zhang, J.; Wang, Z.; Xia, H.; Matyjaszewski, K. Temporal control in mechanically controlled atom transfer radical polymerization using low ppm of Cu catalyst. ACS Macro Lett. 2017, 6, 546–549. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, M.J.; Johnson, J.A. Light-controlled radical polymerization: mechanisms, methods, and applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef] [PubMed]
| Entry | Monomer (% vol) | Initiator | (CuIIBr2)/Laq/Lorg | Surfactant (% wt to monomer) | Eapp | Catalyst Concentration | Đ | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Laq | Lorg | Ppm [CuL]/[M] | Ppm (by wt) | |||||||
| 1 | nBA (20) | EBiB | BPY | - | SDS (4.6) | Epc | 1000 | 2,503 | 9.30 | [76] |
| 2 | nBA (20) | EBiB | BPMEA | - | SDS (4.6) | Epc | 1000 | 2,503 | 4.62 | [76] |
| 3 | nBA (20) | EBiB | BPY | BPMODA | SDS (4.6) | Epc | 1000 | 2,503 | 1.78 | [76] |
| 4 | nBA (20) | EBiB | BPMEA | BPMODA | SDS (4.6) | Epc | 1000 | 2,503 | 1.50 | [76] |
| 5 | nBA (20) | EBiB | TPMA | BPMODA | SDS (4.6) | Epc | 1000 | 2,503 | 2.53 | [76] |
| 6 | nBA (20) | EBiB | BPY | BPMODA* | SDS (4.6) | Epc | 1000 | 2,503 | 1.26 | [76] |
| 7 | nBA (20) | EBiB | BPMEA | BPMODA* | SDS (4.6) | Epc | 1000 | 2,503 | 1.19 | [76] |
| 8 | nBA (20) | EBiB | TPMA | BPMODA* | SDS (4.6) | Epc | 1000 | 2,503 | 1.32 | [76] |
| 9 | nBA (20) | EBiB | TPMA | - | Brij-98 (6.2) | Epc-0.03V | - | 2,181 | 4.77 | [38] |
| 10 | nBA (20) | EBiB | TPMA | - | SDS (6.2) | Epc-0.03V | 321–720 | 2,181 | 1.09–1.26 | [38] |
| 11 | nBA (20) | EBiB | TPMA *2 | - | SDS (6.2) | Epc-0.03V | - | 2,181 | 1.32 | [38] |
| 12 | nBA (20) | EBiB | Me6TREN | - | SDS (6.2) | Epc-0.03V | - | 2,181 | 1.94 | [38] |
| Entry | Monomer (% vol) | Initiator | (CuIIBr2)/Laq/Lorg | Surfactant (% wt to Monomer) | Reducing Agent | Temp. | Catalyst Concentration | Đ | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Laq | Lorg | Ppm [CuL]/[M] | Ppm (by wt) | ||||||||
| 1 | nBA (20) | EBiB | - | BPMODA* | Brij-98 (2.3) | Sn(EH)2 | 80 °C | 250–2,000 | 670 b | 1.15–1.51 | [86] |
| 2 | nBMA (20) | EBPA | - | DOD-BPED* | Brij-98 (2.3) | AsAc | 80 °C | 50–250 | - | 1.23–1.39 | [87] |
| 3 | nBA (20) | EBiB | TPMA | - | SDS (1.15–9.2) | AsAc | 65 °C | 144–719 | 216 c | - | [19] |
| 4 | nBMA (20) | EBPA | TPMA | - | SDS (4.6) | AsAc | 65 °C | 50–800 | 216 c | 1.2–1.42 | [19] |
| 5 | nBMA (20) | EBPA | BPMODA* | - | SDS (4.6) | AsAc | 65 °C | 800 | - | 1.18 | [19] |
| 6 | nBA (20) | EBiB/EBPA | TPMA | - | SDBS (4.6) | AsAc | 65 °C | 704 | 216 c | - | [19] |
| 7 | nBA (20) | EBiB/EBPA | TPMA | - | SDS+SDA (4.6 + 0.5) | AsAc | 65 °C | 704 | 216 c | - | [19] |
| 8 | nBA (17) | Rib-Br2 | TPMA | - | SDS (6.2) | Rib-Br2 | 65 °C | 700–1,000 | 186–267 | 1.39–2.05 | [66] |
| 9 d | nBA (17) | Rib-Br2 | TPMA | - | SDS (6.2) | Rib-Br2 | 65 °C | 1,000 | 266 | 1.19 | [66] |
| Entry | Monomer (% vol) | Initiator | CuIIBr2/Laq | Surfactant (% wt to monomer) | λ (nm), Intensity (mW/cm2) | Catalyst Concentration | Đ | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Ppm [CuL]/[M] | Ppm (by wt) | ||||||||
| 1 | nBMA (5-50) | EBPA | TPMA | SDS (1.2–18.4) | 370 (5) | 800–100 | 220 | 1.24–1.76 | [34] |
| 2 | nBA (20) | EBiB | TPMA | SDS (4.6) | 370 (5) | 800 | 220 | 1.3 | [34] |
| 3 | MMA (20) | PEO2K-BPA | TPMA | SDS (18.4) | 394 (2.6) | 400 | - | 1.75 | [35] |
| 4 | EMA (20) | PEO2K-BPA | TPMA | SDS (18.4) | 394 (2.6) | 400 | - | 1.24 | [35] |
| 5 | nBMA (20) | PEO2K-BPA | TPMA | SDS (18.4) | 394 (2.6) | 400 | 190 | 1.09 | [35] |
| 6 | LMA (20) | PEO2K-BPA | TPMA | SDS (18.4) | 394 (2.6) | 400 | - | - | [35] |
| 7 | nBA (20) | PEO2K-BiB | TPMA | SDS (18.4) | 394 (2.6) | 400 | - | 1.13 | [35] |
| Entry | Monomer (% vol) | Initiator | CuIIBr2/Laq | Surfactant (% wt tom) | Frequency of Sonication (kHz) | Catalyst Concentration | Đ | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Ppm [CuL]/[M] | Ppm (by wt) | ||||||||
| 1 | nBA (20) | EBiB | TPMA | SDS (6.2) | 40 | 717 | 189 | 1.26–1.28 | [43] |
| 2 | MMA (20) | EBiB | TPMA | SDS (6.2) | 40 | 717 | 244 | 1.27 | [43] |
| 3 | MMA (20) | EBPA | TPMA | SDS (6.2) | 40 | 717 | 243 | 1.6 | [43] |
| 4 | nBA (20) | PnBA-Br | TPMA | SDS (6.2) | 40 | 585 | 621 | 1.41 | [43] |
| 5 | tBA (20) | PnBA-Br | TPMA | SDS (6.2) | 40 | 588 | 623 | 1.27 | [43] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surmacz, K.; Chmielarz, P. Low Ppm Atom Transfer Radical Polymerization in (Mini)Emulsion Systems. Materials 2020, 13, 1717. https://doi.org/10.3390/ma13071717
Surmacz K, Chmielarz P. Low Ppm Atom Transfer Radical Polymerization in (Mini)Emulsion Systems. Materials. 2020; 13(7):1717. https://doi.org/10.3390/ma13071717
Chicago/Turabian StyleSurmacz, Karolina, and Paweł Chmielarz. 2020. "Low Ppm Atom Transfer Radical Polymerization in (Mini)Emulsion Systems" Materials 13, no. 7: 1717. https://doi.org/10.3390/ma13071717
APA StyleSurmacz, K., & Chmielarz, P. (2020). Low Ppm Atom Transfer Radical Polymerization in (Mini)Emulsion Systems. Materials, 13(7), 1717. https://doi.org/10.3390/ma13071717






