Abstract
In this paper, lignite activated coke was used as adsorbent for dynamic column adsorption experiments to remove sulfamethoxazole from aqueous solution. The effects of column height, flow rate, initial concentration, pH and humic acids concentration on the dynamic adsorption penetration curve and mass transfer zone length were investigated. Results showed penetration time would be prolonged significantly by increasing column height, while inhibited by the increasement of initial concentration and flow rate. Thomas and Yoon-Nelson model and the Adams-Bohart model were used to elucidate the adsorption mechanism, high coefficients of R2 > 0.95 were obtained in Thomas model for most of the adsorption entries, which revealed that the adsorption rate could probably be dominated by mass transfer at the interface. The average change rates of mass transfer zone length to the changes of each parameters, such as initial concentration, the column height, the flow rate and pH, were 0.0003, 0.6474, 0.0076, 0.0073 and 0.0191 respectively, revealed that column height may play a vital role in dynamic column adsorption efficiency. These findings suggested that lignite activated coke can effectively remove sulfamethoxazole contaminants from wastewater in practice.
Keywords:
lignite activated coke; sulfamethoxazole; dynamic; adsorption; penetration curve; modeling 1. Introduction
Pharmaceuticals and personal care products (PPCPs) have become emerging pollutants due to the large amount of human consumption and usage [1,2,3,4]. Relating to the properties of high polarity and low volatility, PPCPs tend to be distributed and migrated to the environment through water phase transfer and food chain diffusions [5,6]. At present, many PPCPs pollutants have been detected in surface water, groundwater, drinking water and sewage, in the level of ng/L to μg/L, which will have potentially toxicological effects on the aquatic organisms [6]. Besides, the accumulation of these chemicals through the food chain may be harmful to human health [7]. Thus, it is necessary to develop effective treatment options to reduce their release into the environment. Up to now, various methods to remove PPCPs from wastewater have been developed, including photocatalysis [8,9], advanced oxidation [10,11], electrocatalysis [12,13], adsorption [14,15] and so on.
Sulfamethoxazole is a typical broad-spectrum antibiotic, which has been used in large quantities due to its inhibiting ability towards bacteria sensitivity [16]. Many methods, such as photocatalysis, advanced and fenton oxidation technology can effectively removal sulfamethoxazole from aqueous solution but the complicated operations, high cost and safety concerns has limited their application [14,16,17,18,19,20]. Therefore, the adsorption method is often used in industrial wastewater treatment due to the convenient operation in the advanced treatment stage. Different materials, such as carbon materials [21,22,23], composite metal materials [24,25,26], graphene oxide [27,28], metal-organic frameworks (MOFs) [29], resins [30] and so on have been reported with considerable adsorption capacity for sulfamethoxazole. However, considering practical use for wastewater treatment, economic materials with good mechanical strength, high adsorption capacity and fast removal efficiency are still being researched for sulfamethoxazole removal from aqueous solution. Lignite activated cokes (abbreviated as LACs), which are produced from carbonaceous materials, have been widely used in water treatment in recent years due to their benefits of high mechanical strength, cheapness, macroporous and mesoporous structures and oxygen-containing organic functional groups [31,32,33]. These have attracted extensive attention to LACs in the field of wastewater treatment in recent years. In this paper, the dynamic adsorption behavior of LACs towards sulfamethoxazole in aqueous solution was investigated.
The effects of column height, initial concentration, flow rate, humic acids and pH on dynamic adsorption behavior were comparatively analyzed to determine the optimized condition for dynamic adsorption. The models of Thomas, Yoon-Nelson and Adams-Bohart were fitted to experiment data and the Thomas has high correlation coefficients. The homogeneous surface diffusion model (HDSM) was fitted to a breakthrough curve and obtained the surface diffusion constant.
2. Materials and Methods
2.1. Materials, Reagents and Tests
Commercial LACs (1–2 mm) with a specific surface area of 790 m2 g−1 was obtained from Beijing Guodian Futong Technology Co., Ltd. (Beijing, China) and purified as follows [34]. Two grams of LACs was poured into a beaker containing 500 mL deionized water, boiled for 30 min, washed with deionized water for several times and dried at 105 °C for 24 h. Sulfamethoxazole (analytical grade) was purchased from Macleans Corporation (>99%, Shanghai, China). Humic acids, NaOH, HCl and NaCl were purchased from Sinopharm (Shanghai, China). These chemicals were used directly without purification.
The concentration of sulfamethoxazole in aqueous solution was measured by Acquity UPLC-H, with a C18 analytical column (1.7 µm × 2.1 mm × 50 mm) (ACQUITYUPLC, Waters, Milford, MA, USA), mobile phase (0.01% acetic acid solution: methanol = 30:70), flow rate (0.35 mL/min), column temperature 303 K, λ = 265 nm and the retention time was 0.7 min.
2.2. Equipment and Conditions of Column Experiments
2.2.1. Column Experimental Device
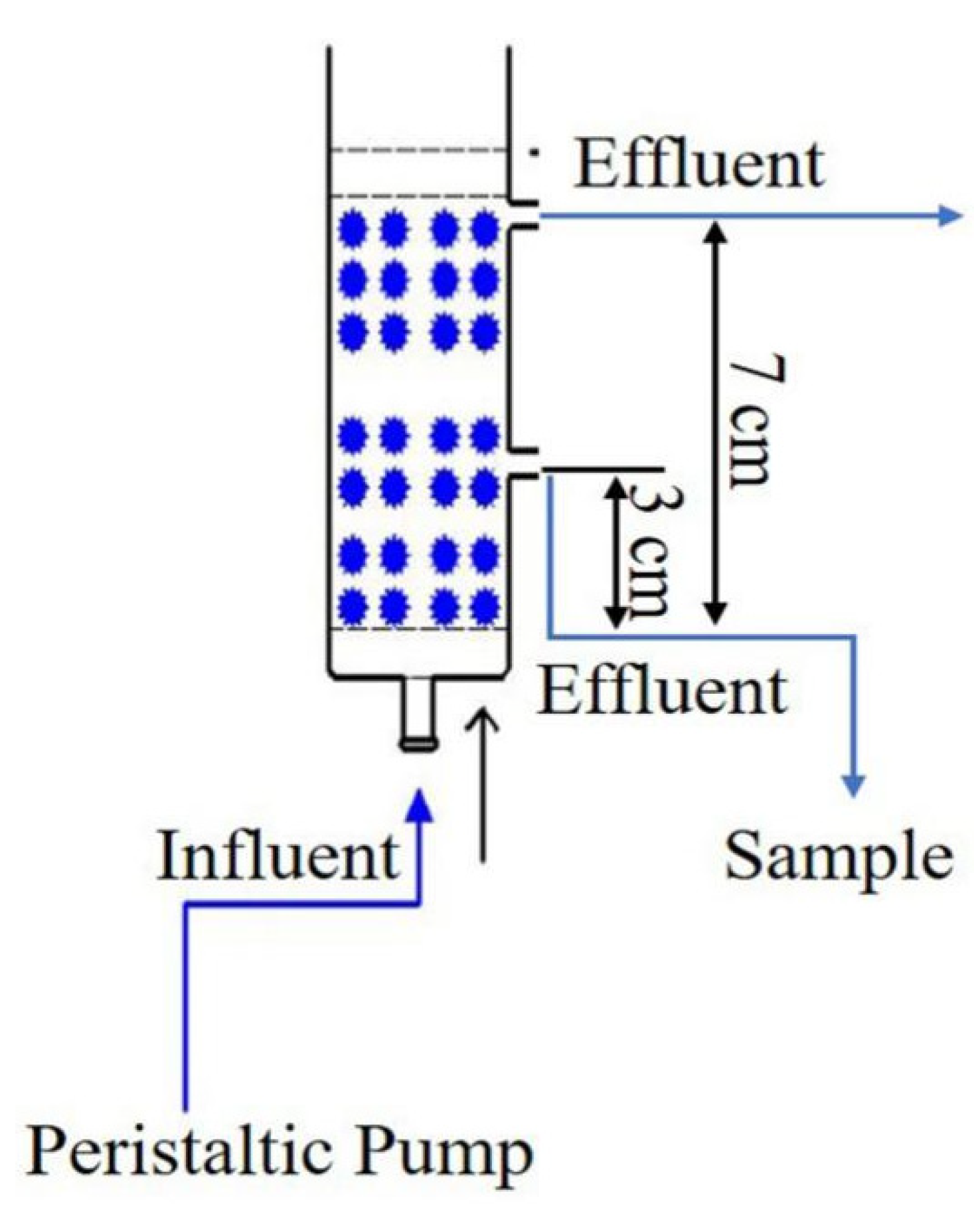
All the dynamic adsorption experiments were operated in a series of abbreviated wet packing glass columns, with an inner diameter of 2.5 cm, as shown in Figure 1. For each column, 0.5 cm glass beads, 3 cm LACs (with particle size of 100 mesh) and 0.5 cm glass beads were successively filled into the column, pressed tightly and uniformly. Prior to the experiment, the deionized water was inflowed into the column completely to exclude the air in the porosity of cokes until the pH of effluent arrived neutral, thus to maintain the stability of pore structures inside the adsorption column. ρ0 is the loading density of activated coke in the column (g/cm3). The aqueous solution of sulfamethoxazole was loaded into the column through the inlet at the bottom of the reactor and suctioned by a peristaltic pump under a steady flow. Samples were taken from the outlet at the fixed time intervals. Except for the column height experiment, the other experiment samples were received from outlets located at a column height of 3 cm. The concentrations of sulfamethoxazole in the sampling solution were tested by UPLC with a retention time of 0.7 min. With this device, the dynamic adsorption behaviors under different operation factors such as pollutant concentration, flow rate, pH and packing column height were investigated.
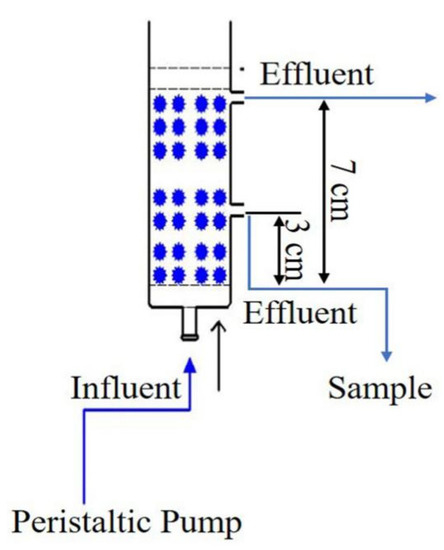
Figure 1.
The experimental device of column adsorption.
2.2.2. Column Experiments
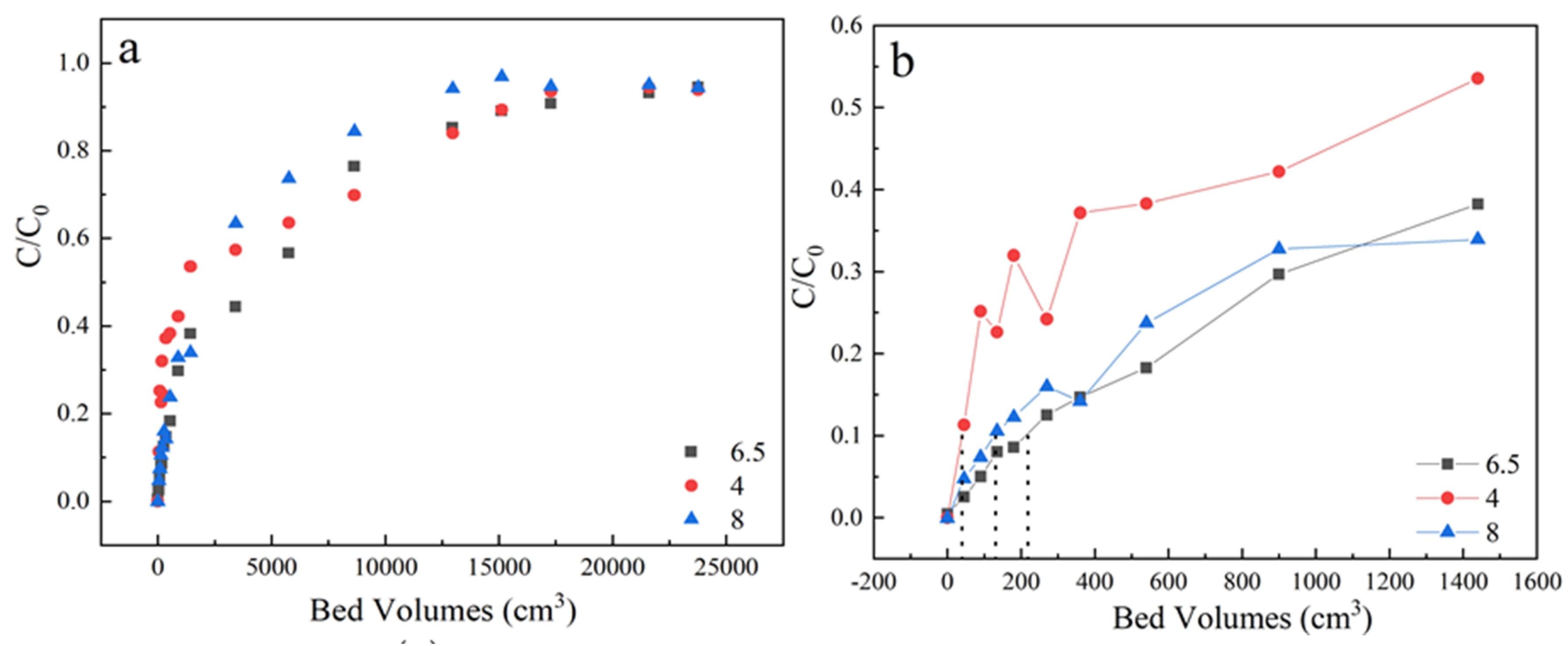
For the column adsorption experiments, the sulfamethoxazole solution (ion strength of 10 mmol/L NaCl) was pumped into the column from bottom to top by a peristaltic pump. The effect of different initial sulfamethoxazole concentrations (35 mg/L “vs.” 75 mg/L), column heights (3 cm “vs.” 7 cm), inlet flow rates (3 mL/min “vs.” 5 mL/min), solution pH (4, 6.5 and 8) and humic acids concentration (0, 0.1, 1 and 10 mg/L) on breakthrough curves were studied.
In the pH effect experiments, sulfamethoxazole solutions (35 mg/L) with initial pH values of 4, 6.5 and 8 were prepared, respectively. Aqueous HCl (0.10 mol/L) or NaOH (0.10 mol/L) were used to adjust the pH. Two outlets were designed in the column with a height of 3 cm and 7 cm respectively. In the column experiments with column height of 7 cm, the lower outlet at 3 cm was sealed. At certain intervals, in eluate, the concentration of the sulfamethoxazole was determined.
Herein, the time with the sampling concentration reaching 10% and 95% of the initial concentration were defined as the adsorption penetration point (tb, min) and the adsorption penetration end time (te, min). The length (H, cm) of the mass transfer zone of the adsorption column was calculated based on the adsorption penetration point time tb and the adsorption penetration end time te as follows (1):
Q represents the flow rate (mL/min); C0 is the inlet sulfamethoxazole concentration (mg/L); q is the amount adsorption capacity (mg/g) of sulfamethoxazole by unit activated coke; ρ0 is the loading density of activated coke in the column (g/cm3). A is the cross-sectional area of the adsorption column (cm2).
The adsorption capacity q is calculated according to Equation (2) [15,35]:
Among them, C0 and Ce were determined by UPLC with a retention time of 0.7 min. q is the dynamic saturated adsorption amount (mg/g); C0 is the inlet concentration of sulfamethoxazole (mg/L); Ce is the outlet concentration of sulfamethoxazole; V (L) is the solution volume; M is the mass weight of the adsorbents (g).
3. Results and Discussion
3.1. Adsorption Performance of Sulfamethoxazole on LACs
3.1.1. Effect of Initial Concentration
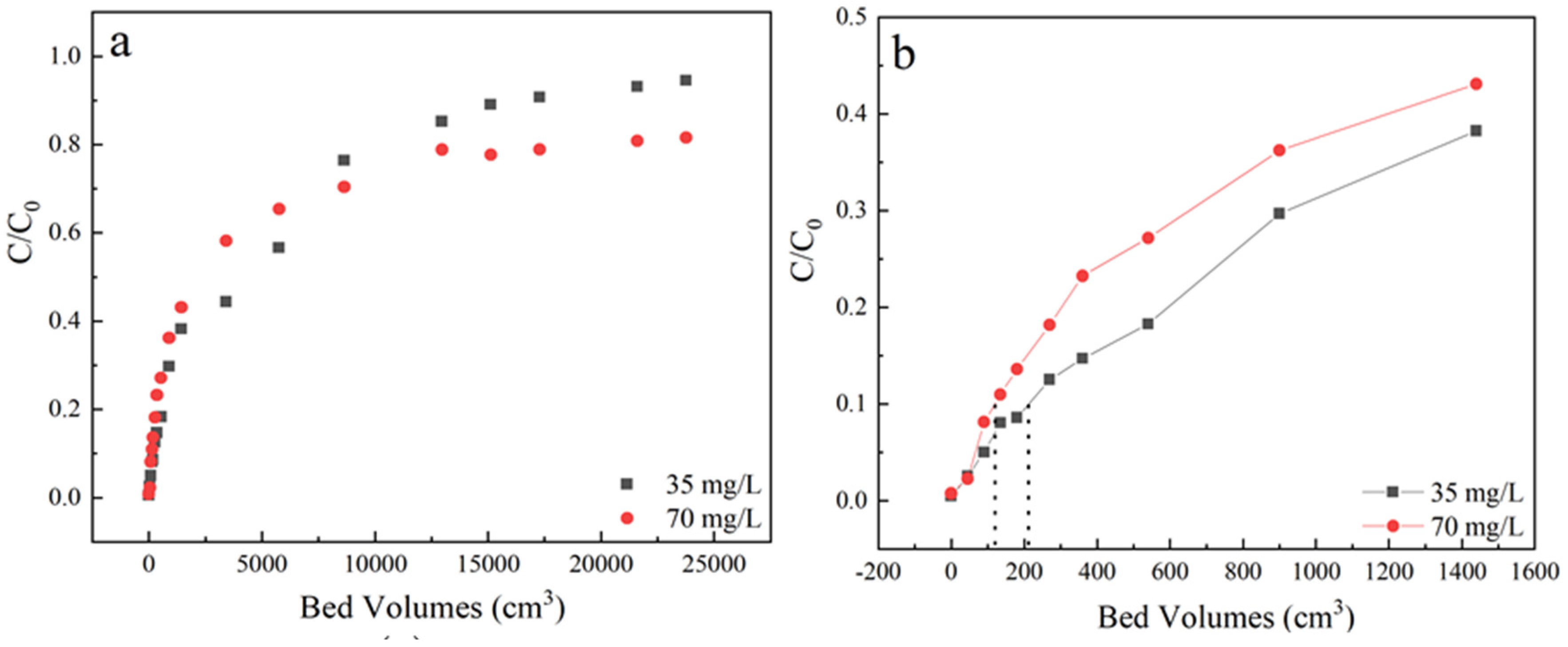
The breakthrough curves of sulfamethoxazole adsorption performance on LACs were described from a series of column adsorption entries to evaluate the enrichment capacity of LACs. At a fixed column height of 3 cm, a flow rate of 3 mL/min, pH 6.5, column adsorption under initial sulfamethoxazole concentration of 35 mg/L and 70 mg/L were comparatively studied. The samples were received from the outlet located at a column height of 3 cm. As shown in Figure 2 and Table 1, with the increase of initial concentration, the bed volume decreased from 210 to 120 cm3.
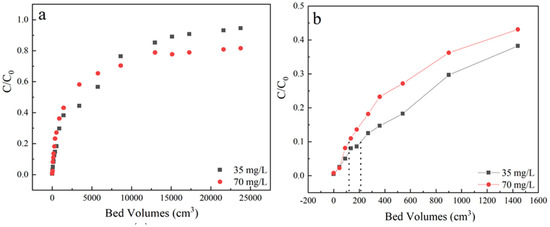
Figure 2.
(a) The effect of different concentration of sulfamethoxazole adsorption breakthrough curves; (b) Partially enlarged screening from 0 to 1600 bed volume. Experiment condition: flow rate of 3 mL/min, column height of 3 cm, pH 6.5, sulfamethoxazole concentration of 35 and 70 mg/L, respectively.

Table 1.
Comparative data of penetration curves of dynamic coke adsorption sulfamethoxazole dynamic column experiments.
The time required to reach 50% of initial concentration was obviously extended from 12 h to 25 h, which may be due to that high initial concentration, increased the sulfamethoxazole concentration difference between activated coke and solutions, making the breakthrough curve slope steeper [36]. On the other hand, it was clear that the time required to reach saturation decreased with the increasing of the initial concentration, as the diffusion rate is controlled by the concentration gradient. As the initial concentration of pollutants increased, the bed utilization rate decreased. Besides, the adsorption penetration end time in the system of 70 mg/L could not be reached, the C/C0 after 140 h was close to 0.8 and arrived in equilibrium, which indicated that a certain retention effect may exist in the liquid flow system of the adsorption column under high sulfamethoxazole initial concentration.
3.1.2. Effect of Column Height
At a fixed flow rate of 3 mL/min, initial sulfamethoxazole concentration of 35 mg/L and pH 6.5, column adsorption under different column heights of 3 cm and 7 cm were studied to assess the effect of column height on the dynamic adsorption. From Figure 3 and Table 1, it was observed that column height had a positive relationship with the bed adsorption capacity. Under a high column height of 7 cm, the bed volume could be extended remarkably from 210 to 395 cm3. Besides, the curve slope became smooth under a higher column length. Undoubtedly, the amounts of LACs increased, the adsorption capacity could be enhanced, and high breakthrough time gave better intraparticle diffusion phenomena. Some references have mentioned that carbon-based materials with high mesopores, large pore volumes and medium specific surface area have the best adsorption effect on sulfamethoxazole [32,37]. LACs contained a number of macropore and mesoporous structures, attributed not only to the increasing of the specific surface area but also enhancing the sulfamethoxazole removal by increasing the spread of contaminant and a capacity of the sorbent material [34]. Moreover, LACs contain many oxygen-containing functional groups, such as phenolic groups, which could provide abundant binding sites for sulfamethoxazole through hydrogen-bonding interactions. The curves shape noted for 3 cm was more upright than 7 cm, this might be because a larger mass transfer region has formed in the longer column, which retarded the arrival of penetration time.
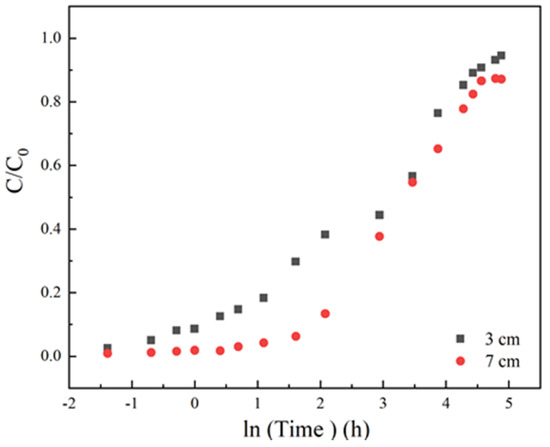
Figure 3.
The adsorption effect of different column height on breakthrough curves, Experiment condition: sulfamethoxazole concentration of 35 mg/L, flow rate of 3 mL/min, pH 6.5, column height 3, 7 cm, respectively.
3.1.3. Effect of Flow Rate
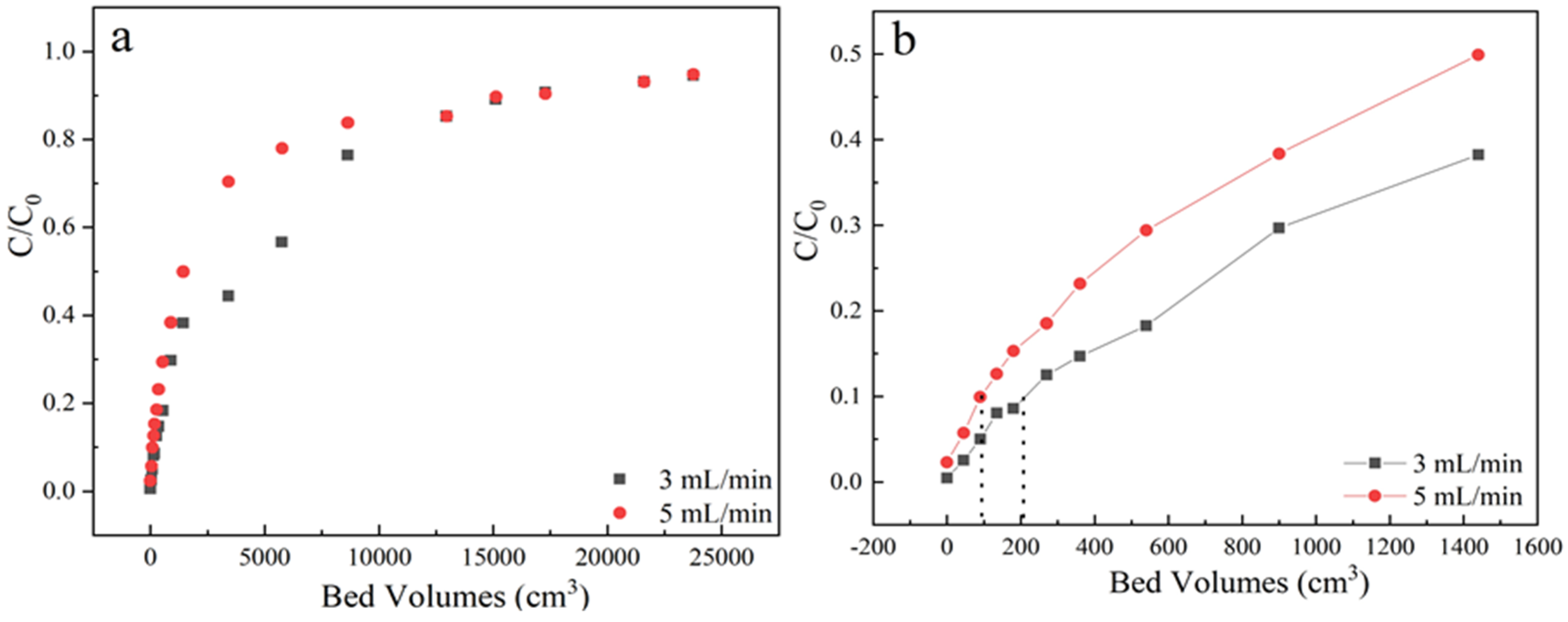
Flow rate is an important parameter for industrial-scale wastewater treatment. The samples were received from the outlet located at a column height of 3 cm, sulfamethoxazole concentration of 35 mg/L at pH 6.5, the adsorption flow rates were varied from 3 mL/min to 5 mL/min, the corresponding dynamic adsorption breakthrough curves were compared in Figure 4 and Table 1. It could be found that the steepness increased with the flow rate, resulting in an earlier breakthrough point volume under 5 mL/min.
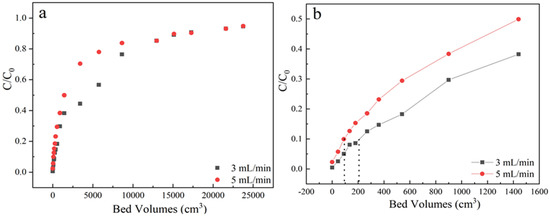
Figure 4.
(a) The adsorption effect of different flow rate on breakthrough curves; (b) Partially enlarged screening from 0 to 1600 bed volume. Experiment condition: sulfamethoxazole concentration of 35 mg/L, pH 6.5, column height of 3 cm, flow rate of 3, 5 mL/min, respectively.
This is because that low flow rate can offer enough time for intra-particle diffusion of pollutants at the interface of adsorbents as well as a binding interaction between sulfamethoxazole and functional groups of LACs. And the flow rate will affect the external film diffusion but not the surface diffusion. Contrarily, high flow rate of 5 mL/min could easily cause the decrease of mass transfer resistance, then the breakpoint time and saturation were quickly reached. Besides, fast flow rate would also reduce the utilization efficiency of the fixed bed before reached to saturation.
3.1.4. Effect of pH
In the actual wastewater treatment process, pH factor will affect the effectiveness of the adsorbent to remove pollutants. So, it is necessary to consider the pH effect on wastewater treatment. Herein, effect of pH was executed at 4, 6.5 and 8 under the condition of 35 mg/L sulfamethoxazole concentration, 3 mL/min flow rate and the samples were received from the outlets, located at a column height of 3 cm.
As shown in Figure 5 and Table 1, the best adsorption performance was obtained at pH 6.5. The pHpzc of activated coke was 6.5 and the pKa of sulfamethoxazole was 5 [38]. At pH 4, electrostatic interaction contributes to the major adsorption mechanism due to the negatively charged sulfamethoxazole species and the positively charged surface of LACs. At pH 6.5, the sulfamethoxazole species were negatively charged while the surface of LACs was nearly uncharged, the hydrogen-bonding interaction played an important role during adsorption because the phenolic groups of LACs and the N, O atoms of sulfamethoxazole species. Similar observations were also reported in diclofenac sodium adsorption on oxidized activated carbon [39]. At pH 8.0, the sulfamethoxazole species and the surface of LACs were both negatively charged, the electrostatic repulsion could inhibit the sulfamethoxazole adsorption, thus a lowered penetration time was obtained.
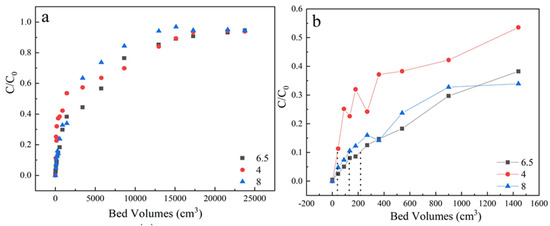
Figure 5.
(a) The adsorption effect of different pH on breakthrough curves, (b) Partially enlarged screening from 0 to 1600 bed volume. Experiment condition: flow rate of 3 mL/min, column height of 3 cm, sulfamethoxazole concentration of 35 mg/L, pH 4, 6.5, 8 respectively.
3.1.5. Effect of Humic Acids
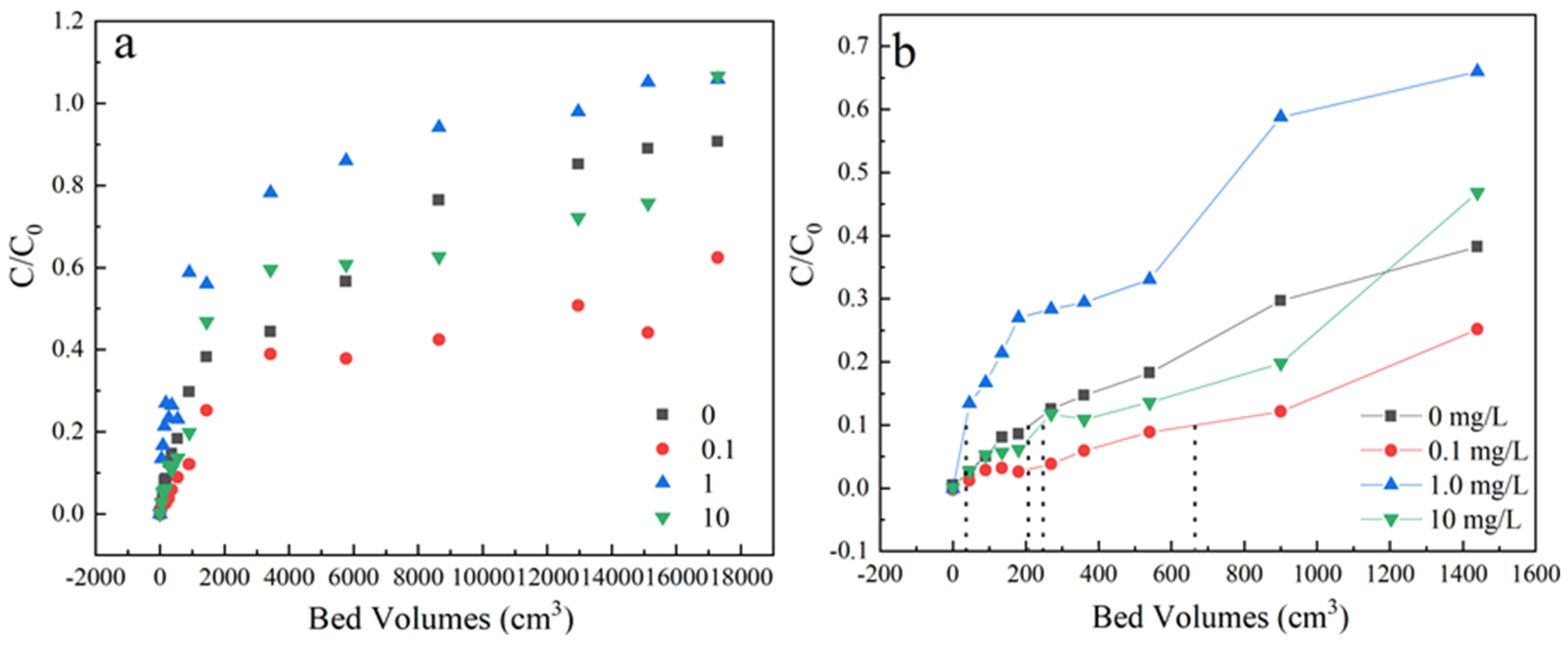
The effect of humic acids was executed at 0, 0.1, 1 and 10 mg/L under the condition of 35 mg/L sulfamethoxazole concentration, 3 mL/min flow rate and the samples were received from the outlets, located at a column height of 3 cm. As shown in Figure 6 and Table 1, the penetration time increase slowly during 0–0.1 mg/L and decreased deeply when organic concentration increased to 1 mg/L. When increasing the concentration of organic matter to 10 mg/L, the adsorption ability increased a little. The slopes of penetration curves under different humic acids concentration follows in the order of 1 > 0 > 10 > 0.1 mg/L. These results illustrated that the existence of humic acids would affect the dynamic adsorption behavior.
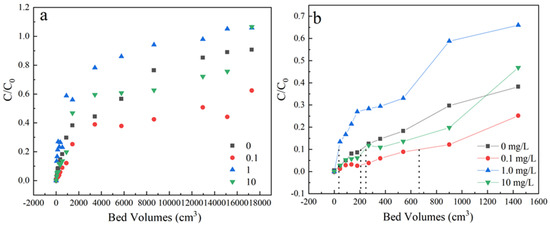
Figure 6.
(a) The adsorption effect of different concentration of human acids on breakthrough curves, (b) Partially enlarged screening from 0 to 1600 bed volume. Experiment condition: flow rate of 3 mL/min, column height of 3 cm, sulfamethoxazole concentration of 35 mg/L, pH 6.5, humic acids concentration= 0, 0.1, 1, 10 mg/L, respectively.
3.2. Breakthrough Curves Models Analysis
To elucidate the adsorption mechanism of sulfamethoxazole on LACs, the Thomas model, Yoon-Nelson and Adams-Bohart model were used to predict the performance and parameters of the fixed-bed column. The Thomas model is derived depending upon second-order kinetics and assumed that the sorption is dominated by mass transfer at the interface not the chemical reaction [40]. The mathematical form is as follows:
C0 and Ct (mg/L) are the concentration of solution in the inlet and outlet at time t (min), kT (mL/(min·mg)) is the Thomas rate constant, Q (mL/min) is the flow rate, qe (mg/g) is the maximum sorption capacity, m (g) is the mass of activated coke.
The Yoon-Nelson model assumes that the rate of decreasing in the probability of adsorption for each adsorbate molecule is directly proportional to adsorbate breakthrough probability and adsorbate adsorption probability [41]. The Yoon-Nelson does not rely on the physical factors bed and adsorbate characteristics [42].
C0 and Ct (mg/L) are the concentrations of solution in the inlet and outlet at time t (min), kY (1/min) is the Yoon-Nelson rate constant, τ (min) is the time to reach 50% of sulfamethoxazole breakthrough. Except for the linearized Equation, Thomas and Yoon-Nelson models are equivalent in terms of mathematical form as described in Reference [43]:
The Adams-Bohart model was based on the surface reaction theory, applied to account for the initial part of the breakthrough curve. The Adams-Bohart model and its mathematical form are as follows [40]:
C0 and Ct (mg/L) are the concentration of solution in the inlet and outlet at time t (min), kA (L/(min·g)) is the Yoon-Nelson rate constant, N0 (g/L) is the sorption capacity of the adsorbent per unit volume of the bed, h (mm) is the column height, v (mm/min) is the flow rate. The analysis of Ce/C0 and t at different experimental conditions was performed.
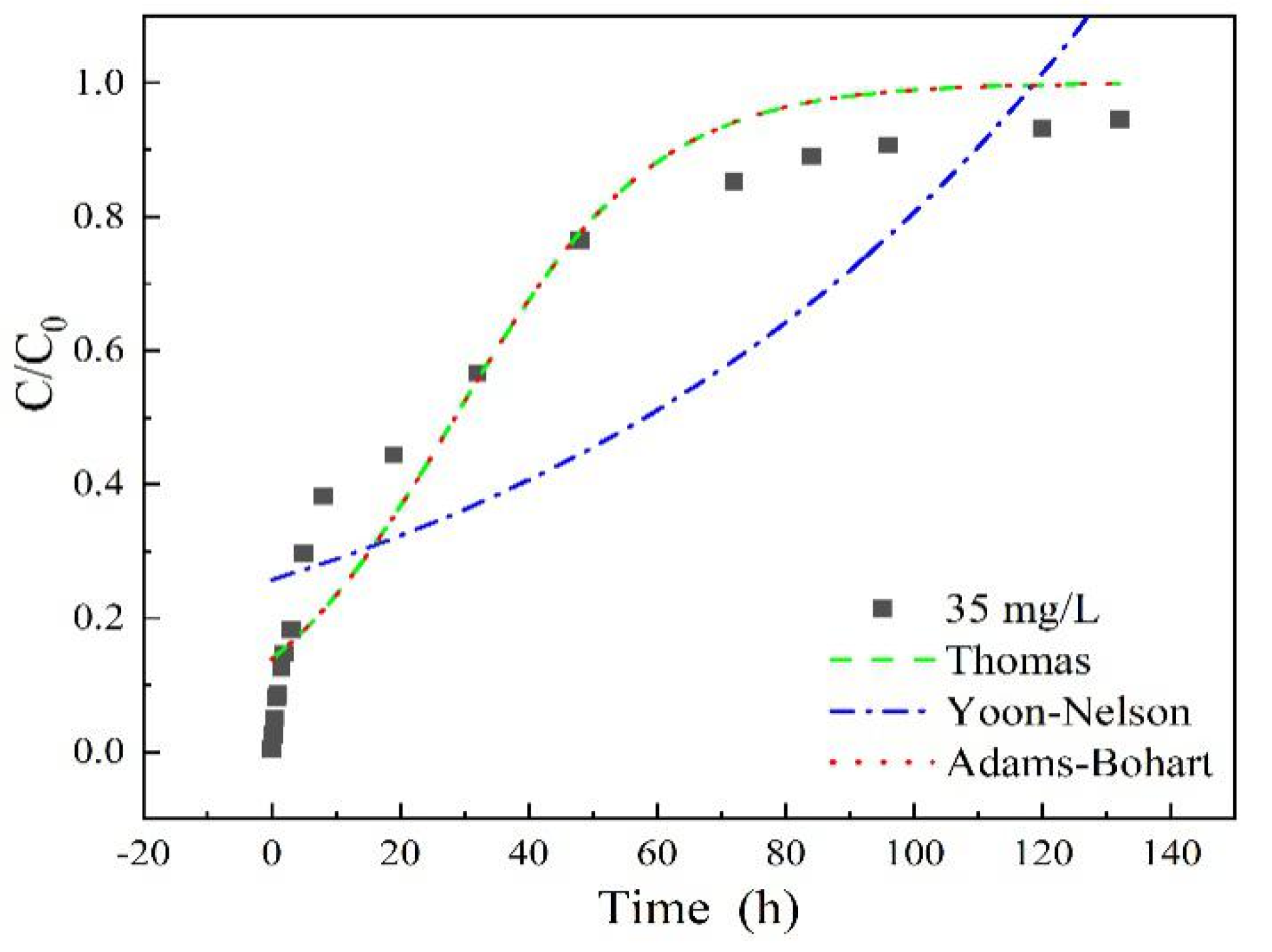
As shown in Figure 7 and Table 2, high correlation coefficients R2 > 0.95 were obtained in the Thomas model for all the adsorption entries (except for the column height of 7 cm), For the Yoon-Nelson model, adsorption entries under low experiment parameters (such as 35 mg/L sulfamethoxazole concentration, 3 mL/min flow rate and 7 cm column height) fitted better than the higher ones, with correlation coefficients R2 > 0.95. While a low R2 value obtained in the Adams-Bohart model indicated worse fitting results of experiment data. These calculation results demonstrated that the adsorption rate could be probably dominated by mass transfer at the interface, due to the microporous and mesoporous structure characteristics of LACs, similar conclusions were also reported in diclofenac sodium adsorption on LACs [34]. The value of the model’s parameters, such as kT, kY, qe and τ depends on experimental conditions such as flow rate, initial concentration and column height. The initial concentration of sulfamethoxazole could obviously affect the maximum sorption capacity, qe, long column height of LACs may inhibit the adsorption rate due to the sharply decreasing kT in 7 cm column height entry.
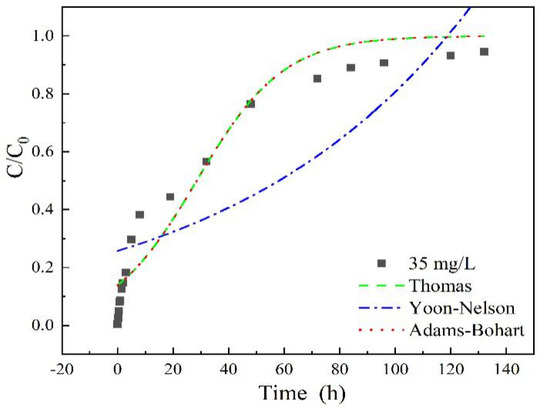
Figure 7.
Adsorption breakthrough curves fitted by different models with sulfamethoxazole initial concentration of 35 mg/L, flow rate of 3 mL/min, pH 6.5, column height of 3 cm.

Table 2.
The summary of experiment parameters calculated from different models under various experimental conditions.
3.3. Homogeneous Surface Diffusion Model (HDSM)
The HSDM model is one of the most widely used dual-resistance diffusion models to predict the fixed bed adsorption process. Through optimizing the fit curve to the experiment breakthrough curve, the surface diffusion coefficient DS can be obtained [44]. The HSDM was used to evaluated the diffusion coefficient of experiment data. The Equations of the HSDM model are listed as follows [45]. Freundlich isotherm parameters (K, 1/n) needed in HSDM model by adsorption isotherm were presented in the Supplementary material (Table S1 and Figure S1) [46].
The mass balance Equation of particle can be represented as:
The moving phase and particle concentration is correlated with Freundlich Equation:
The initial and boundary conditions are:
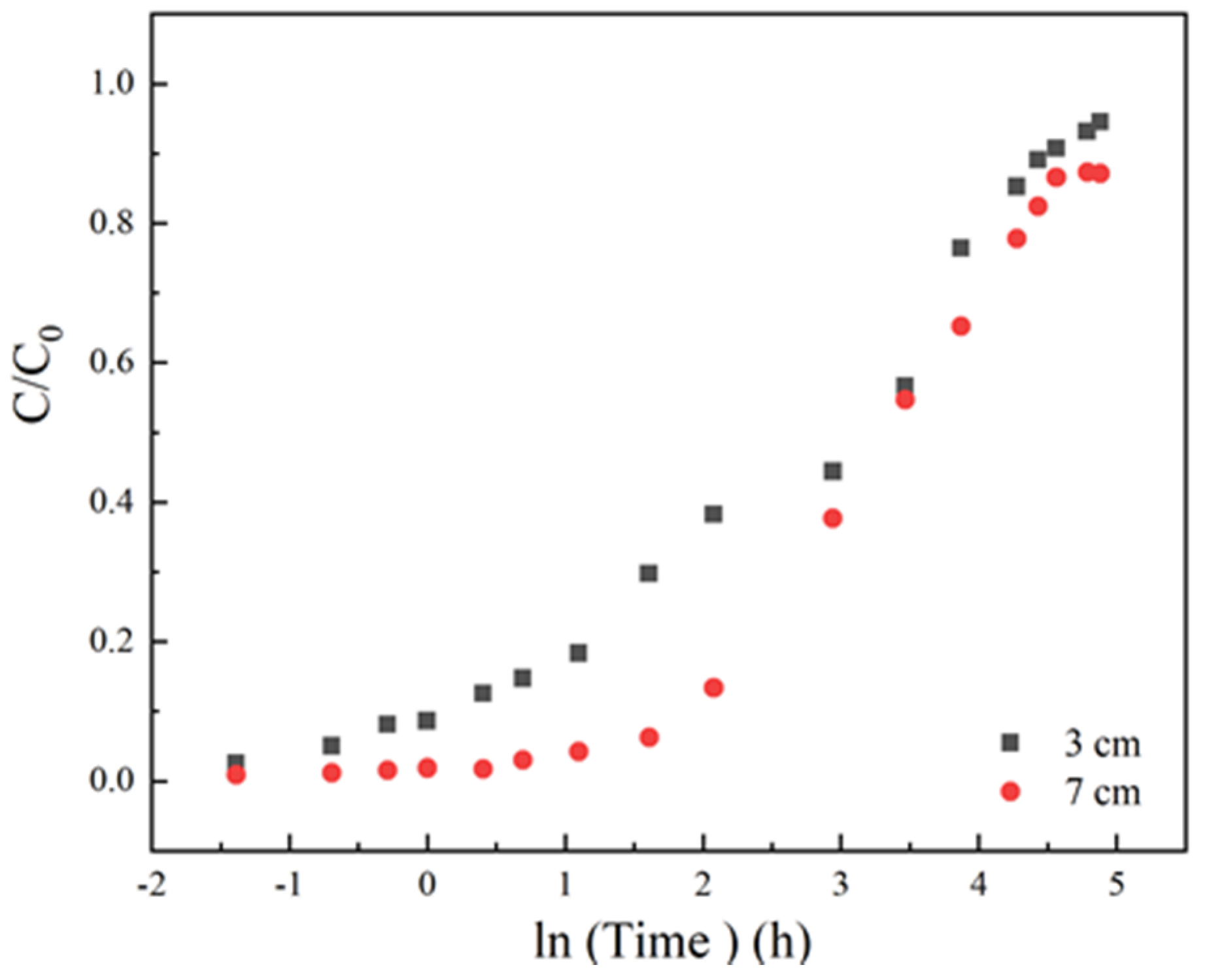
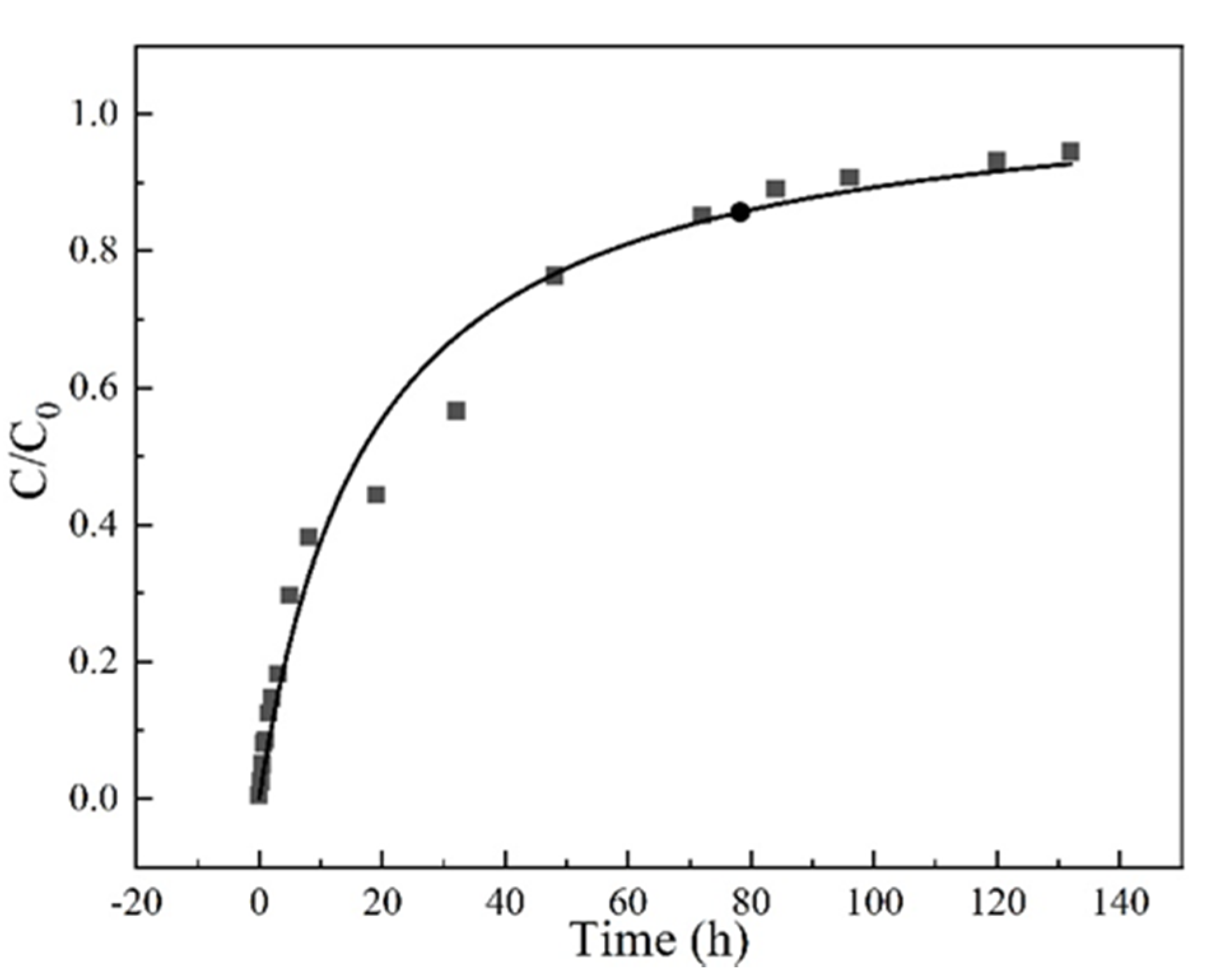
The surface diffusion coefficient (Ds) depends on the nature of adsorbents. So, the experimental breakthrough curves at a concentration of 35 mg/L, a flow rate at 3 mL/min, a column height of 3 cm and pH 6.5 conditions were fitted with the HSDM, as shown in Figure 8. The determined surface diffusion coefficient is 2.39 × 10−9 cm2/min.
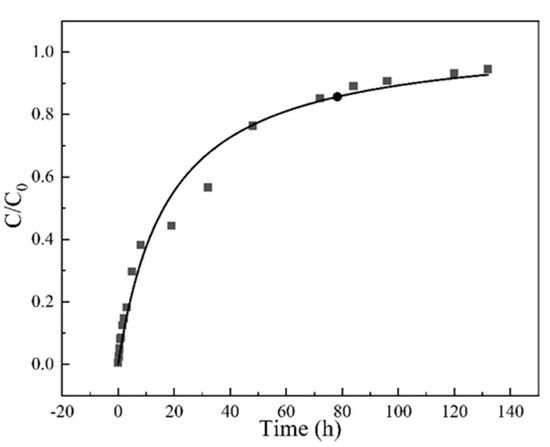
Figure 8.
The homogeneous surface diffusion model (HDSM) diffusion model fitted breakthrough curve Experiment condition: sulfamethoxazole concentration of 35 mg/L, flow rate of 3 mL/min, pH 6.5, column height 3 cm.
The sum of square of error (SSE) between the experimental data and the theoretical simulation was used to evaluate the feasibility of the model. As shown in Table 3, the value of SSE is 0.04, demonstrating that the proposed HSDM model can successfully describe the adsorption and diffusion behavior in the fixed bed.

Table 3.
The SSE of HSDM models.
3.4. Evaluation of Factors Effect
In order to investigate the optimized parameters for dynamic adsorption of sulfamethoxazole on LACs, the effects of initial concentration, column height, flow rate, pH and organic matter on the penetration time and the length of the mass transfer zone were compared systematically. As shown in Table 4, the absolute value of average rate (Δtb/Δx or ΔH/Δx) were calculated from the increments of penetration time (Δtb), mass transfer zone length (ΔH) for each factor increments (Δx).

Table 4.
Analysis of each factor on the length of the mass transfer zone of the activated coke adsorption sulfamethoxazole dynamic column experiment.
The average change rates of the penetration time to the changes of adsorption parameters (Δtb/Δx), Δx represents initial concentration, column height, flow rate and pH, were 0.86, 98.25, 20, 12.25, respectively. Thus, column height may play a vital role in penetration time. The same results were also gained for ΔH/Δx.
The average change rates of the length of the mass transfer zone with respect to the initial concentration, the column height, the flow rate, pH and organic matter were 0.0003, 0.6474, 0.0076, 0.0073 and 0.0191, respectively. Therefore, the change of the initial concentration has the lowest effect on the average rate of change in the length of the mass transfer zone and the column height has the greatest effect on it. These calculations were in accordance with the studies in the above adsorption effects sections.
4. Conclusions
Dynamic column adsorption of sulfamethoxazole by lignite activated coke from aqueous solution were conducted under different initial sulfamethoxazole concentrations, flow rates, column height and pH to investigate the optimized adsorption conditions for wastewater treatment. Results demonstrated that activated coke has high adsorption efficiency for sulfamethoxazole removal from aqueous solution. Increasing the concentration of pollutants and the flow rate will shorten the penetration time, while opposite tendency was found in the column height effect. The best adsorption performance was obtained at pH 6.5 due to the possible hydrogen bonding interaction between sulfamethoxazole species and LACs. The adsorption capacity of activated coke increased by increase in the column height. The Thomas model could best describe the column adsorption behavior with R2 > 0.95 for nearly all adsorption entries. The column height of the activated cokes played a vital role in long penetration time and mass transfer zone.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/13/7/1785/s1, Figure S1: Adsorption isotherms of sulfamethoxazole. Condition: under low initial concentrations of 1~50 mg/L. Adsorbent dosage = 0.5 g/L, pH = 6.5 ± 0.1, [NaCl] = 10 mmol/L, Table S1: Adsorption isotherms parameters.
Author Contributions
Data curation and writing, J.H. and K.C.; Formal analysis, Z.S., M.L. and P.G.; Funding acquisition, L.W.; Methodology, J.H. and K.C.; Software, Z.S., M.L. and P.G.; Writing—original draft, H.L.; Fund supporting and Designing, L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of China, (No. 51678025 & 51978032); Great Wall Scholars Program (CIT&TCD20170313); Youth Top Talents Training Program (CIT&TCD201804052); Science & Technology Foundation of POWERCHINA Co., Ltd (DJ-ZDXM 201619).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perez-Lemus, N.; Lopez-Serna, R.; Perez-Elvira, S.I.; Barrado, E. Analytical methodologies for the determination of pharmaceuticals and personal care products (PPCPs) in sewage sludge: A critical review. Anal. Chim. Acta 2019, 1083, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, Y.; Shi, B.; Meng, J.; He, B.; Yang, H.; Yoon, S.J. Anthropogenic impacts on the contamination of pharmaceuticals and personal care products (PPCPs) in the coastal environments of the Yellow and Bohai seas. Environ. Int. 2020, 135, 105306. [Google Scholar] [CrossRef] [PubMed]
- Tarpani, R.R.Z.; Azapagic, A. Life cycle environmental impacts of advanced wastewater treatment techniques for removal of pharmaceuticals and personal care products (PPCPs). J. Environ. Manag. 2018, 215, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yin, H.; Wu, Q. Occurrence and health risk assessment of pharmaceutical and personal care products (PPCPs) in tap water of Shanghai. Ecotoxicol. Environ. Saf. 2019, 183, 109497. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: An overview. Radiat. Phys. Chem. 2016, 125, 56–64. [Google Scholar] [CrossRef]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596, 303–320. [Google Scholar] [CrossRef]
- Khan, M.; Fung, C.S.L.; Kumar, A.; He, J.; Lo, I.M.C. Unravelling mechanistic reasons for differences in performance of different Ti-and Bi-based magnetic photocatalysts in photocatalytic degradation of PPCPs. Sci. Total Environ. 2019, 686, 878–887. [Google Scholar] [CrossRef]
- Zeng, L.; Li, S.; Li, X.; Li, J.; Fan, S.; Chen, X. Visible-light-driven sonophotocatalysis and peroxymonosulfate activation over 3D Urchin-like MoS2/C nanoparticles for accelerating levofloxacin elimination: Optimization and kinetic study. Chem. Eng. J. 2019, 378, 122039. [Google Scholar] [CrossRef]
- Alkhuraiji, T.S. Advanced oxidation process based on water radiolysis to degrade and mineralize diclofenac in aqueous solutions. Sci. Total Environ. 2019, 688, 708–717. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Leiknes, T.O. Oxidation of Refractory Benzothiazoles with PMS/CuFe2O4: Kinetics and Transformation Intermediates. Environ. Sci. Technol. 2016, 50, 564–5073. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, Y.; Liu, H.H.; Zhang, F.; Wang, S. Room-temperature air oxidation of organic pollutants via electrocatalysis by nanoscaled Co-CoO on graphite felt anode. Environ. Int. 2019, 131, 104977. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhong, S.; Song, Y.; Wang, B.; Zhang, F. Degradation of tetracycline hydrochloride by electro-activated persulfate oxidation. J. Electroanal. Chem. 2018, 809, 74–79. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Ma, T.; Zhao, S.; Rong, H. Uniform and stable immobilization of metal-organic frameworks into chitosan matrix for enhanced tetracycline removal from water. Chem. Eng. J. 2020, 382. [Google Scholar] [CrossRef]
- Zhu, G.; Sun, Q.; Wang, C.; Yang, Z.; Xue, Q. Removal of Sulfamethoxazole, Sulfathiazole and Sulfamethazine in their Mixed Solution by UV/H2O2 Process. Int. J. Environ. Res. Public Health 2019, 16, 1797. [Google Scholar] [CrossRef]
- Ling, C.; Yue, C.; Yuan, R.; Qiu, J.; Liu, F.Q.; Zhu, J.J. Enhanced removal of sulfamethoxazole by an ovelcomposite of TiO2 nanocrystals in situ wrapped-Bi2O4 microrods undersimulated solar irradiation. Chem. Eng. J. 2020, 384, 123278. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Li, Y.; Li, W.; Zhou, L. Insight into heterogeneous catalytic degradation of sulfamethazine by peroxymonosulfate activated with CuCo2O4 derived from bimetallic oxalate. Chem. Eng. J. 2020, 384, 123257. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, Y.; Huang, D.; Lai, C.; Zeng, G.; Huang, J.; Xiong, W. Prussian blue analogue derived magnetic Cu-Fe oxide as a recyclable photoFenton catalyst for the efficient removal of sulfamethazine at near neutral pH values. Chem. Eng. J. 2019, 362, 865–876. [Google Scholar] [CrossRef]
- Liu, X.; Huang, F.; Yu, Y.; Jiang, Y.; Zhao, K.; He, Y.; Zhang, Y. Determination and toxicity evaluation of the generated byproducts from sulfamethazine degradation during catalytic oxidation process. Chemosphere 2019, 226, 103–109. [Google Scholar] [CrossRef]
- Zbair, M.; Ahsaine, H.A.; Anfar, Z. Porous carbon by microwave assisted pyrolysis: An effective and low-cost adsorbent for sulfamethoxazole adsorption and optimization using response surface methodology. J. Clean Prod. 2018, 202, 571–581. [Google Scholar] [CrossRef]
- Lawal, I.A.; Lawal, M.M.; Akpotu, S.O.; Moodley, B. Theoretical and experimental adsorption studies of sulfamethoxazole and ketoprofen on synthesized ionic liquids modified CNTs. Ecotox. Environ. Safe. 2018, 161, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Prasannamedha, G.; Kumar, P.S. A review on contamination and removal of sulfamethoxazole from aqueous solution using cleaner techniques: Present and future perspective. J. Clean Prod. 2020, 250, 119553. [Google Scholar] [CrossRef]
- Heo, J.; Yoon, Y.; Lee, G.; Kim, Y.; Park, C.M. Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4-biochar composite. Bioresour. Technol. 2019, 281, 179–187. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, X.; Chen, B.; Ma, J.; Niu, X.; Zhou, S. Enhanced adsorption of sulfamethoxazole from aqueous solution by Fe-impregnated graphited biochar. J. Clean Prod. 2020, 256, 120662. [Google Scholar] [CrossRef]
- Li, S.; Wang, F.; Pan, W.; Yang, X.; Gao, Q.; Sun, W.; Ni, J. Molecular insights into the effects of Cu(II) on sulfamethoxazole and 17β-estradiol adsorption by carbon nanotubes/CoFe2O4 composites. Chem. Eng. J. 2019, 373, 995–1002. [Google Scholar] [CrossRef]
- Ogunleye, D.T.; Akpotu, S.O.; Moodley, B. Adsorption of sulfamethoxazole and reactive blue 19 using graphene oxide modified with imidazolium based ionic liquid. Environ. Technol. Innov. 2020, 17, 100616. [Google Scholar] [CrossRef]
- Rostamian, R.; Behnejad, H. A comparative adsorption study of sulfamethoxazole onto graphene and graphene oxide nanosheets through equilibrium, kinetic and thermodynamic modeling. Process Saf. Environ. Protect. 2016, 102, 20–29. [Google Scholar] [CrossRef]
- Yu, K.; Ahmed, I.; Won, D.-I.; Lee, W.I.; Ahn, W.-S. Highly efficient adsorptive removal of sulfamethoxazole from aqueous solutions by porphyrinic MOF-525 and MOF-545. Chemosphere 2020, 250, 126133. [Google Scholar] [CrossRef]
- Qiu, H.; Ling, C.; Yuan, R.; Liu, F.; Li, A. Bridging effects behind the coadsorption of copper and sulfamethoxazole by a polyamine-modified resin. Chem. Eng. J. 2019, 362, 422–429. [Google Scholar] [CrossRef]
- Zheng, M.; Han, Y.; Xu, C.; Zhang, Z.; Han, H. Selective adsorption and bioavailability relevance of the cyclic organics in anaerobic pretreated coal pyrolysis wastewater by lignite activated coke. Sci. Total Environ. 2019, 653, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, L.; Liu, H.; Lan, H.; Qu, J. Improvement of aqueous mercury adsorption on activated coke by thiol-functionalization. Chem. Eng. J. 2013, 228, 925–934. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Liu, Q.; Wang, X.; Zhang, Z. Facile and economical synthesis of porous activated semi-cokes for highly efficient and fast removal of microcystin-LR. J. Hazard. Mater. 2015, 299, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Du, C.; He, H.; Yang, Z.; Li, H. Effective adsorption of diclofenac sodium from neutral aqueous solution by low-cost lignite activated cokes. J. Hazard. Mater. 2020, 384, 121284. [Google Scholar] [CrossRef]
- Zhuang, S.; Liu, Y.; Wang, J. Covalent organic frameworks as efficient adsorbent for sulfamerazine removal from aqueous solution. J. Hazard. Mater. 2020, 383, 121126. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saha, P.D. Batch and continuous (fixed-bed column) biosorption of Cu (II) by Tamarindus indicafruit shell. Korean J. Chem. Eng. 2013, 30, 369–378. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Hameed, B.H. Fixed-bed adsorption of reactive azo dye onto granular activated carbon prepared from waste. J. Hazard. Mater. 2010, 175, 298–303. [Google Scholar] [CrossRef]
- Chen, K.L.; Liu, L.C.; Chen, W.R. Adsorption of sulfamethoxazole and sulfapyridine antibiotics in high organic content soils. Environ. Pollut. 2017, 231 Pt 1, 1163–1171. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Seo, P.W.; Jhung, S.H. Adsorption of diclofenac sodium from water using oxidized activated carbon. Chem. Eng. J. 2016, 301, 27–34. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Wang, C.; Ying, Z.; Huo, M.; Yang, W. Effective column adsorption of triclosan from pure water and wastewater treatment plant effluent by using magnetic porous reduced graphene oxide. J. Hazard. Mater. 2020, 386, 121942. [Google Scholar] [CrossRef]
- Nithya, K.; Sathish, A.; Kumar, P.S. Packed bed column optimization and modeling studies for removal of chromium ions using chemically modified Lantana camara adsorbent. Process Saf. Environ. Protect. 2020, 33, 101069. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Albayati, T.M.; Alrubaye, J.M. Adsorption of the methyl green dye pollutant from aqueous solution using mesoporous materials MCM-41 in a fixed-bed column. Heliyon 2020, 6, e03253. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Luo, J.; An, Q.; Xiao, Z.; Wang, H.; Cai, W.; Li, Z. Efficiently selective adsorption of Pb(II) with functionalized alginate-based adsorbent in batch/column systems: Mechanism and application simulation. J. Clean Prod. 2020, 250, 119585. [Google Scholar] [CrossRef]
- Weber, W.J.J.; Liu, K.T. Determination of mass transport parameters for fixed-bed. Chem. Eng. Commun. 1980, 6, 49–60. [Google Scholar] [CrossRef]
- Zheng, M.; Hu, H.; Ye, Z.; Huang, Q.; Chen, X. Adsorption desulfurization performance and adsorption-diffusion study of B2O3 modified Ag-CeOx/TiO2-SiO2. J. Hazard. Mater. 2019, 362, 424–435. [Google Scholar] [CrossRef]
- Yu, J.; Yang, F.C.; Hung, W.N.; Liu, C.L.; Yang, M.; Lin, T.F. Prediction of powdered activated carbon doses for 2-MIB removal in drinking water treatment using a simplified HSDM approach. Chemosphere 2016, 156, 374–382. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).