The Effect of Temperature and Milling Process on Steel Scale Utilized as a Pigment for Ceramic Glaze
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Part 1: The Glazes with the Original Scale and Pre-Calcined Scale
2.3. Part 2: The Glazes Prepared with the Scale Milled in Two Different Types of Mill Devices
2.4. Characterization Methods
3. Results and Discussion
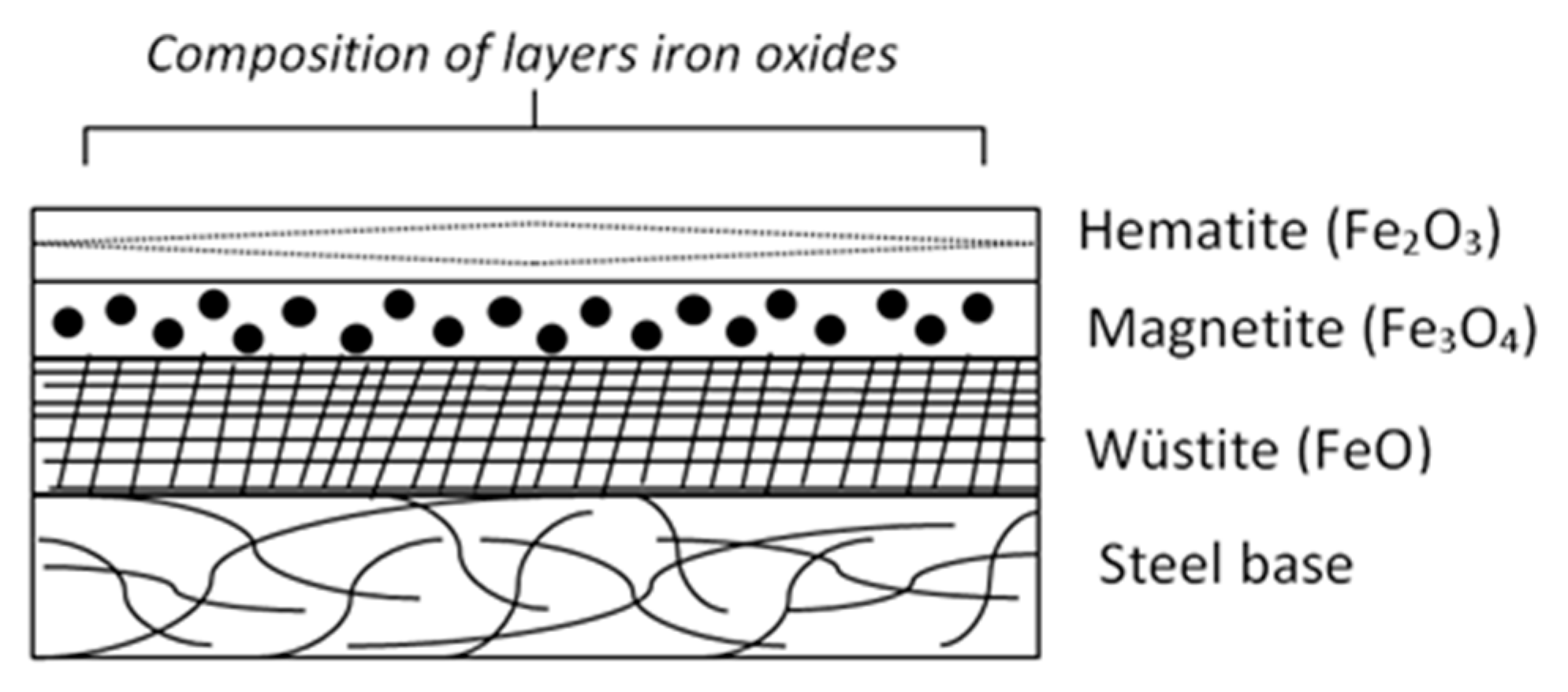
3.1. Characterization of Raw Materials and Thermal Treatment of Scale
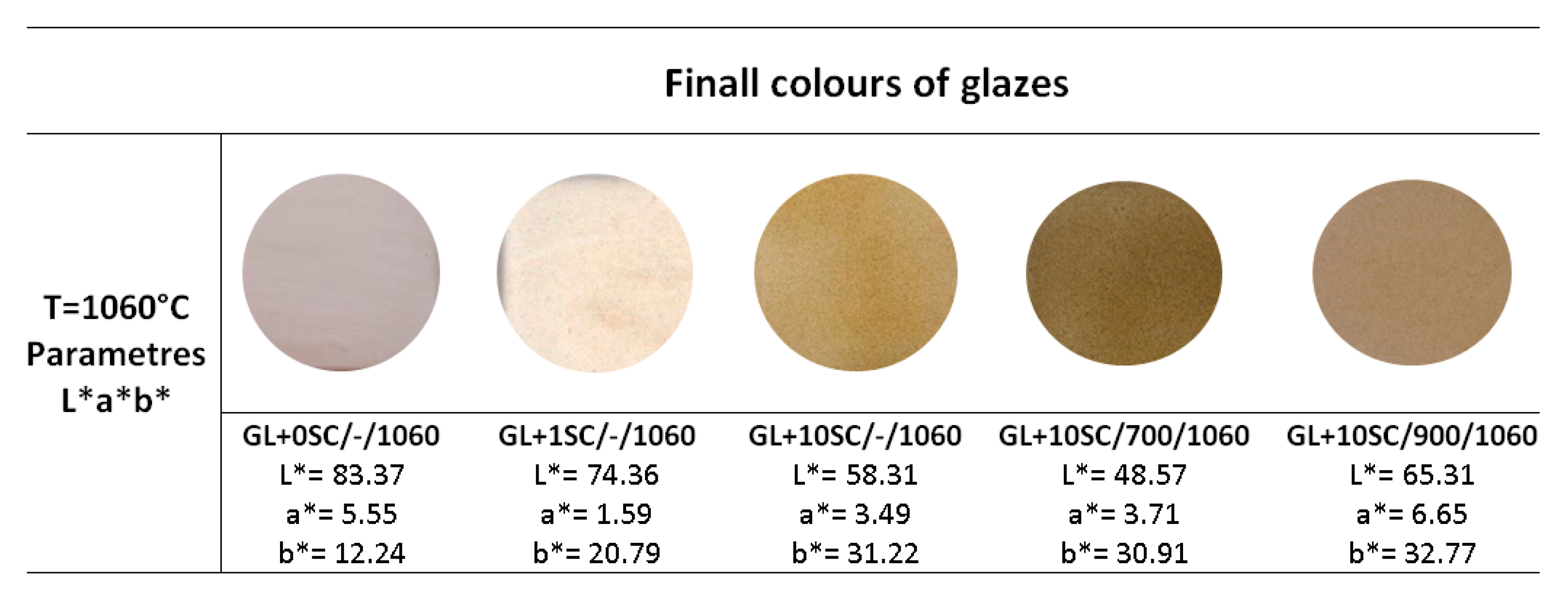
3.2. The Glazes Prepared with the Original Scale and Scale Fired at 700 and 900 °C
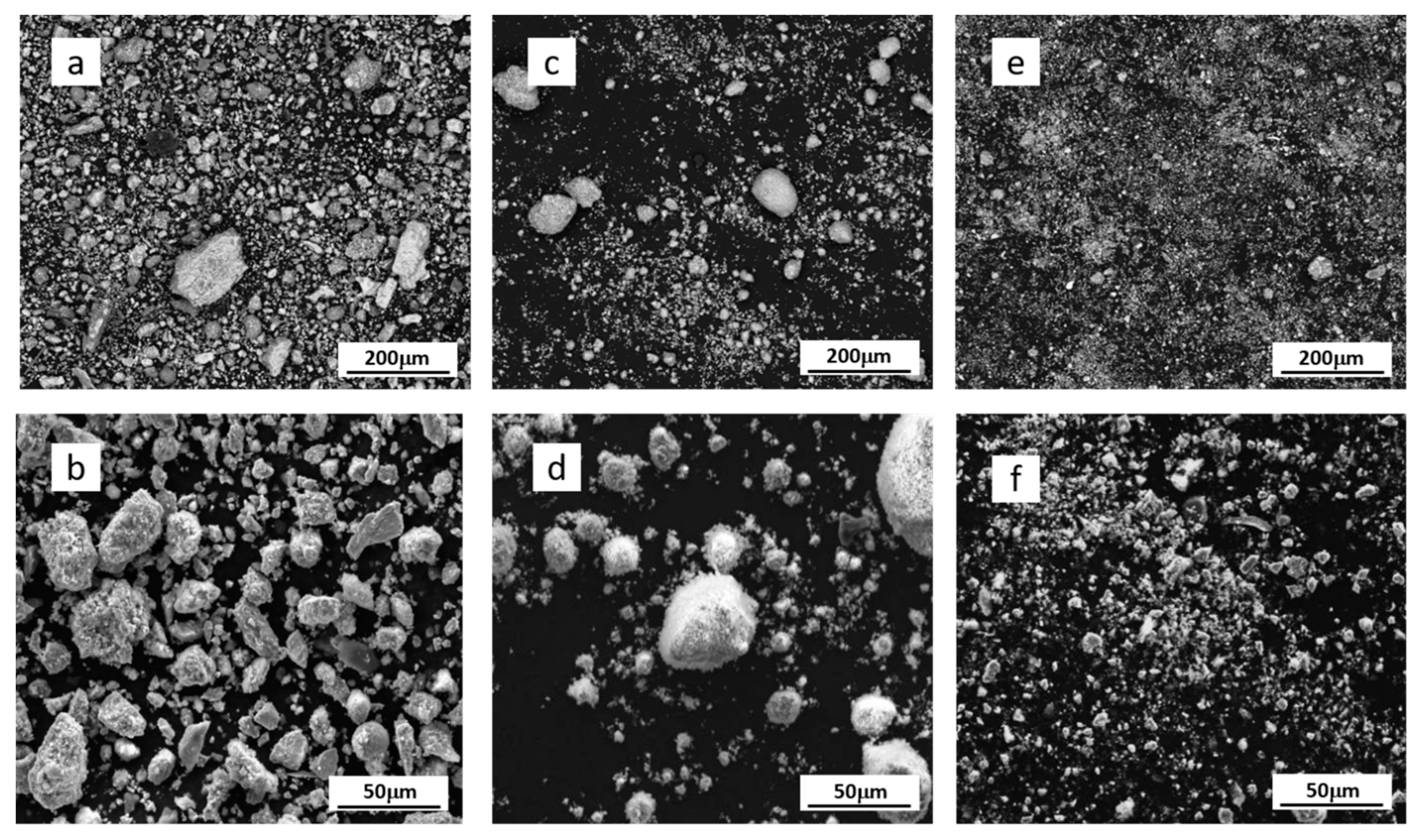
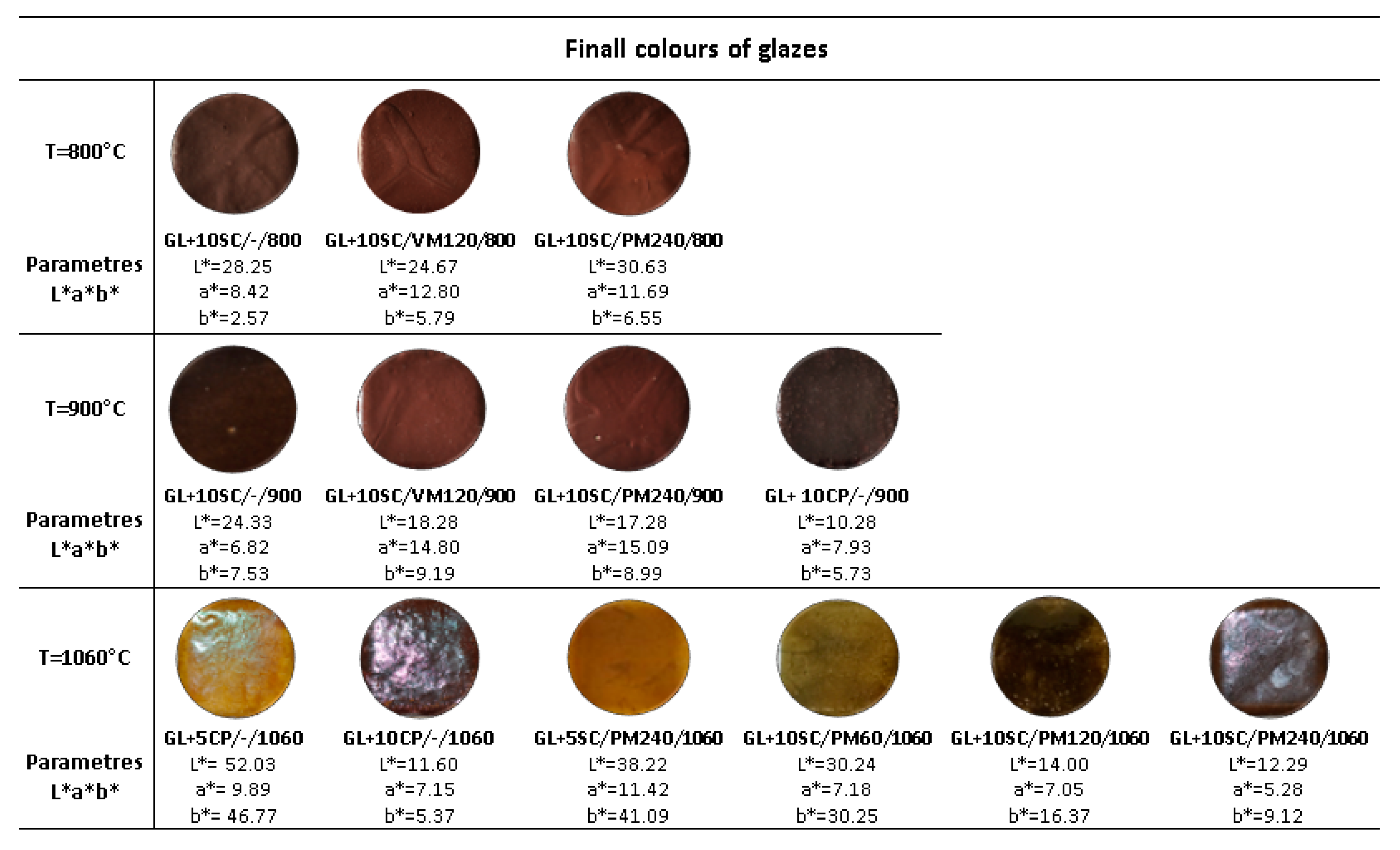
3.3. The Glazes Prepared with Grinded Scale
4. Conclusions
- Mill scale pre-calcinated at different temperatures (700 and 900 °C) changes its original black color to brown, because of the phase transformation of Fe3O4 to Fe2O3.
- The glaze with thermally treated scale applied using spraying method and subsequently fired at 1060 °C did not show any visible defect.
- Different amount of thermally treated scale (1 and 10 wt.%) added to the commercial glaze had an influence on the glaze saturation.
- Glazes with mechanically treated scale applied by dipping:
- Milling for 60, 120 or 240 min reduced the size of the scale particles and changed the morphology of the particles.
- Different firing temperature (800, 900 and 1060 °C) of glaze containing the mechanically treated scale formed the glazes with various colors—dark red, brown color, yellow-brown.
- Firing of the glazes at the temperatures 800 and 900 °C led to the formation of defects (pinholing and crazing) on the surface of glaze, and the surface exhibited the matt character.
- Glazes prepared at the temperature 1060 °C were compact, without any evident defects and the surface had a glossy character.
- The glazes prepared with milled scale fired at 1060 °C reached similar quality as the glazes prepared with the commercial pigment.
Author Contributions
Funding
Conflicts of Interest
References
- Ma, N.; Houser, J.B.; Wood, L.A. Production of cleaner mill scale by dynamic separation of the mill scale from the fast-moving flume water at a hot rolling mill. J. Clean. Prod. 2018, 176, 889–894. [Google Scholar] [CrossRef]
- World Steel Association. Available online: https://www.worldsteel.org/media-centre/press-releases/2019/Global-crude-steel-output-increases-by-4.6--in-2018.html (accessed on 7 January 2020).
- Paswan, D.N.; Malathi, M.; Minj, R.K.; Bandopadhyay, D. Mill Scale: A Potential Raw Material for Iron and Steel Making. Steelworld 2015, 21, 54–56. [Google Scholar]
- Anand, R.S.; Kumar, P.; Paswan, D.N. Conversion of Sponge Iron from Low Grade Iron ore and Mill Scale through Simulating Tunnel Kiln Condition. J. Mech. Civ. Eng. 2016, 13, 49–54. [Google Scholar] [CrossRef]
- Tominaga, J.; Wakimoto, K.Y.; Mori, T.; Murakami, M.; Yoshimura, T. Manufacture of Wire Rods with Good Descaling Property. Trans. Iron Steel Inst. Jpn. 1982, 22, 646–656. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Classification of dye and pigments. Dyes and Pigments; Springer: Heidelberg, Germany, 2016. [Google Scholar]
- Shena, L.; Qiaoa, Y.; Tan, Y.G.J. Preparation and formation mechanism of nano-iron oxide black pigment from blast furnace flue dust. Ceram. Int. 2013, 39, 737–744. [Google Scholar] [CrossRef]
- Penniman, J.R.S.; Zoph, N.M. Process of Manufacturing Iron Compounds. U.S. Patent No. 1,327,061, 20 June 1917. [Google Scholar]
- Westerhaus, A.; Ganter, K.-W. Color Intensive Iron Oxide Black Pigments Obtained by the Nitrobenzene Reduction Process and a Process for Its Preparation. U.S. Patent No. 4,966,641, 18 June 1986. [Google Scholar]
- Martinez, I.; Garcia-Lobato, M.A.; Perry, D.L. Study of the properties of iron oxide nanostructure. In Research in Nanotechnology Developments; Nova Science Publishers: Hauppauge, NY, USA, 2009. [Google Scholar]
- Opuchovic, O.; Kareiva, A. Historical hematite pigment: Synthesis by an aqueous sol–gel method, characterization and application for the colouration of ceramic glazes. Ceram. Int. 2015, 41, 4504–4513. [Google Scholar] [CrossRef]
- Eppler, A.R. Encyclopedia of Chemical Technology; Othmer, K., Ed.; John Wiley & Son: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Monrós, G. Pigment, Ceramic, Encyclopedia of Color Science and Technology; Ronnier, L., Ed.; Springer: Berlin, Germany, 2014; ISBN1 978-1-4419-8070-0. ISBN2 978-3-642-27851-8. [Google Scholar]
- CPMA Classification and Chemical Description of the Complex Inorganic Color Pigments, 4th ed; Dry Color Manufaturers Association: Alexandria, Egypt, 2010.
- Ozel, E.; Turan, S. Production and characterisation of iron–chromium pigments and their interactions with transparent glazes. J. Eur. Ceram. Soc. 2003, 23, 2097–2104. [Google Scholar] [CrossRef]
- Escardino, A.; Mestre, S.; Barba, A.; Beltran, V.; Blasco, A. Synthesis mechanism of an iron-chromium ceramic pigments. J. Am. Ceram. Soc. 2000, 83, 29–32. [Google Scholar] [CrossRef]
- Levy, D.; Artioli, G. Thermal expansion of chromite and zinc spinels. Mat. Sci. For. 1998, 278–281, 390–395. [Google Scholar] [CrossRef]
- Selvaraj, M.; Palraj, S.; Maruthan, K.; Venkatachari, G. Physico-chemical properties of ceramic pigments for high-temperature application. Prog. Org. Coat. 2008, 62, 326–330. [Google Scholar] [CrossRef]
- Cunha, J.D.; Melo, D.M.A.; Martinalli, A.E.; Melo, M.A.F.; Miara, I.; Cunba, S.D. Ceramic pigment obtained by polymeric precursors. Dyes Pigment. 2005, 65, 11–14. [Google Scholar] [CrossRef]
- Legodi, M.A.; de Waal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dyes Pigment. 2007, 74, 161–168. [Google Scholar] [CrossRef]
- Yilmaza, S.O.; Tekerb, T. Experimental research on mechanism and process of direct iron making reduction of mechanically milling scale with coal. J. Alloy. Compd. 2015, 650, 741–747. [Google Scholar] [CrossRef]
- Linderoth, S.; Jiang, J.Z.; Morup, S. Mechanical milling of Fe3O4/SiO2: Formation of an amorphous Fe (II)-Si-O-containing phase. Mater. Sci. Forum 1997, 235, 205. [Google Scholar] [CrossRef]
- Wang, H.; He, Q.; Yao, B.; Wen, G.; Wang, F.; Xu, Y.; Li, Y. Transformation of α-Fe2O3 to Fe3O4 Realized by Mechanochemical Reaction of α-Fe2O3 and SrCO3. Metall. Mater. Trans. B 2012, 43, 1574–1578. [Google Scholar] [CrossRef]
- Can, M.M.; Ozcan, S.; Ceylan, A.; Firat, T. Effect of milling time on the synthesis of magnetite nanoparticles by wet milling. Mater. Sci. Eng. B 2010, 172, 72–75. [Google Scholar] [CrossRef]
- Schanda, J. (Ed.) Colorimetry: Understanding the CIE System; John Wiley: Hoboken, NJ, USA, 2007; ISBN 9780470049044. [Google Scholar]
- Gulrajani, M.L. (Ed.) Colour Measurement: Principles, Advances and Industrial Applications; Woodhead Publishing: Philadelphia, PA, USA, 2010; ISBN 9780081014424. [Google Scholar]
- EasyRGB. Available online: https://www.easyrgb.com (accessed on 10 January 2020).
- Reinhard, E.; Pouli, T. Colour Spaces for Colour Transfer. In International Workshop on Computational Color Imaging. CCIW 2011: Computational Color Imaging; Springer: Berlin, Germany, 2011; pp. 1–15. [Google Scholar]
- Munsell, A.H. Atlas of the Munsell Colour System, Malden, Mass; Wadsworth, Howland & Co., inc., Printers: New York, NY, USA, 1915. [Google Scholar]
- Markov, L.; Blaskov, V.; Klissurski, D.; Nikolov, S. The thermal decomposition mechanism of iron (III) hydroxide carbonate to α-Fe2O3. J. Mater. Sci. 1990, 25, 3096–3100. [Google Scholar] [CrossRef]
- Ullrich, A.; Rölle, N.; Horn, S. From wustite to hematite: Thermal transformation of differently sized iron oxide nanoparticles in air. J. Nanopart. Res. 2019, 21, 168. [Google Scholar] [CrossRef]
- Mozaffari, M.; Gheisari, M.; Niyaifar, M.; Amighian, J. Magnetic properties of mechanochemically prepared iron–wüstite (Fe–FeyO) nanocomposites. J. Magn. Magn. Mater. 2019, 321, 2981–2984. [Google Scholar] [CrossRef]



| Samples | Amount of the Materials (wt.%) | Pre-Calcination Temperature | |
|---|---|---|---|
| Transparent Glaze (GL) | Scale (SC) | ||
| GL+0SC/-/1060 | 100 | 0 | − |
| GL+1SC/-/1060 | 99 | 1 | − |
| GL+10SC/-/1060 | 90 | 10 | − |
| GL+10SC/700/1060 | 90 | 10 | 700 °C |
| GL+10SC/900/1060 | 90 | 10 | 900 °C |
| Samples | Amount of the Materials (wt.%) | Temperature of Firing (°C) | Milling Time (min) | ||||
|---|---|---|---|---|---|---|---|
| Transparent Glaze (GL) | Scale (SC) | Fepren (CP) | 800 | 900 | 1060 | ||
| GL+10SC/-/800 | 90 | 10 | x | − | − | − | |
| GL+10SC/-/900 | 90 | 10 | − | x | − | − | |
| GL+10SC/VM120/800 | 90 | 10 | x | − | − | 120 | |
| GL+10SC/VM120/900 | 90 | 10 | − | x | − | 120 | |
| GL+10SC/PM60/1060 | 90 | 10 | − | − | x | 60 | |
| GL+10SC/PM120/1060 | 90 | 10 | − | − | x | 120 | |
| GL+10SC/PM240/800 | 90 | 10 | x | − | − | 240 | |
| GL+10SC/PM240/900 | 90 | 10 | − | x | − | 240 | |
| GL+10SC/PM240/1060 | 90 | 10 | − | − | x | 240 | |
| GL+5SC/PM/240/1060 | 95 | 5 | − | − | x | 240 | |
| GL+5CP/-/1060 | 95 | − | 5 | − | − | x | − |
| GL+10CP/-/900 | 90 | − | 10 | − | x | − | − |
| GL+10CP/-/1060 | 90 | 10 | − | − | x | − | |
| Oxides | Amount of the Materials (wt.%) | ||
|---|---|---|---|
| Scale (SC) | Transparent Glaze (GL) | Ceramic Slurry | |
| Na2O | 0.42 | 5.5 | 1.22 |
| MgO | 0.78 | <0.3 | 2.83 |
| Al2O3 | 1.97 | 9.1 | 12.42 |
| SiO2 | 10.2 | 80.1 | 69.57 |
| P2O5 | 0.21 | <0.01 | <0.0012 |
| SO3 | 0.58 | 0.12 | 0.19 |
| K2O | 0.40 | 0.88 | 2.08 |
| CaO | 6.3 | 0.30 | 4.52 |
| TiO2 | 0.086 | 0.13 | 0.56 |
| MnO | 0.67 | 0.006 | 0.07 |
| Fe2O3 | 73.6 | 0.22 | 4.09 |
| BaO | − | 0.054 | 0.05 |
| Loss on ignition | 4.2 | 1.40 | 1.4 |
| Samples | Type of Device | Milling Time (min) | Granulometry µm | ||
|---|---|---|---|---|---|
| d10 | d50 | d90 | |||
| SC | − | 1.78 | 13.20 | 64.80 | |
| VM60 | vibratory disc mill | 60 | 0.85 | 4.44 | 21.60 |
| VM120 | 120 | 1.32 | 6.04 | 50.30 | |
| PM60 | planetary ball mill | 60 | 1.76 | 6.05 | 27.70 |
| PM120 | 120 | 2.07 | 6.04 | 19.40 | |
| PM240 | 240 | 1.23 | 4.69 | 15.10 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovčačíková, H.; Vlček, J.; Matějka, V.; Juřica, J.; Maierová, P.; Mlčoch, P. The Effect of Temperature and Milling Process on Steel Scale Utilized as a Pigment for Ceramic Glaze. Materials 2020, 13, 1814. https://doi.org/10.3390/ma13081814
Ovčačíková H, Vlček J, Matějka V, Juřica J, Maierová P, Mlčoch P. The Effect of Temperature and Milling Process on Steel Scale Utilized as a Pigment for Ceramic Glaze. Materials. 2020; 13(8):1814. https://doi.org/10.3390/ma13081814
Chicago/Turabian StyleOvčačíková, Hana, Jozef Vlček, Vlastimil Matějka, Jan Juřica, Petra Maierová, and Petr Mlčoch. 2020. "The Effect of Temperature and Milling Process on Steel Scale Utilized as a Pigment for Ceramic Glaze" Materials 13, no. 8: 1814. https://doi.org/10.3390/ma13081814






