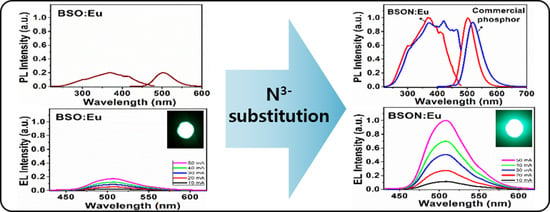
Highly Luminous Ba2SiO4−δN2/3δ:Eu2+ Phosphor for NUV-LEDs: Origin of PL-Enhancement by N3−-Substitution
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
3.1. Rietveld Refinements
3.2. Secondary Ion Mass Spectrometry: Average N3− Content of BSON:Eu2+
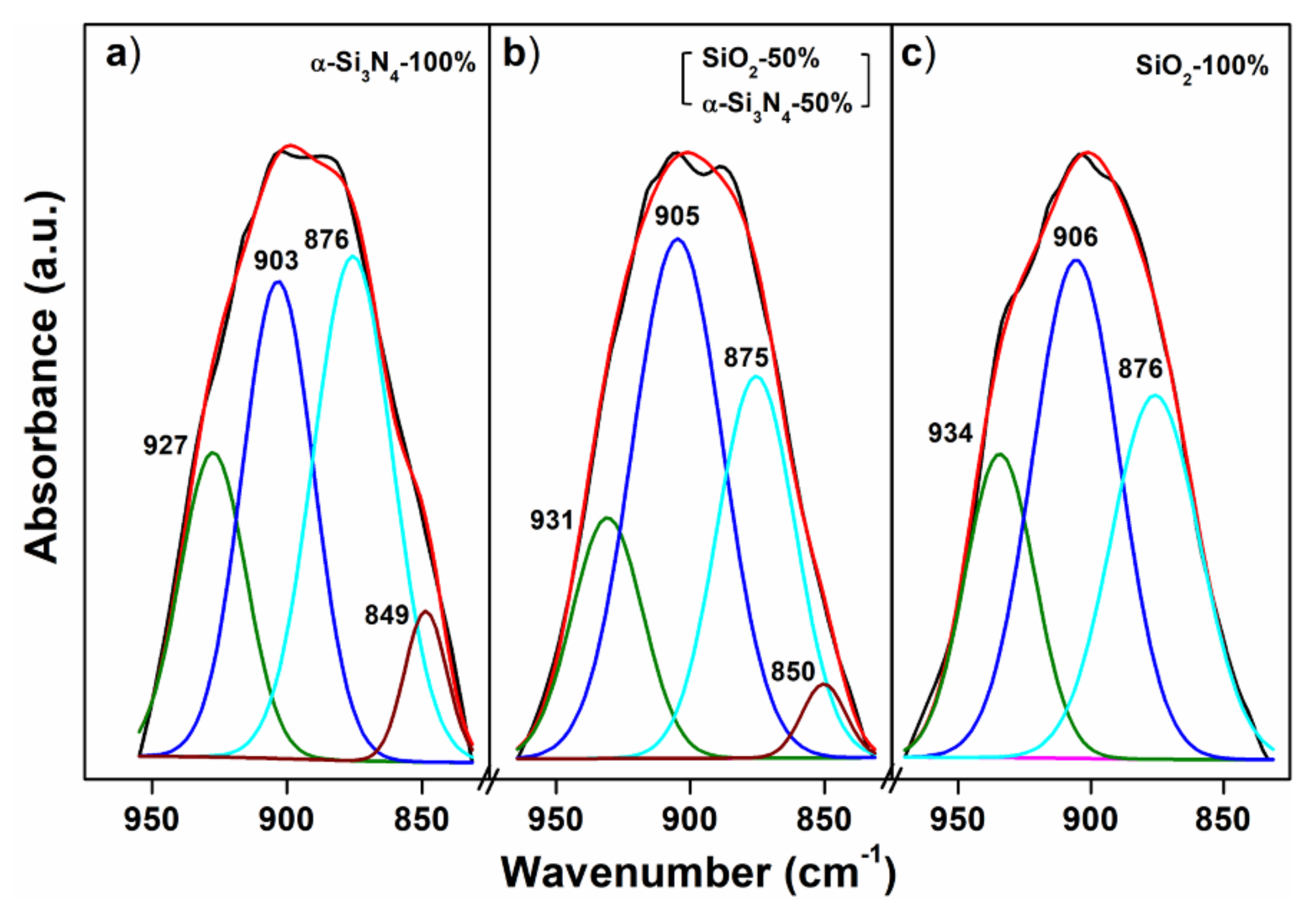
3.3. Infrared Spectroscopy: The Evidence of N3− Substitution
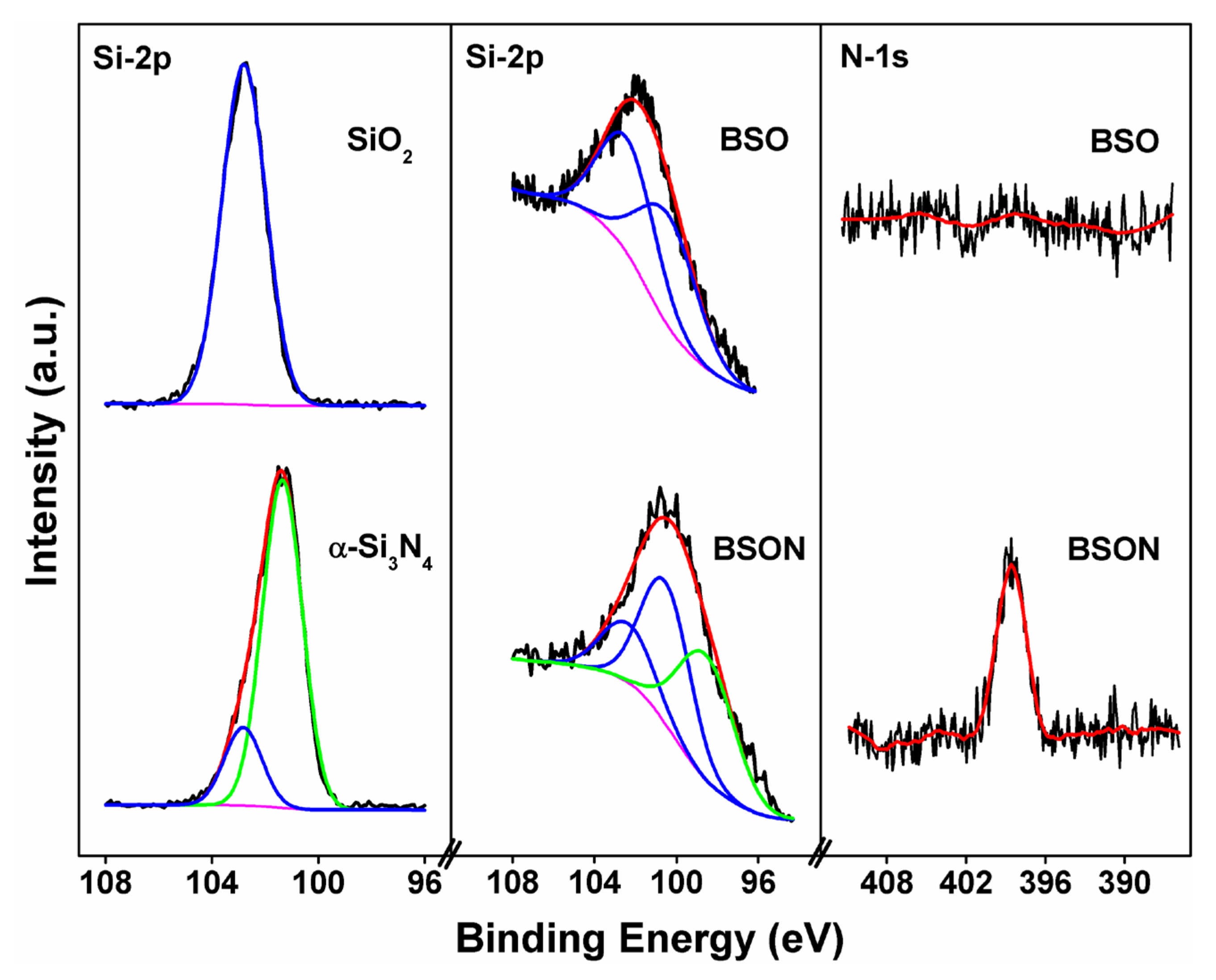
3.4. X-Ray Photoelectron Spectroscopy: The Evidence of N3− Substitution
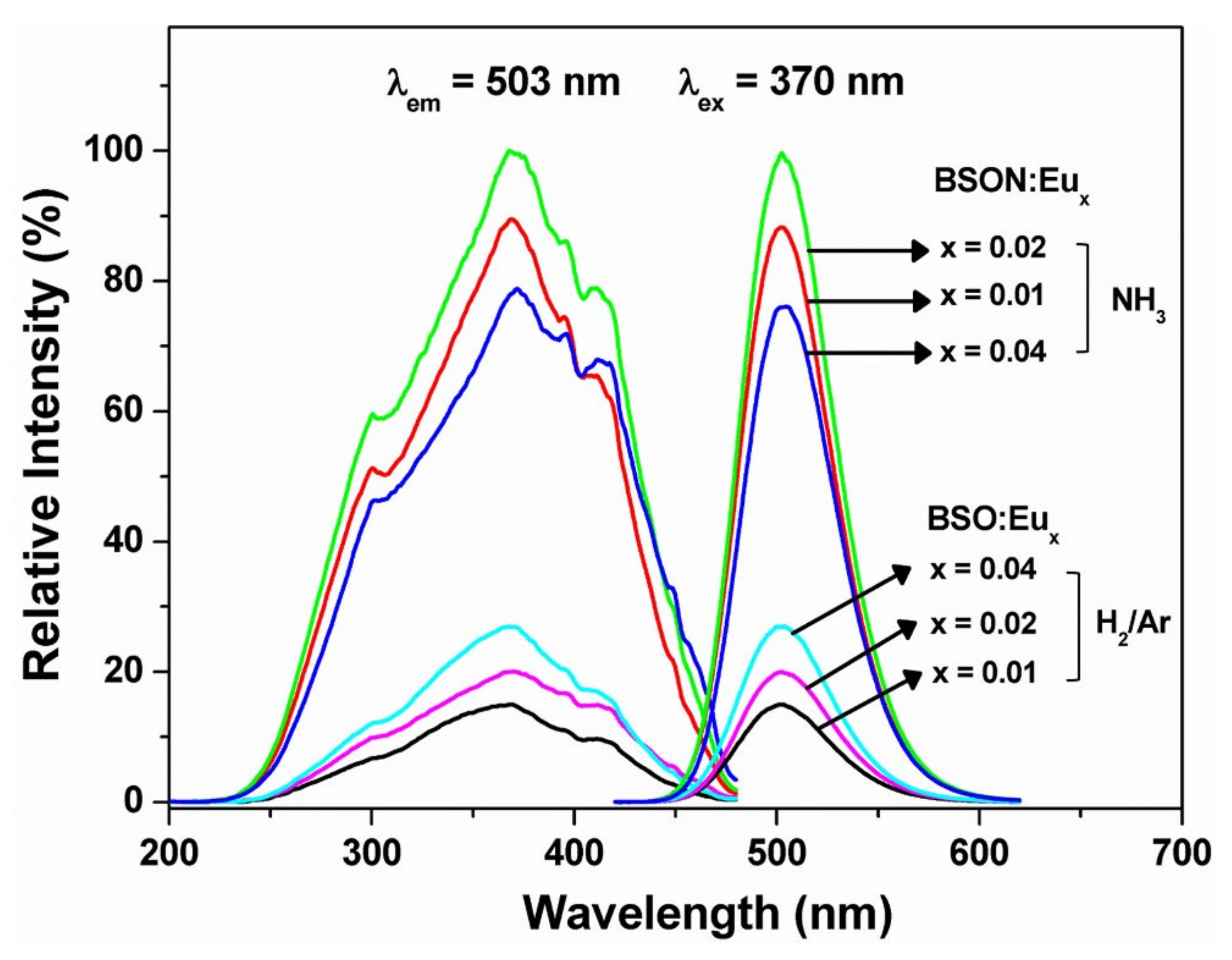
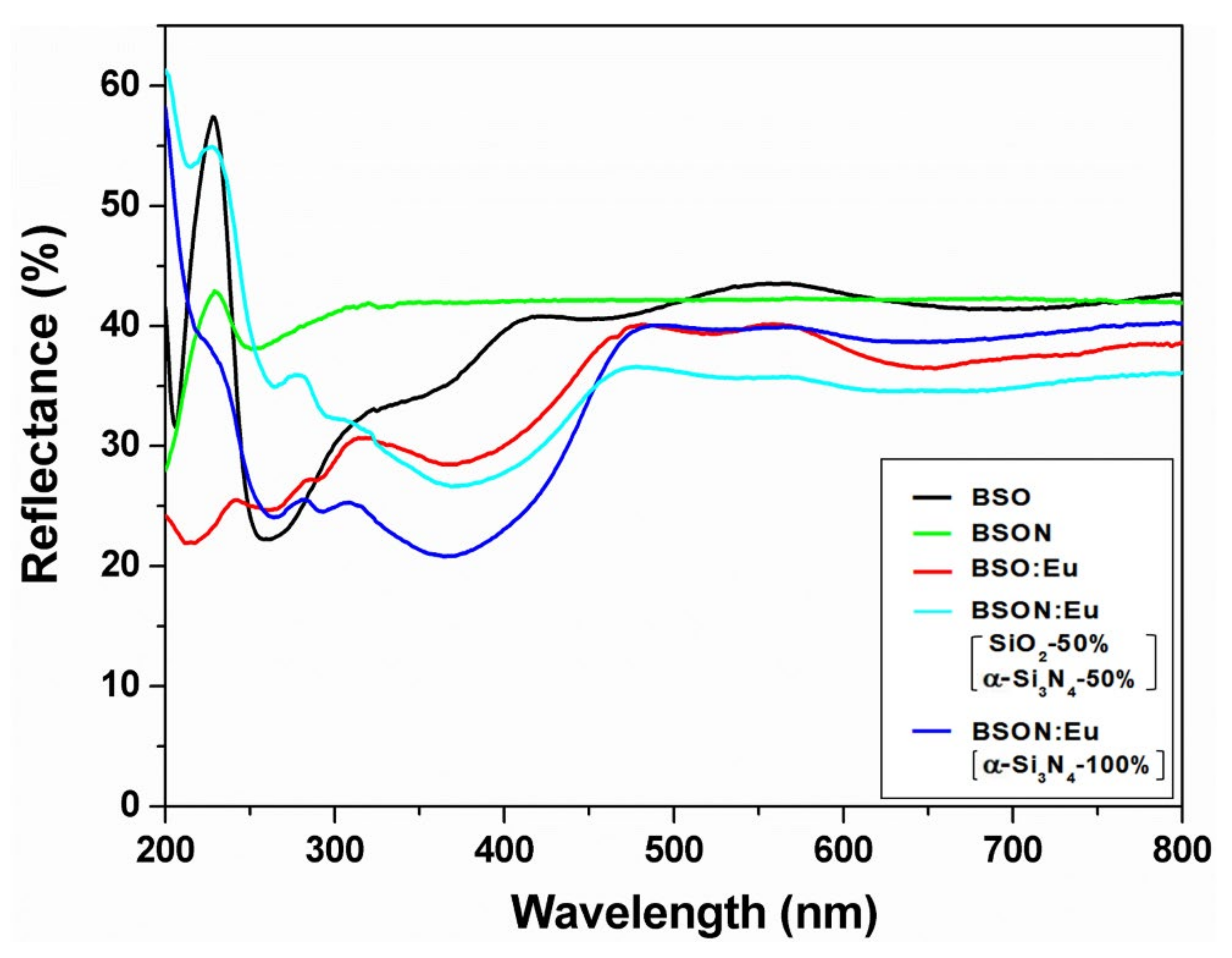
3.5. Photoluminescence Spectra
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nakamura, S.; Senoh, M.; Mukai, T. High-power InGaN/GaN double-heterostructure violet light emitting diodes. Appl. Phys. Lett. 1993, 62, 2390–2392. [Google Scholar] [CrossRef]
- Li, Y.Q.; Delsing, A.; De With, G.; Hintzen, H. Luminescence properties of Eu2+-activated alkaline-earth silicon-oxynitride MSi2O2-δN2+ 2/3δ (M = Ca, Sr, Ba): A promising class of novel LED conversion phosphors. Chem. Mater. 2005, 17, 3242–3248. [Google Scholar] [CrossRef]
- Yun, B.-G.; Miyamoto, Y.; Yamamoto, H. Luminescence Properties of (Sr1−uBau)Si2O2N2:Eu2+, Yellow or Orange Phosphors for White LEDs, Synthesized with (Sr1−uBau)2SiO4:Eu2+ as a Precursor. J. Electrochem. Soc. 2007, 154, J320–J325. [Google Scholar] [CrossRef]
- Zhang, R.; Numata, M.; Maeda, T.; Akazawa, Y.; Murai, K.-I.; Moriga, T. Preparation And Luminescence Properties Of Eu2+-Activated Ba-Six-O-N Phosphors. Int. J. Mod. Phys. B 2010, 24, 3221–3225. [Google Scholar] [CrossRef]
- Song, Y.; Choi, T.; Senthil, K.; Masaki, T.; Yoon, D. Photoluminescence properties of green-emitting Eu2+-activated Ba3Si6O12N2 oxynitride phosphor for white LED applications. Mater. Lett. 2011, 65, 3399–3401. [Google Scholar] [CrossRef]
- Fang, Y.; Li, Y.; Xie, R.; Hirosaki, N.; Takade, T.; Li, X.; Qiu, T. Structure and photoluminescence properties of Ce3+-doped novel silicon-oxynitride Ba4−zMzSi8O20−3xN2x (M= Mg, Ca, Sr). J. Solid State Chem. 2011, 184, 1405–1414. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Q.; Li, Y.; Wang, H. Simultaneous tuning for excitation and emission of N doped Sr2SiO4: Eu for white light LEDs. J. Alloys Compd. 2011, 509, L109–L112. [Google Scholar] [CrossRef]
- Chen, W.-T.; Sheu, H.-S.; Liu, R.-S.; Attfield, J.P. Cation-size-mismatch tuning of photoluminescence in oxynitride phosphors. J. Am. Chem. Soc. 2012, 134, 8022–8025. [Google Scholar] [CrossRef]
- Li, Y.; Hirosaki, N.; Xie, R.; Takeda, T.; Mitomo, M. Crystal and electronic structures, luminescence properties of Eu2+-doped Si6−zAlzOzN8−z and MySi6−zAlz−yOz+yN8−z−y (M= 2Li, Mg, Ca, Sr, Ba). J. Solid State Chem. 2008, 181, 3200–3210. [Google Scholar] [CrossRef]
- Huheey, J.E.; Keiter, E.A.; Keiter, R.L. Inorganic Chemistry. Principles of Structure and Reactivity; Harper Collins: New York, NY, USA, 1993. [Google Scholar]
- Grobe, H. Bariumorthosilicate Ba2SiO4. Cryst. Struct. Commun. 1974, 3, 599–602. [Google Scholar]
- Walker, A.V. Why is SIMS underused in chemical and biological analysis? Challenges and opportunities. Anal. Chem. 2008, 80, 8865–8870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seki, S.; Tamura, H.; Sumiya, H. Quantitative SIMS analysis of nitrogen using in situ internal implantation. Appl. Surf. Sci. 1999, 147, 14–18. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, K.-W.; Jin, J.S.; Kang, S.-G.; Seo, D.-K.; Park, J.-C. Remarkable flux effect of Li-codoping on highly enhanced luminescence of orthosilicate Ba2SiO4:Eu2+ phosphors for NUV-LEDs: Autonomous impurity purification by eutectic Li2CO3 melts. RSC Adv. 2015, 5, 105339–105346. [Google Scholar] [CrossRef]
- Iyi, N.; Matsumoto, T.; Kaneko, Y.; Kitamura, K. Deintercalation of carbonate ions from a hydrotalcite-like compound: Enhanced decarbonation using acid−salt mixed solution. Chem. Mater. 2004, 16, 2926–2932. [Google Scholar] [CrossRef]
- Vaysse, C.; Guerlou-Demourgues, L.; Delmas, C. Thermal evolution of carbonate pillared layered hydroxides with (Ni, L) (L = Fe, Co) based slabs: Grafting or nongrafting of carbonate anions? Inorg. Chem. 2002, 41, 6905–6913. [Google Scholar] [CrossRef]
- Cumberland, S.; Strouse, G. Analysis of the nature of oxyanion adsorption on gold nanomaterial surfaces. Langmuir 2002, 18, 269–276. [Google Scholar] [CrossRef]
- Zheng, J.; Song, X.; Li, X.; Pu, Y. Large-scale production of amorphous silicon oxynitride nanowires by nickel-catalyzed transformation of silicon wafers in NH3 plasma. J. Phys. Chem. C 2008, 112, 27–34. [Google Scholar] [CrossRef]
- Zhu, H.L.; Han, F.D.; Bi, J.Q.; Bai, Y.J.; Qi, Y.X.; Pang, L.L.; Wang, C.G.; Li, S.J.; Lu, C.W. Facile synthesis of Si3N4 nanocrystals via an organic–inorganic reaction route. J. Am. Ceram. Soc. 2009, 92, 535–538. [Google Scholar] [CrossRef]
- Wang, K.; Günthner, M.; Motz, G.N.; Flinn, B.D.; Bordia, R.K. Control of surface energy of silicon oxynitride films. Langmuir 2013, 29, 2889–2896. [Google Scholar] [CrossRef]
- Scott, J.; Porto, S. Longitudinal and transverse optical lattice vibrations in quartz. Phys. Rev. 1967, 161, 903. [Google Scholar] [CrossRef]
- Etchepare, J.; Merian, M.; Smetankine, L. Vibrational normal modes of SiO2. I. α and β quartz. J. Chem. Phys. 1974, 60, 1873–1876. [Google Scholar] [CrossRef]
- Ocaña, M.; Fornes, V.; García-Ramos, J.V.; Serna, C. Polarization effects in the infrared spectra of α-quartz and α-cristobalite. Phys. Chem. Miner. 1987, 14, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Shluger, A. On the nonequivalency of Si-O bonds in silicon dioxide. J. Phys. Chem. Solids 1986, 47, 659–664. [Google Scholar] [CrossRef]
- Matsunaga, K.; Iwamoto, Y. Molecular dynamics study of atomic structure and diffusion behavior in amorphous silicon nitride containing boron. J. Am. Ceram. Soc. 2001, 84, 2213–2219. [Google Scholar] [CrossRef]
- Luo, Y.R. Comprehensive Handbook of Chemical Bond Energies; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kim, D.; Park, D.; Oh, N.; Kim, J.; Jeong, E.D.; Kim, S.-J.; Kim, S.; Park, J.-C. Luminescent properties of rare earth fully activated apatites, LiRE9(SiO4)6O2 (RE = Ce, Eu, and Tb): Site selective crystal field effect. Inorg. Chem. 2015, 54, 1325–1336. [Google Scholar] [CrossRef]
- Handke, M.; Urban, M. IR and Raman spectra of alkaline earth metals orthosilicates. J. Mol. Struct. 1982, 79, 353–356. [Google Scholar] [CrossRef]
- Pires, A.M.; Davolos, M.R. Luminescence of europium (III) and manganese (II) in barium and zinc orthosilicate. Chem. Mater. 2001, 13, 21–27. [Google Scholar] [CrossRef]
- Wang, F.; Liu, G.; Rothwell, S.; Nevius, M.; Tejeda, A.; Taleb-Ibrahimi, A.; Feldman, L.C.; Cohen, P.I.; Conrad, E. Wide-gap semiconducting graphene from nitrogen-seeded SiC. Nano Lett. 2013, 10, 4827–4832. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.-Q.; Lu, Q.-Y.; Tang, K.-B.; Qian, Y.-T.; Zhou, G.-E.; Liu, X.-M.; Wu, J.-X. A New Rapid Reduction− Carbonization Route to Nanocrystalline β-SiC. Chem. Mater. 1999, 11, 2369–2371. [Google Scholar] [CrossRef]
- Hong, T.E.; Jung, J.-H.; Yeo, S.; Cheon, T.; Bae, S.I.; Kim, S.-H.; Yeo, S.J.; Kim, H.-S.; Chung, T.-M.; Park, B.K. Highly Conformal Amorphous W–Si–N Thin Films by Plasma-Enhanced Atomic Layer Deposition as a Diffusion Barrier for Cu Metallization. J. Phys. Chem. C 2015, 119, 1548–1556. [Google Scholar] [CrossRef]
- Lamagna, L.; Wiemer, C.; Perego, M.; Spiga, S.; Rodriguez, J.; Santiago Coll, D.; Grillo, M.E.; Klejna, S.; Elliott, S.D. Mechanisms for substrate-enhanced growth during the early stages of atomic layer deposition of alumina onto silicon nitride surfaces. Chem. Mater. 2012, 24, 1080–1090. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J. Redox deposition of birnessite-type manganese oxide on silicon carbide microspheres for use as supercapacitor electrodes. ACS Appl. Mater. Interfaces 2014, 6, 9036–9045. [Google Scholar] [CrossRef]
- Tshabalala, M.; Dejene, F.; Pitale, S.S.; Swart, H.; Ntwaeaborwa, O. Generation of white-light from Dy3+ doped Sr2SiO4 phosphor. Phys. B Condens. Matter 2014, 439, 126–129. [Google Scholar] [CrossRef]
- Bender, S.; Franke, R.; Hartmann, E.; Lansmann, V.; Jansen, M.; Hormes, J. X-ray absorption and photoemission electron spectroscopic investigation of crystalline and amorphous barium silicates. J. Non-Cryst. Solids 2002, 298, 99–108. [Google Scholar] [CrossRef]
- Zhang, S.; Nakai, Y.; Tsuboi, T.; Huang, Y.; Seo, H.J. Luminescence and microstructural features of Eu-activated LiBaPO4 phosphor. Chem. Mater. 2011, 23, 1216–1224. [Google Scholar] [CrossRef]
- Im, W.B.; Kim, Y.-I.; Yoo, H.S.; Jeon, D.Y. Luminescent and Structural Properties of (Sr1−x,Bax)3MgSi2O8: Eu2+: Effects of Ba Content on the Eu2+ Site Preference for Thermal Stability. Inorg. Chem. 2008, 48, 557–564. [Google Scholar] [CrossRef]
- Inoue, K.; Hirosaki, N.; Xie, R.-J.; Takeda, T. Highly efficient and thermally stable blue-emitting AlN:Eu2+ phosphor for ultraviolet white light-emitting diodes. J. Phys. Chem. C 2009, 113, 9392–9397. [Google Scholar] [CrossRef]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta Metal. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Feng, I.-W.; Li, J.; Sedhain, A.; Lin, J.; Jiang, H.; Zavada, J. Enhancing erbium emission by strain engineering in GaN heteroepitaxial layers. Appl. Phys. Lett. 2010, 96, 031908. [Google Scholar] [CrossRef]
- Singh, L.; Singh, N.; Singh, T. Enhancement of Luminescence Intensity in Dy3+ Ions Doped YVO4 Nanomaterials by Ba2+ Ion Codoping and YVO4:2Dy/Fe3O4 Nanohybrid for Hyperthermia Application. J. Nanomed. Nanotechnol. 2017, 8, 1000445. [Google Scholar]
- Yang, Q.; Wang, W.; Xu, S.; Wang, Z.L. Enhancing light emission of ZnO microwire-based diodes by piezo-phototronic effect. Nano Lett. 2011, 11, 4012–4017. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.; Hao, Z.; Ren, X.; Luo, Y.; Wang, X.; Zhang, J. Enhanced phosphorescence in N contained Ba2SiO4:Eu2+ for X-ray and cathode ray tubes. Opt. Mater. 2010, 32, 1042–1045. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Huang, C.-H.; Lee, T.-J.; Liu, W.-R.; Yeh, Y.-T.; Jang, S.-M.; Liu, R.-S. Eu2+-activated silicon-oxynitride Ca3Si2O4N2: A green-emitting phosphor for white LEDs. Opt. Express 2011, 19, A331–A339. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Bae, J.-S.; Hong, T.E.; Hui, K.N.; Kim, S.; Kim, C.H.; Park, J.-C. Color-Tunable and Highly Luminous N3–-Doped Ba2–xCaxSiO4−δN2/3δ:Eu2+ (0.0≤ x≤ 1.0) Phosphors for White NUV-LED. ACS Appl. Mater. Interfaces 2016, 8, 17371–17381. [Google Scholar] [CrossRef]
- Lv, W.; Jiao, M.; Zhao, Q.; Shao, B.; Lü, W.; You, H. Ba1.3Ca0. 7SiO4:Eu2+,Mn2+: A promising single-phase, color-tunable phosphor for near-ultraviolet white-light-emitting diodes. Inorg. Chem. 2014, 53, 11007–11014. [Google Scholar] [CrossRef]

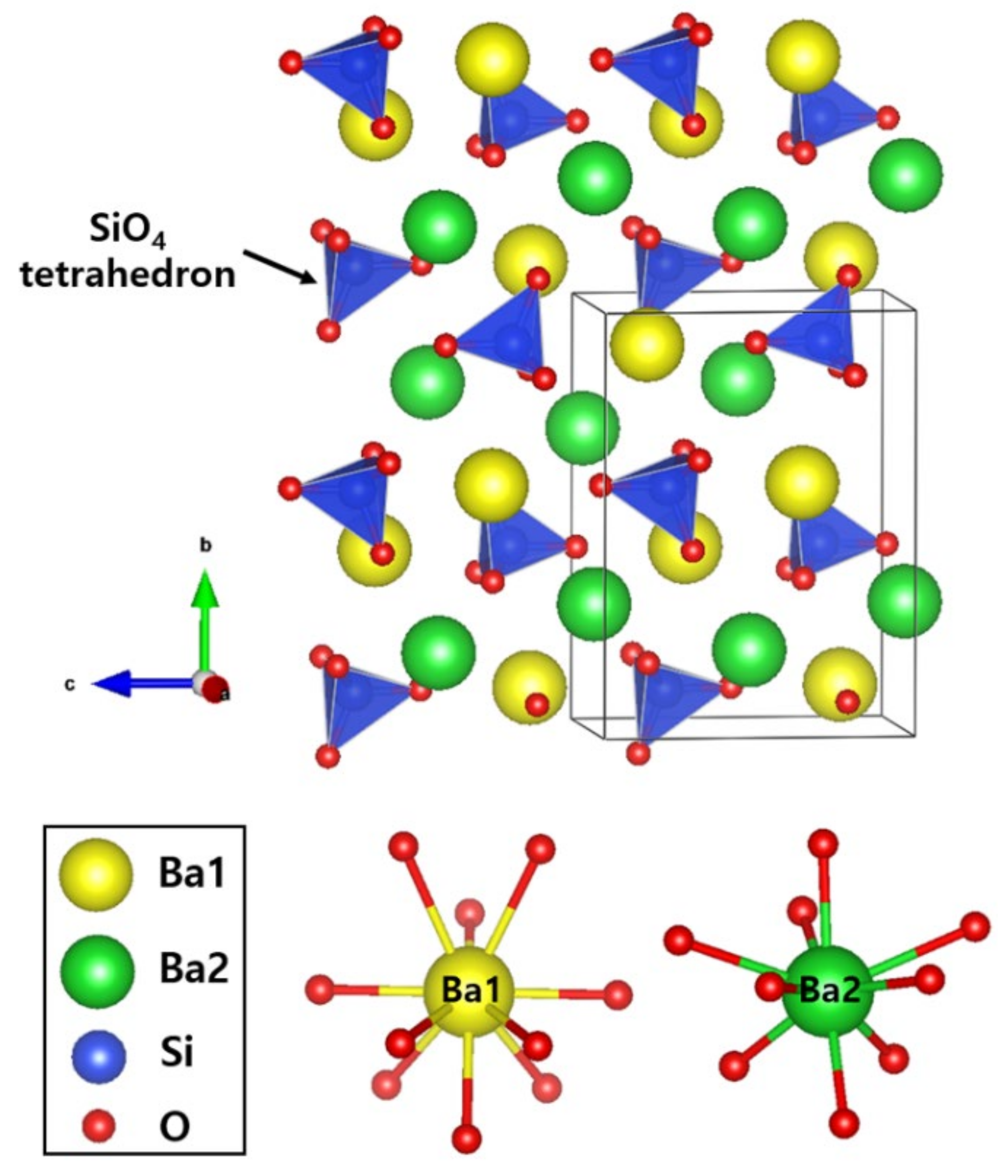
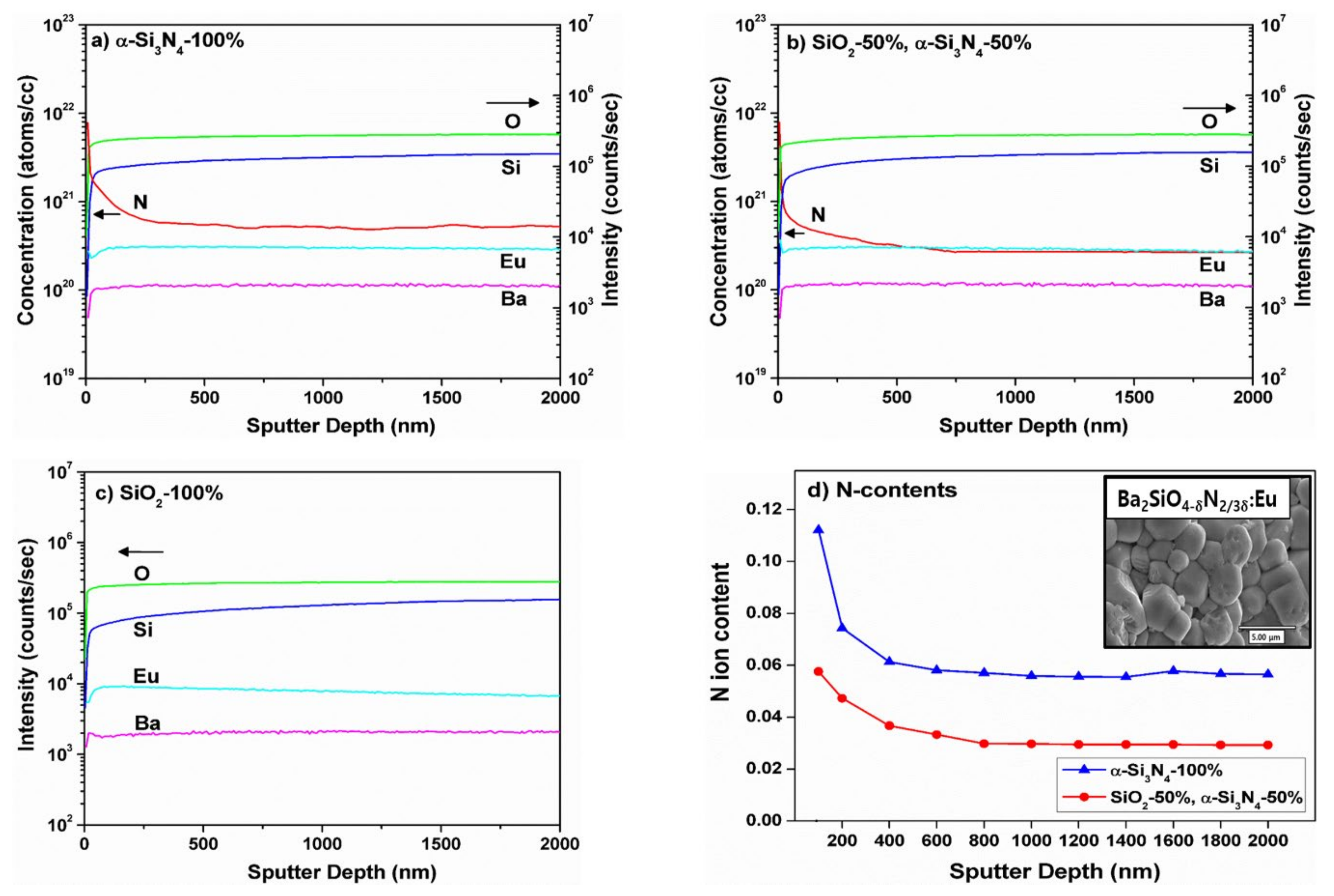
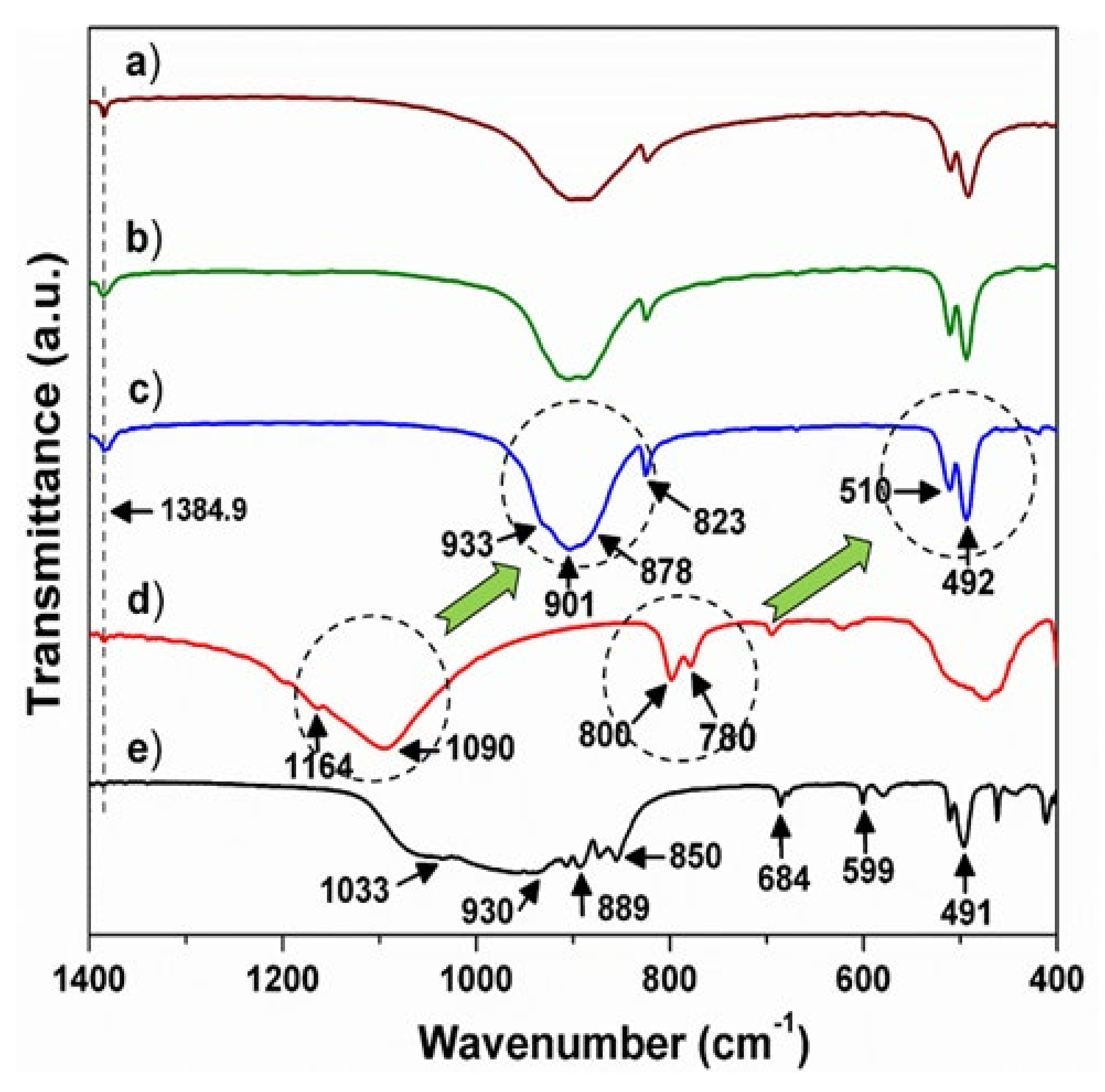


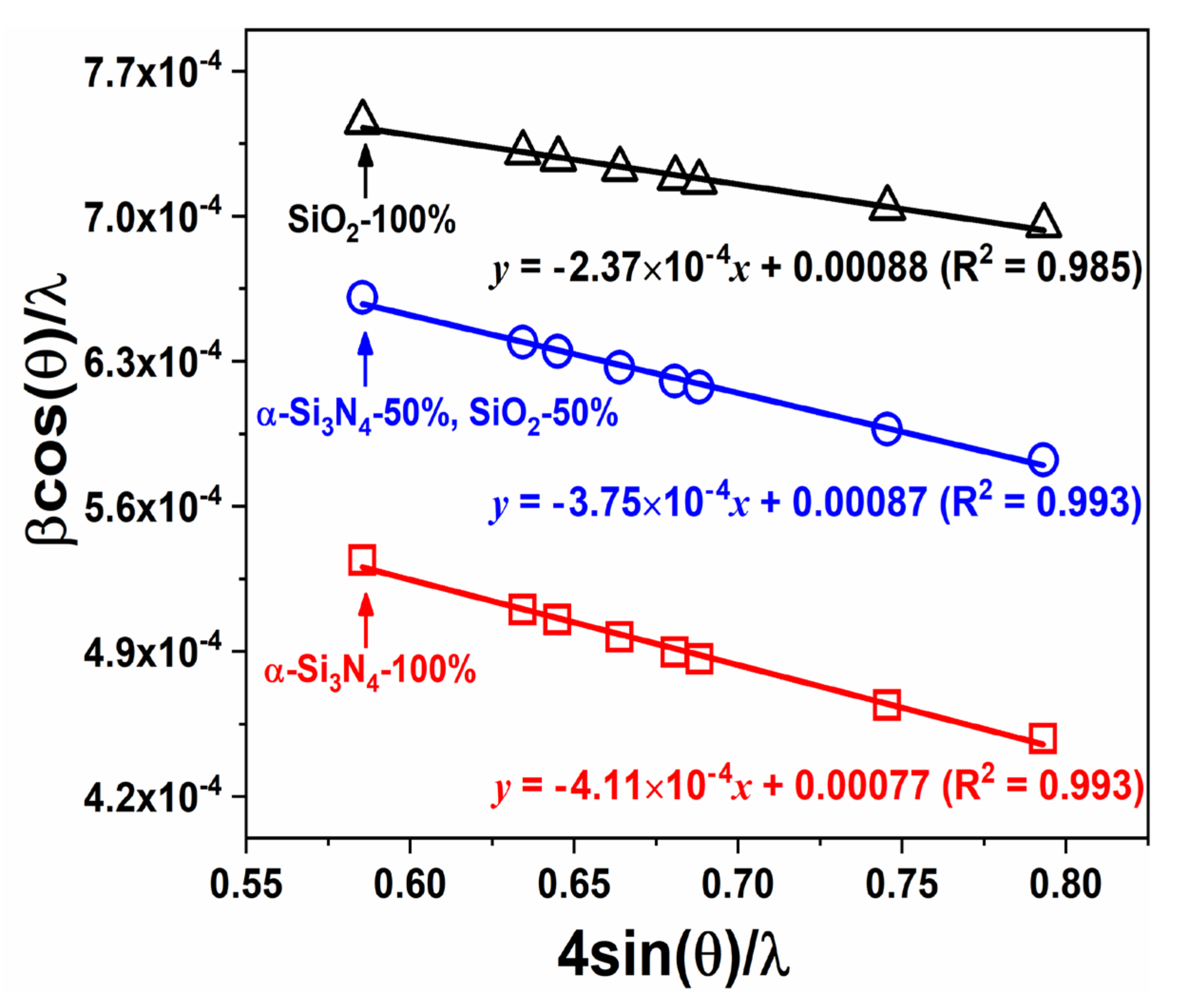

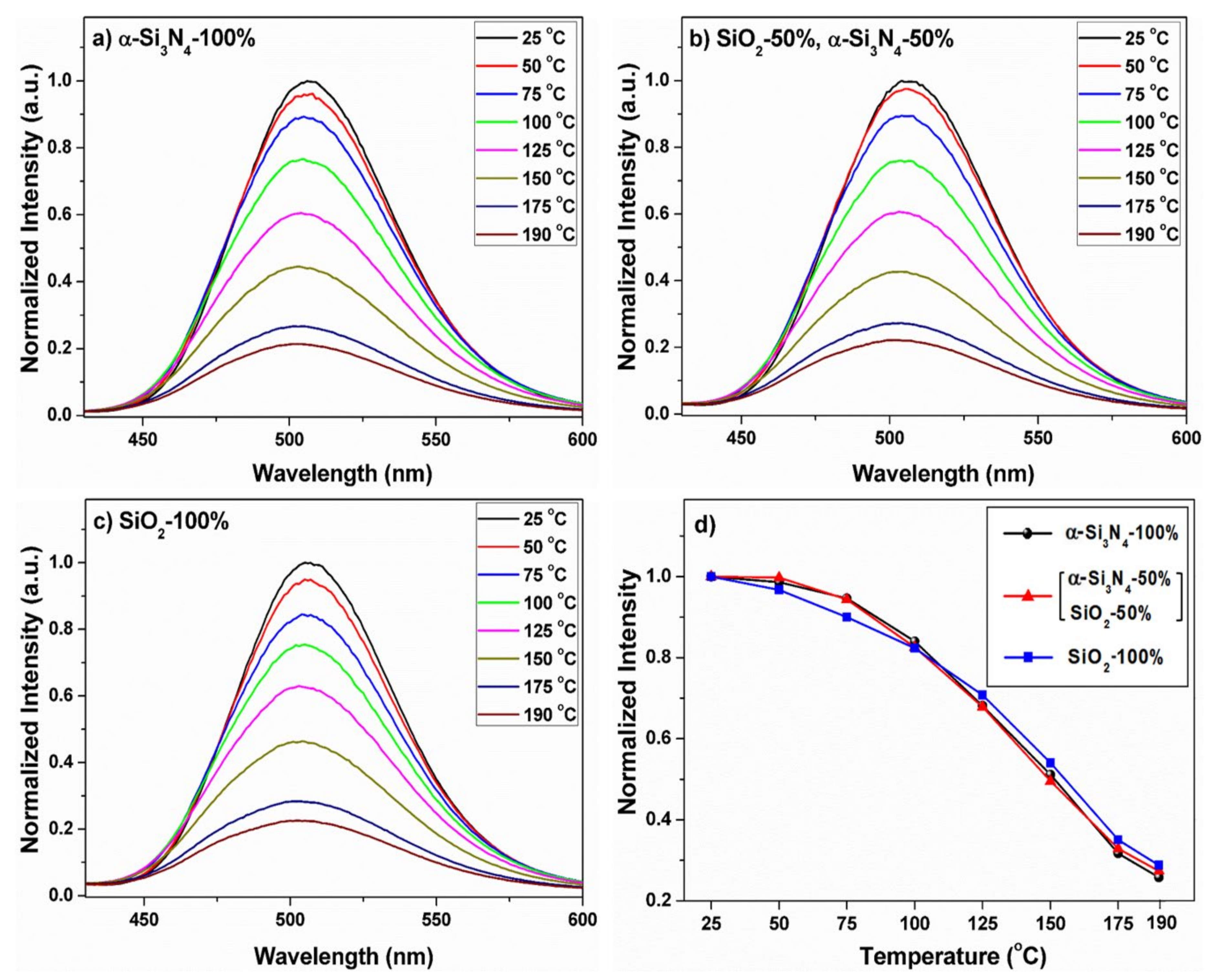
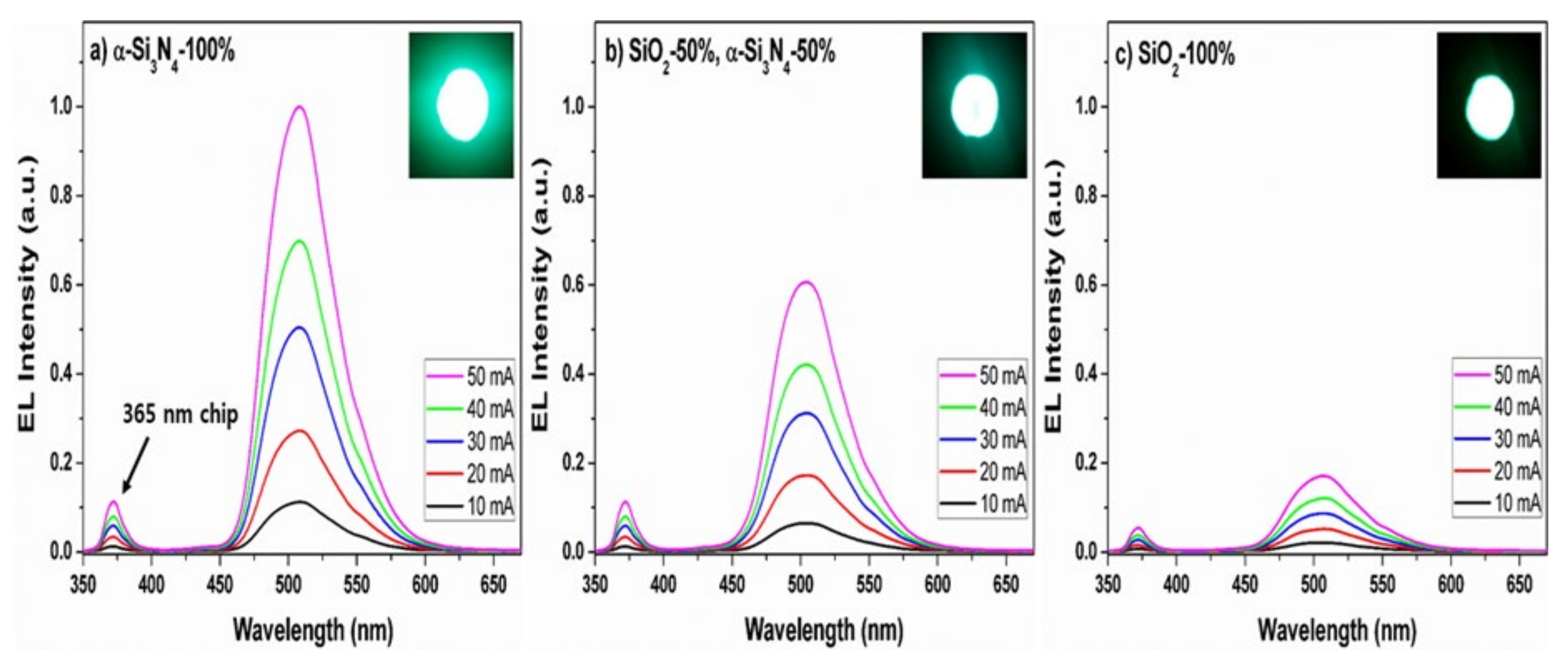
| Compound | BSO:Eu | BSON:Eu |
|---|---|---|
| Space Group | Pmcn | Pmcn |
| Crystal System | Orthorhombic | Orthorhombic |
| Z | 4 | 4 |
| a(Å) | 5.809(6) | 5.806(7) |
| b(Å) | 10.204(1) | 10.198(2) |
| c(Å) | 7.500(6) | 7.497(1)) |
| V(Å3) | 444.65(1) | 443.96(2) |
| RBragg (%) | 5.33 | 9.37 |
| Rwp (%) | 10.6 | 13.9 |
| Rexp (%) | 6.07 | 6.85 |
| χ2 (Rwp2/Rexp2) | 3.07 | 4.13 |
| Compound | BSO:Eu | BSON:Eu |
|---|---|---|
| Ba1–O1 | 2.591 | 2.542 |
| Ba1–O2 (× 2) | 2.918 | 2.922 |
| Ba1–O3 (× 2) | 2.941 | 2.921 |
| Ba1–O3 (× 2) | 3.009 | 3.051 |
| Ba1–O2 | 3.101 | 3.132 |
| Ba1–O3 (× 2) | 3.160 | 3.121 |
| Average | 2.975 | 2.970 |
| Ba2–O3 (× 2) | 2.658 | 2.621 |
| Ba2–O2 | 2.681 | 2.662 |
| Ba2–O2 | 2.781 | 2.752 |
| Ba2–O3 (× 2) | 2.810 | 2.761 |
| Ba2–O1 | 2.831 | 2.881 |
| Ba2–O1 (× 2) | 3.129 | 3.115 |
| Average | 2.832 | 2.810 |
| Si–O3 (× 2) | 1.636 | 1.681 |
| Si–O1 | 1.681 | 1.752 |
| Si–O2 | 1.682 | 1.642 |
| Average | 1.659 | 1.689 |
| O1–Si–O2 | 111.1 | 112.2 |
| O1–Si–O3 (× 2) | 112.1 | 109.1 |
| O2–Si–O3 (× 2) | 107.2 | 109.1 |
| O3–Si–O3 (× 2) | 106.8 | 109.1 |
| Compound | Maximum IPL Ratio (%) 1 | Maximum ILED-PL Ratio (%) 1, 50 mA | Quantum Yield (%) |
|---|---|---|---|
| BSO: | 19 | 21 | 21 |
| BSON: | 100 | 100 | 71 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kim, T.H.; Hong, T.E.; Bae, J.-S.; Kim, C.H.; Kim, J.; Kim, S.-J.; Jeon, K.-W.; Park, J.-C. Highly Luminous Ba2SiO4−δN2/3δ:Eu2+ Phosphor for NUV-LEDs: Origin of PL-Enhancement by N3−-Substitution. Materials 2020, 13, 1859. https://doi.org/10.3390/ma13081859
Kim D, Kim TH, Hong TE, Bae J-S, Kim CH, Kim J, Kim S-J, Jeon K-W, Park J-C. Highly Luminous Ba2SiO4−δN2/3δ:Eu2+ Phosphor for NUV-LEDs: Origin of PL-Enhancement by N3−-Substitution. Materials. 2020; 13(8):1859. https://doi.org/10.3390/ma13081859
Chicago/Turabian StyleKim, Donghyeon, Tae Hun Kim, Tae Eun Hong, Jong-Seong Bae, Chang Hae Kim, Jaegyeom Kim, Seung-Joo Kim, Ki-Wan Jeon, and Jung-Chul Park. 2020. "Highly Luminous Ba2SiO4−δN2/3δ:Eu2+ Phosphor for NUV-LEDs: Origin of PL-Enhancement by N3−-Substitution" Materials 13, no. 8: 1859. https://doi.org/10.3390/ma13081859
APA StyleKim, D., Kim, T. H., Hong, T. E., Bae, J. -S., Kim, C. H., Kim, J., Kim, S. -J., Jeon, K. -W., & Park, J. -C. (2020). Highly Luminous Ba2SiO4−δN2/3δ:Eu2+ Phosphor for NUV-LEDs: Origin of PL-Enhancement by N3−-Substitution. Materials, 13(8), 1859. https://doi.org/10.3390/ma13081859