NiO Grained-Flowers and Nanoparticles for Ethanol Sensing
Abstract
:1. Introduction
2. Materials and Methods
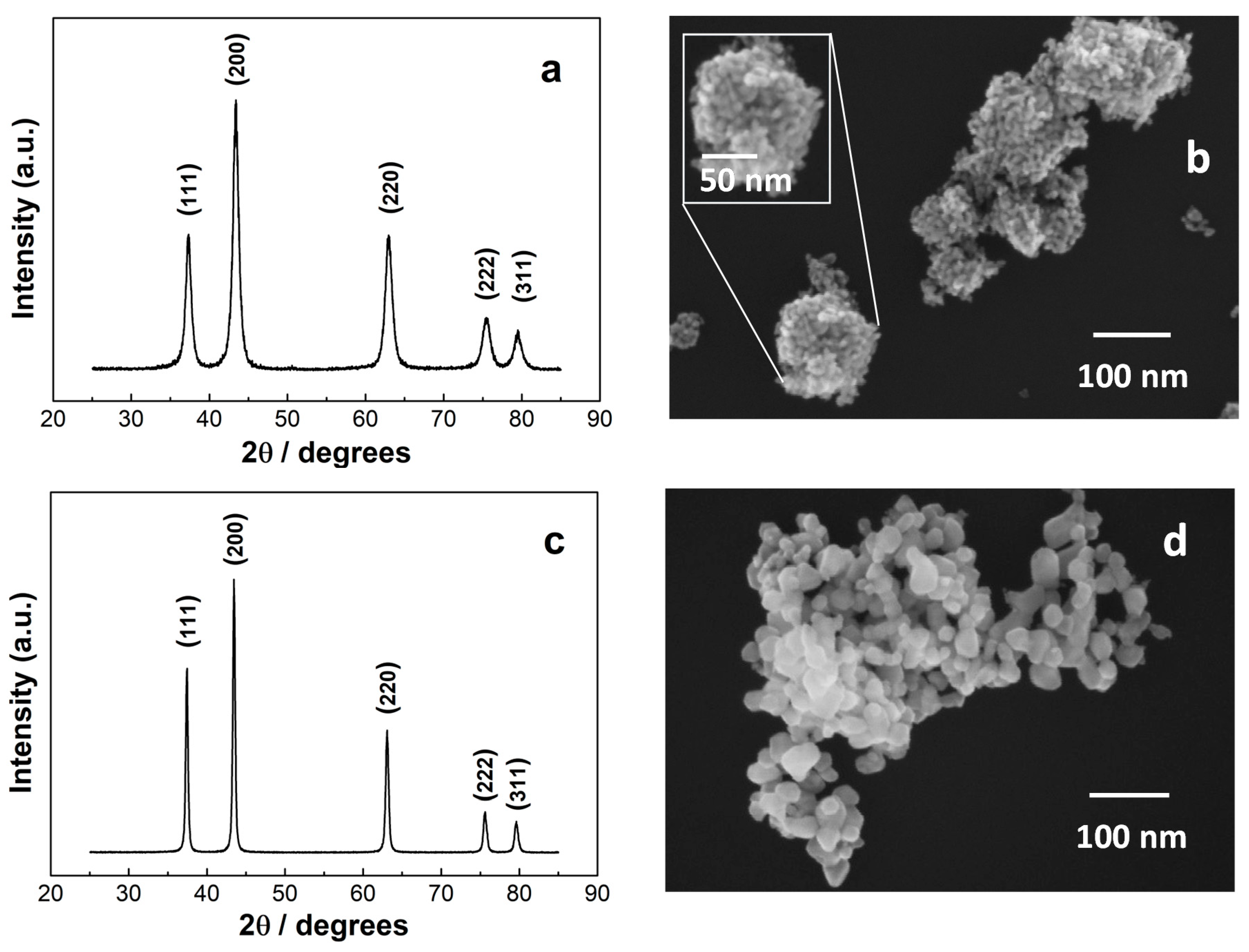
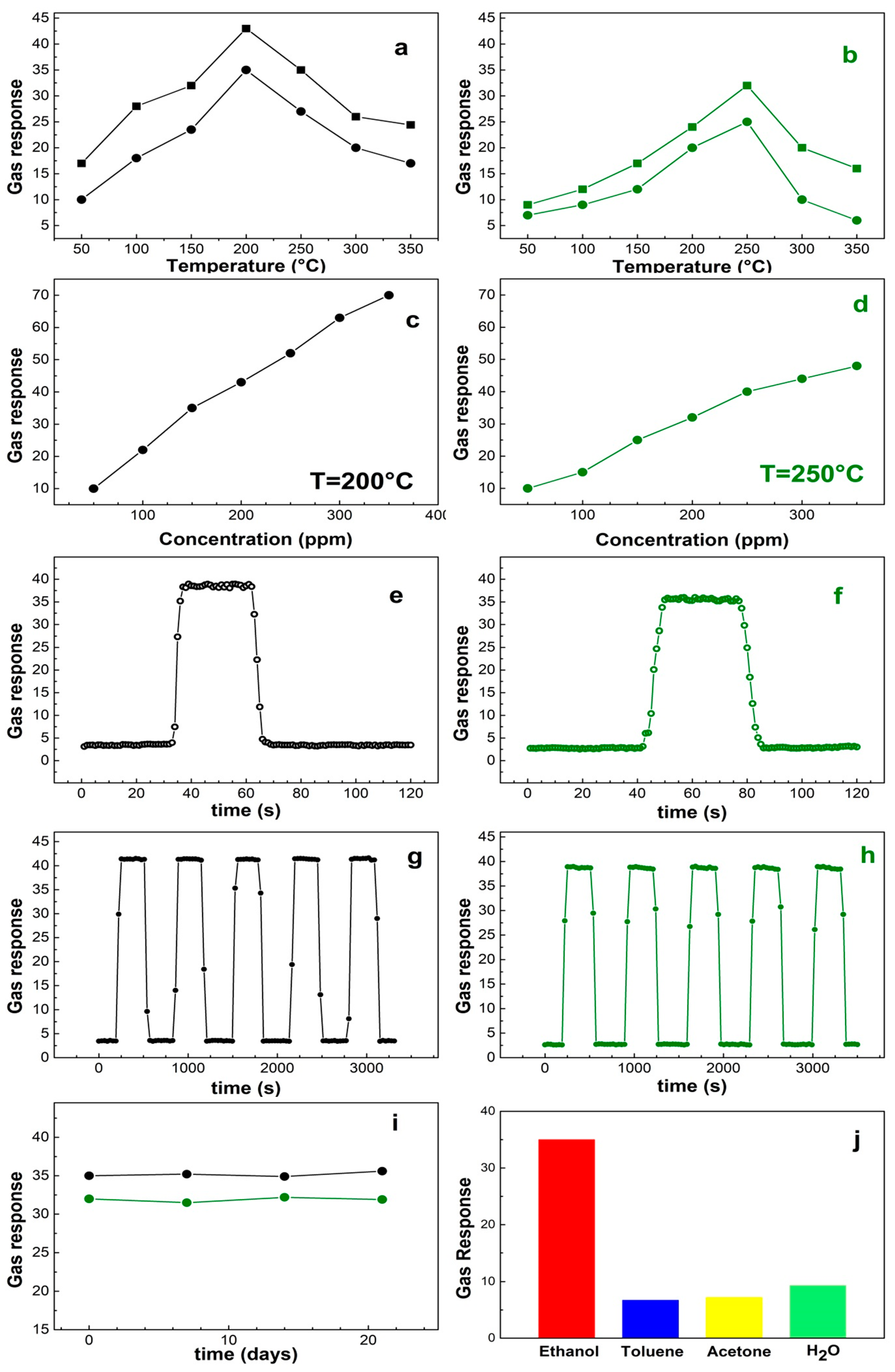
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tomer, V.K.; Duhan, S. Ordered mesoporous Ag-doped TiO2/SnO2 nanocomposite based highly sensitive and selective VOC sensors. J. Mater. Chem. A 2016, 4, 1033–1043. [Google Scholar] [CrossRef]
- Santra, S.; Guha, P.; Ali, S.; Hiralal, P.; Unalan, H.; Covington, J.A.; Amaratunga, G.; Milne, W.; Gardner, J.; Udrea, F. ZnO nanowires grown on SOI CMOS substrate for ethanol sensing. Sens. Actuators B Chem. 2010, 146, 559–565. [Google Scholar] [CrossRef]
- Valentini, F.; Roscioli, D.; Carbone, M.; Conte, V.; Floris, B.; Palleschi, G.; Flammini, R.; Bauer, E.M.; Nasillo, G.; Caponetti, E. Oxidized Graphene in Ionic Liquids for Assembling Chemically Modified Electrodes: A Structural and Electrochemical Characterization Study. Anal. Chem. 2012, 84, 5823–5831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sau, S.; Chakraborty, S.; Das, T.; Pal, M. Ethanol Sensing Properties of Nanocrystalline α-MoO3. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Boroujerdi, R.; Abdelkader, A.; Paul, R. State of the Art in Alcohol Sensing with 2D Materials. Nano-Micro Lett. 2020, 12, 33. [Google Scholar] [CrossRef] [Green Version]
- Valentini, F.; Carbone, M.; Palleschi, G. Carbon nanostructured materials for applications in nano-medicine, cultural heritage, and electrochemical biosensors. Anal. Bioanal. Chem. 2013, 405, 451–465. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Liu, H.; Zhao, W.; Ming, A.; Wei, F. Preparation of porous Co3O4 and its response to ethanol with low energy consumption. RCS Adv. 2020, 1, 2191–2197. [Google Scholar] [CrossRef] [Green Version]
- Valentini, F.; Carbone, M.; Palleschi, G. Graphene oxide nanoribbons (GNO), reduced graphene nanoribbons (GNR), and multi-layers of oxidized graphene functionalized with ionic liquids (GO-IL) for assembly of miniaturized electrochemical devices. Anal. Bioanal. Chem. 2013, 405, 3449–3474. [Google Scholar] [CrossRef]
- Kekec, N.C.; Ekiz, F.; Udum, Y.A.; Hizliates, C.G.; Ergun, Y.; Toppare, L. A novel conducting polymer based platform for ethanol sensing. Sens. Actuators B Chem. 2014, 193, 306–314. [Google Scholar] [CrossRef]
- Cinti, S.; Basso, M.; Moscone, D.; Arduini, F. A paper-based nanomodified electrochemical biosensor for ethanol detection in beers. Anal. Chim. Acta 2017, 960, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, R.; Carbone, M.; Panero, S.; Sadun, C. Conductivity and Structure of Poly(ethylene glycol) Complexes Using Energy Dispersive X-ray Diffraction. J. Phys. Chem. B 1999, 103, 10348–10355. [Google Scholar] [CrossRef]
- Valentini, F.; Roscioli, D.; Carbone, M.; Conte, V.; Floris, B.; Bauer, E.M.; DiTaranto, N.; Sabbatini, L.; Caponetti, E.; Martino, D.F.C. Graphene and ionic liquids new gel paste electrodes for caffeic acid quantification. Sens. Actuators B Chem. 2015, 212, 248–255. [Google Scholar] [CrossRef]
- Erfkamp, J.; Guenther, M.; Gerlach, G. Hydrogel-Based Sensors for Ethanol Detection in Alcoholic Beverages. Sensors 2019, 19, 1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.; Edler, K.J.; Bowen, C.; Mintova, S.; Burrows, A. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Liu, H.; Zhang, Q. Preparation of NiO nanoflakes under different calcination temperatures and their supercapacitive and optical properties. Appl. Surf. Sci. 2017, 392, 1097–1106. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [Green Version]
- Carbone, M.; Missori, M.; Micheli, L.; Tagliatesta, P.; Bauer, E.M. NiO Pseudocapacitance and Optical Properties: Does the Shape Win? Materials 2020, 13, 1417. [Google Scholar] [CrossRef] [Green Version]
- Carbone, M.; Nesticò, A.; Bellucci, N.; Micheli, L.; Palleschi, G. Enhanced performances of sensors based on screen printed electrodes modified with nanosized NiO particles. Electrochimica Acta 2017, 246, 580–587. [Google Scholar] [CrossRef]
- Gao, Q.; Zeng, W.; Miao, R. Synthesis of multifarious hierarchical flower-like NiO and their gas-sensing properties. J. Mater. Sci.-Mater. El. 2016, 27, 9410–9416. [Google Scholar] [CrossRef]
- Miao, B.; Zeng, W.; Lin, L.; Xu, S. Characterization and gas-sensing properties of NiO nanowires prepared through hydrothermal method. Phys. E: Low-dimensional Syst. Nanostructures 2013, 52, 40–45. [Google Scholar] [CrossRef]
- Lin, L.; Liu, T.; Miao, B.; Zeng, W. Synthesis of NiO nanostructures from 1D to 3D and researches of their gas-sensing properties. Mater. Res. Bull. 2013, 48, 449–454. [Google Scholar] [CrossRef]
- Miao, R.; Zeng, W. Hydrothermal synthesis of flake-flower NiO architectures: Structure, growth and gas-sensing properties. Mater. Lett. 2016, 171, 200–203. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Q.; Lu, Z.; Wei, Z.; Zeng, W. The novel 2D honeycomb-like NiO nanoplates assembled by nanosheet arrays with excellent gas sensing performance. Mater. Lett. 2019, 255, 126523. [Google Scholar]
- Zhao, C.; Fu, J.; Zhang, Z.; Xie, E. Enhanced ethanol sensing performance of porous ultrathin NiO nanosheets with neck-connected networks. RSC Adv. 2013, 3, 4018–4023. [Google Scholar] [CrossRef]
- Carbone, M. Cu Zn Co nanosized mixed oxides prepared from hydroxycarbonate precursors. J. Alloy. Compd. 2016, 688, 202–209. [Google Scholar] [CrossRef]
- Carbone, M. Zn defective ZnCo2O4 nanorods as high capacity anode for lithium ion batteries. J. Electroanal. Chem. 2018, 815, 151–157. [Google Scholar] [CrossRef]
- Carbone, M.; Bauer, E.M.; Micheli, L.; Missori, M. NiO morphology dependent optical and electrochemical properties. Colloids Surfaces A: Physicochem. Eng. Asp. 2017, 532, 178–182. [Google Scholar] [CrossRef]
- Kim, S.-I.; Lee, J.-S.; Ahn, H.-J.; Song, H.-K.; Jang, J.-H. Facile Route to an Efficient NiO Supercapacitor with a Three-Dimensional Nanonetwork Morphology. ACS Appl. Mater. Interfaces 2013, 5, 1596–1603. [Google Scholar] [CrossRef]
- Nakate, U.; Lee, G.H.; Ahmad, R.; Patil, P.; Bhopate, D.; Hahn, Y.; Yu, Y.; Suh, E.-K. Hydrothermal synthesis of p-type nanocrystalline NiO nanoplates for high response and low concentration hydrogen gas sensor application. Ceram. Int. 2018, 44, 15721–15729. [Google Scholar] [CrossRef]
- Hoa, N.D.; Van Tong, P.; Hung, C.M.; Van Duy, N.; Van Hieu, N. Urea mediated synthesis of Ni(OH)2 nanowires and their conversion into NiO nanostructure for hydrogen gas-sensing application. Int. J. Hydrogen Energy 2018, 43, 9446–9453. [Google Scholar] [CrossRef]
- Srikesh, G.; Samson Nesaraj, A. Synthesis and Characterization of Phase Pure NiO Nanoparticles via the Combustion Route using Different Organic Fuels for Electrochemical Capacitor Applications. J. Electrochem. Sci. Technol. 2015, 6, 16–25. [Google Scholar] [CrossRef]
- Querejeta-Fernández, A.; Parras, M.; Varela, A.; Del Monte, F.; García-Hernández, M.; González-Calbet, J.M. Urea-Melt Assisted Synthesis of Ni/NiO Nanoparticles Exhibiting Structural Disorder and Exchange Bias. Chem. Mater. 2010, 22, 6529–6541. [Google Scholar] [CrossRef]
- The Joint Committee on Powder Diffraction Standards, ASTM File No. 14-117.
- The Joint Committee on Powder Diffraction Standards, ASTM File No. 04-0835.
- Wales, D.J.; Parker, R.M.; Gates, J.C.; Grossel, M.C.; Smith, P.G.R. An investigation into relative humidity measurement using analuminosilicate sol–gel thin film as the active layer in an integratedoptical Bragg grating refractometer. Sens. Actuators B 2013, 188, 857–866. [Google Scholar] [CrossRef]
- Azim-Araghi, M.E.; Krier, A. Thin film (ClAlPc) phthalocyanine gas sensors. Sel. Top. Adv. Solid State Fibre Opt. Sens. 2000, 11, 215–243. [Google Scholar]
- Wang, H.; Li, Q.; Zheng, X.; Wang, C.; Ma, J.; Yan, B.; Du, Z.; Li, M.; Wang, W.; Fan, H. 3D porous flower-like ZnO microstructures loaded by large-size Ag and their ultrahigh sensitivity to ethanol. J. Alloys Compd. 2020, 829, 154453. [Google Scholar] [CrossRef]
- Duan, W.J.; Lu, S.H.; Wu, Z.L.; Wang, Y. Size Effects on Properties of NiO Nanoparticles Grown in Alkalisalts. J. Phys. Chem. C 2012, 116, 26043–26051. [Google Scholar] [CrossRef]

| Sample | Surface Area (m2g−1) |
|---|---|
| Grained-flowers | 240.3 |
| Nanoparticles | 170.1 |
| Sample | Ethanol (ppm) | Operating Temperature | Rg/Ra | Response/Recovery Time (s) | Ref. |
|---|---|---|---|---|---|
| Grained-flower | 150 | 200 | 35 | 3/6 | This work |
| Nanoparticles | 200 | 250 | 32 | 4/8 | This work |
| UT-Nanosheet | 200 | 200 | 3.1 | N/A | [24] |
| Flake-flower | 400 | 300 | 32 | 4/8 | [23] |
| Nanoblocks | 500 | 300 | 1.1 | 5/5 | [20] |
| Nanorods | 500 | 300 | 1.4 | 4/5 | [20] |
| Nanowires | 500 | 300 | 3.4 | 5/6 | [20] |
| Nanosheet | 50 | 350 | 1.8 | 6/9 | [21] |
| Nanobulks | 50 | 350 | 1.5 | 5/7 | [21] |
| Nanospheres | 50 | 350 | 2.5 | 8/5 | [21] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, M.; Tagliatesta, P. NiO Grained-Flowers and Nanoparticles for Ethanol Sensing. Materials 2020, 13, 1880. https://doi.org/10.3390/ma13081880
Carbone M, Tagliatesta P. NiO Grained-Flowers and Nanoparticles for Ethanol Sensing. Materials. 2020; 13(8):1880. https://doi.org/10.3390/ma13081880
Chicago/Turabian StyleCarbone, Marilena, and Pietro Tagliatesta. 2020. "NiO Grained-Flowers and Nanoparticles for Ethanol Sensing" Materials 13, no. 8: 1880. https://doi.org/10.3390/ma13081880
APA StyleCarbone, M., & Tagliatesta, P. (2020). NiO Grained-Flowers and Nanoparticles for Ethanol Sensing. Materials, 13(8), 1880. https://doi.org/10.3390/ma13081880






