Compatibilization Effect of Ionic Liquid-Based Surfactants on Physicochemical Properties of PBS/Rice Starch Blends: An Initial Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PBS/RS Blends
2.3. Characterization of PBS/RS Blends
2.3.1. Mechanical Characterization
2.3.2. Chemical Characterization
2.3.3. Thermal Characterization
3. Results and Discussion
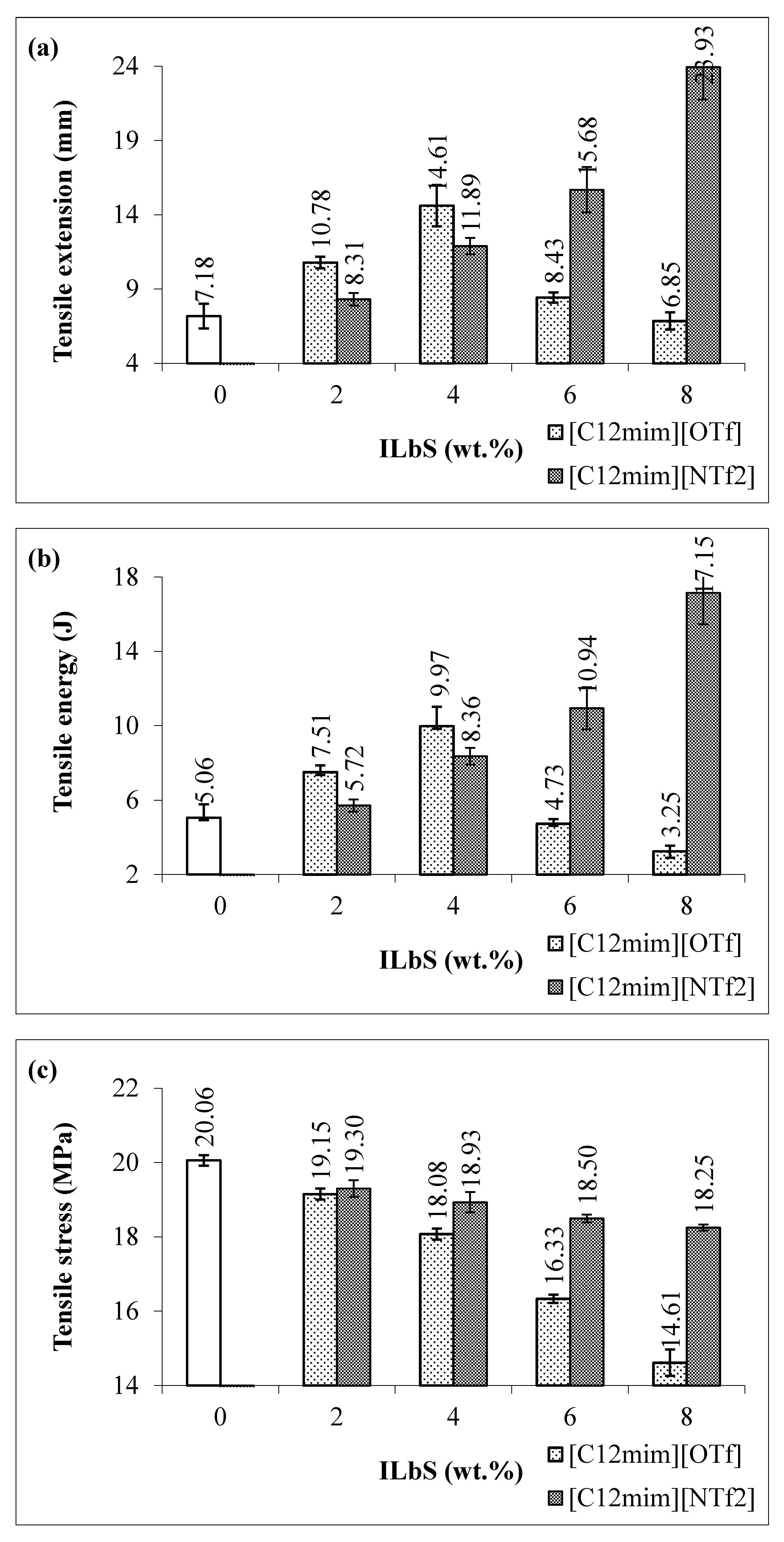
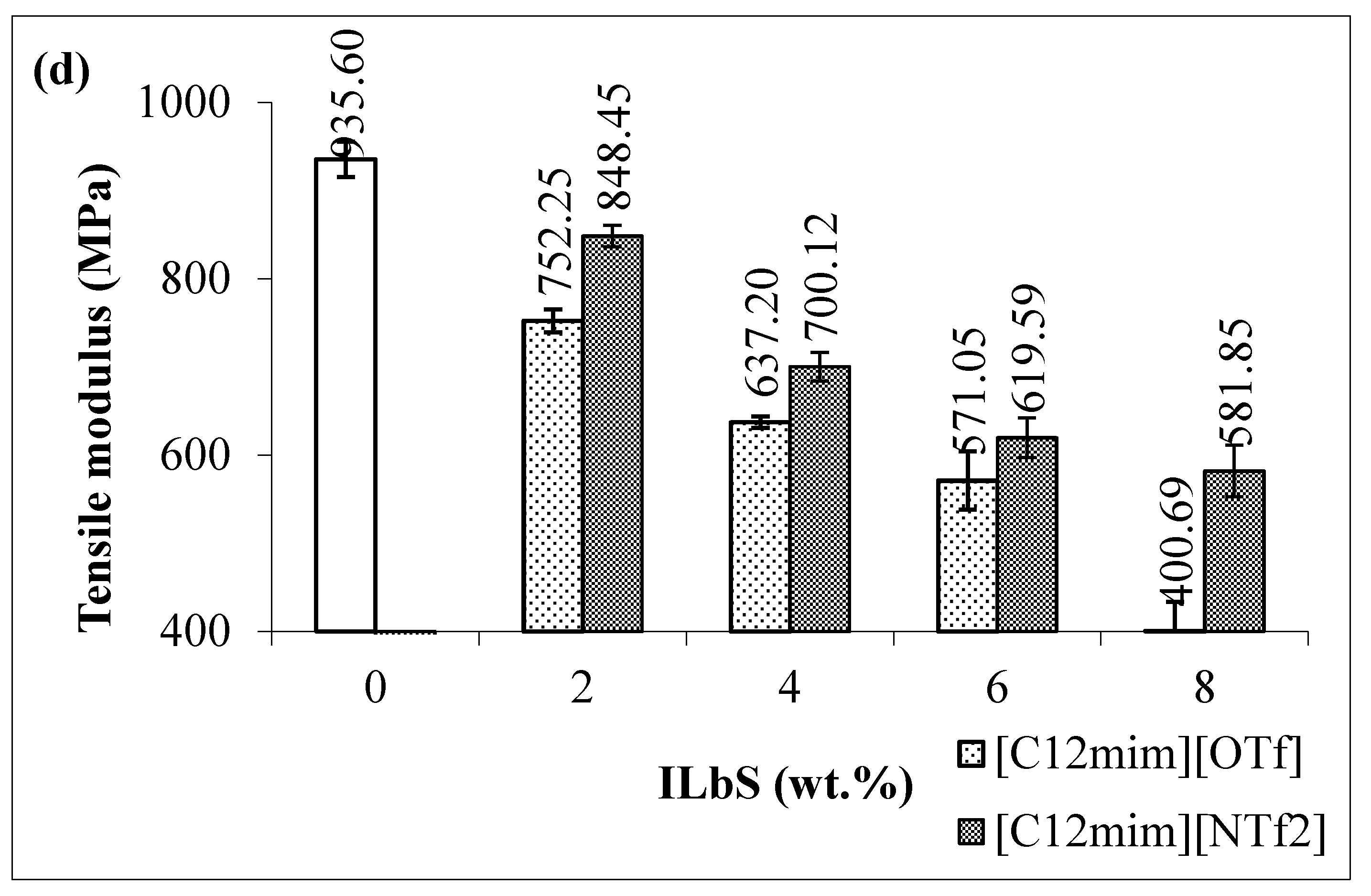
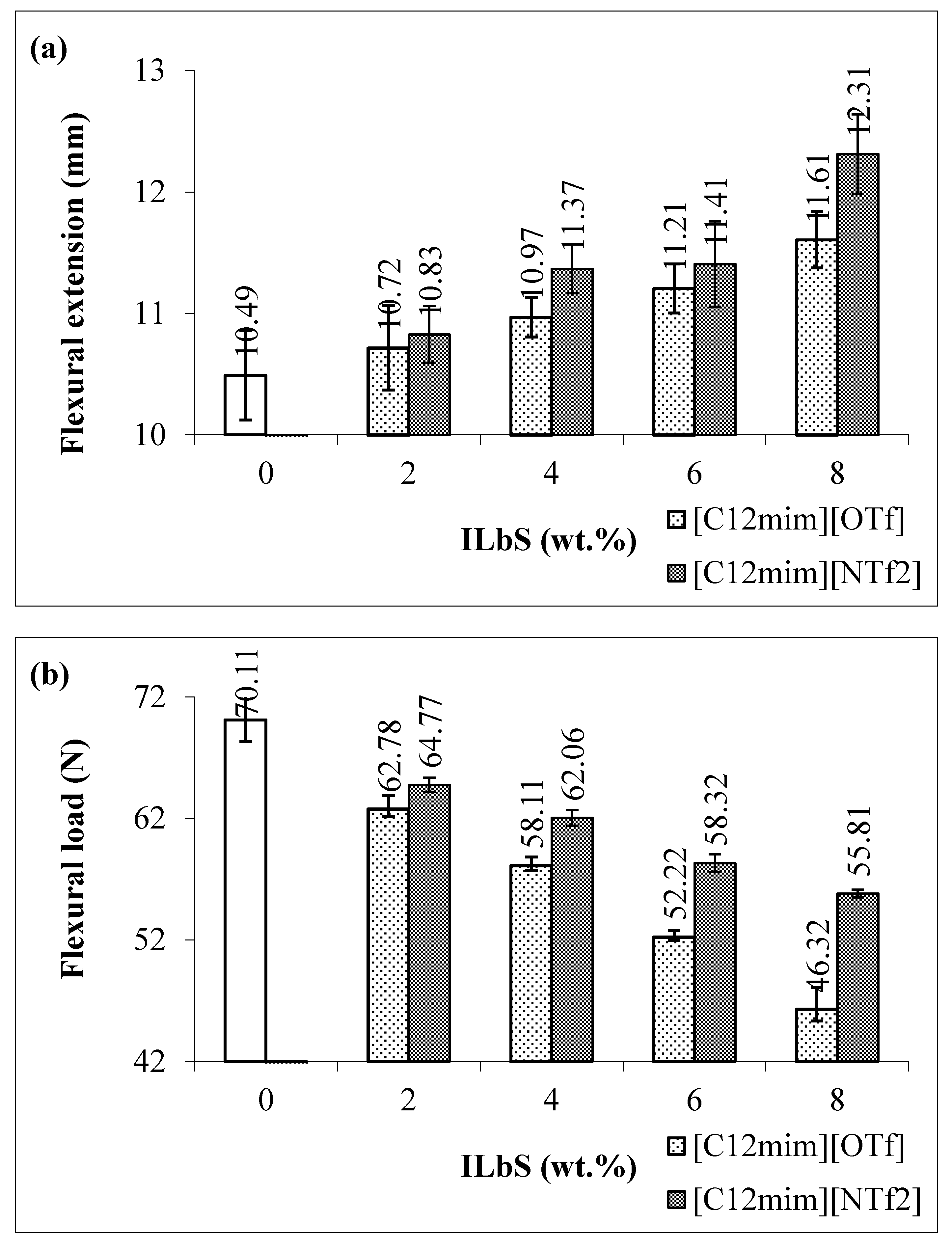
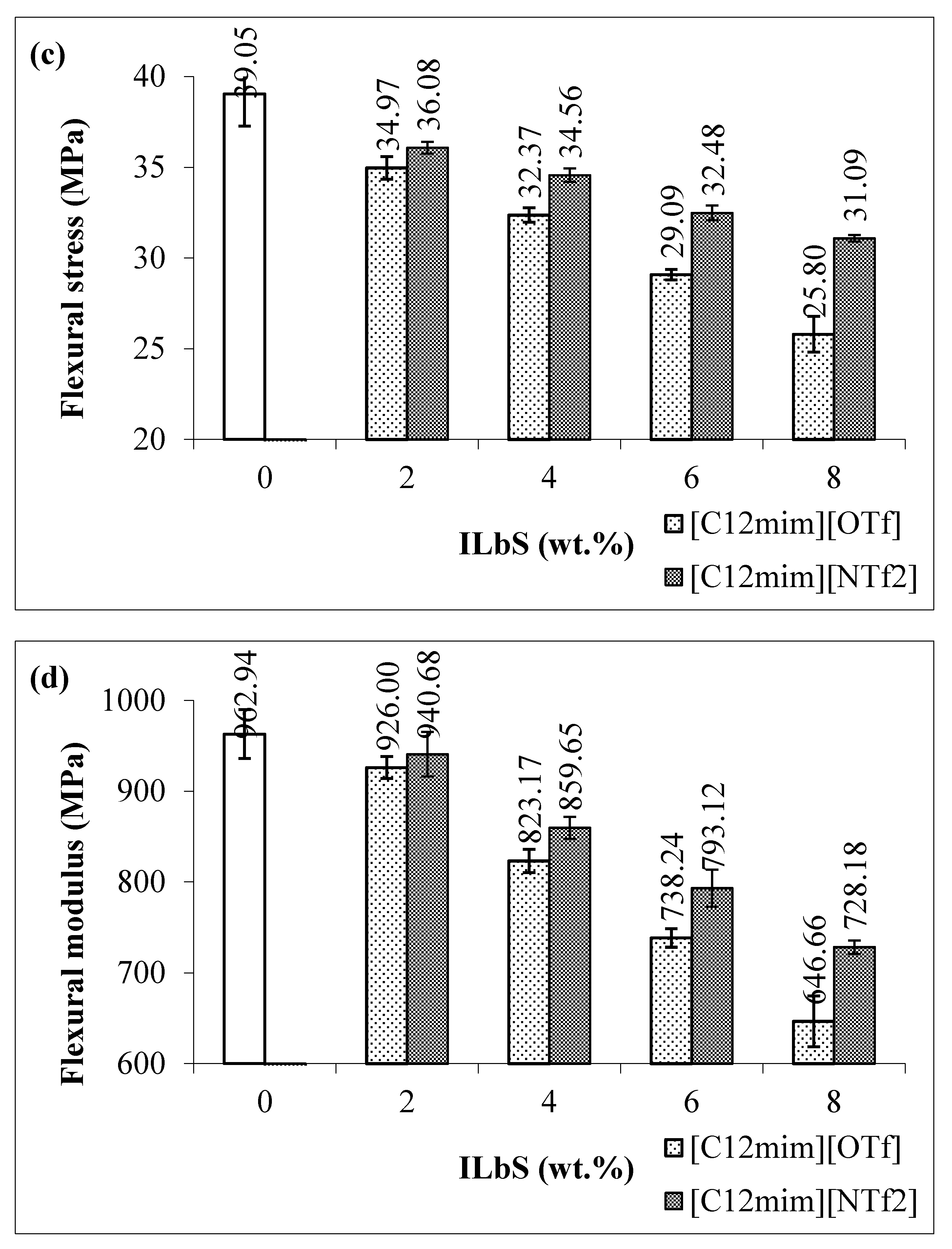
3.1. Tensile and Flexural Results
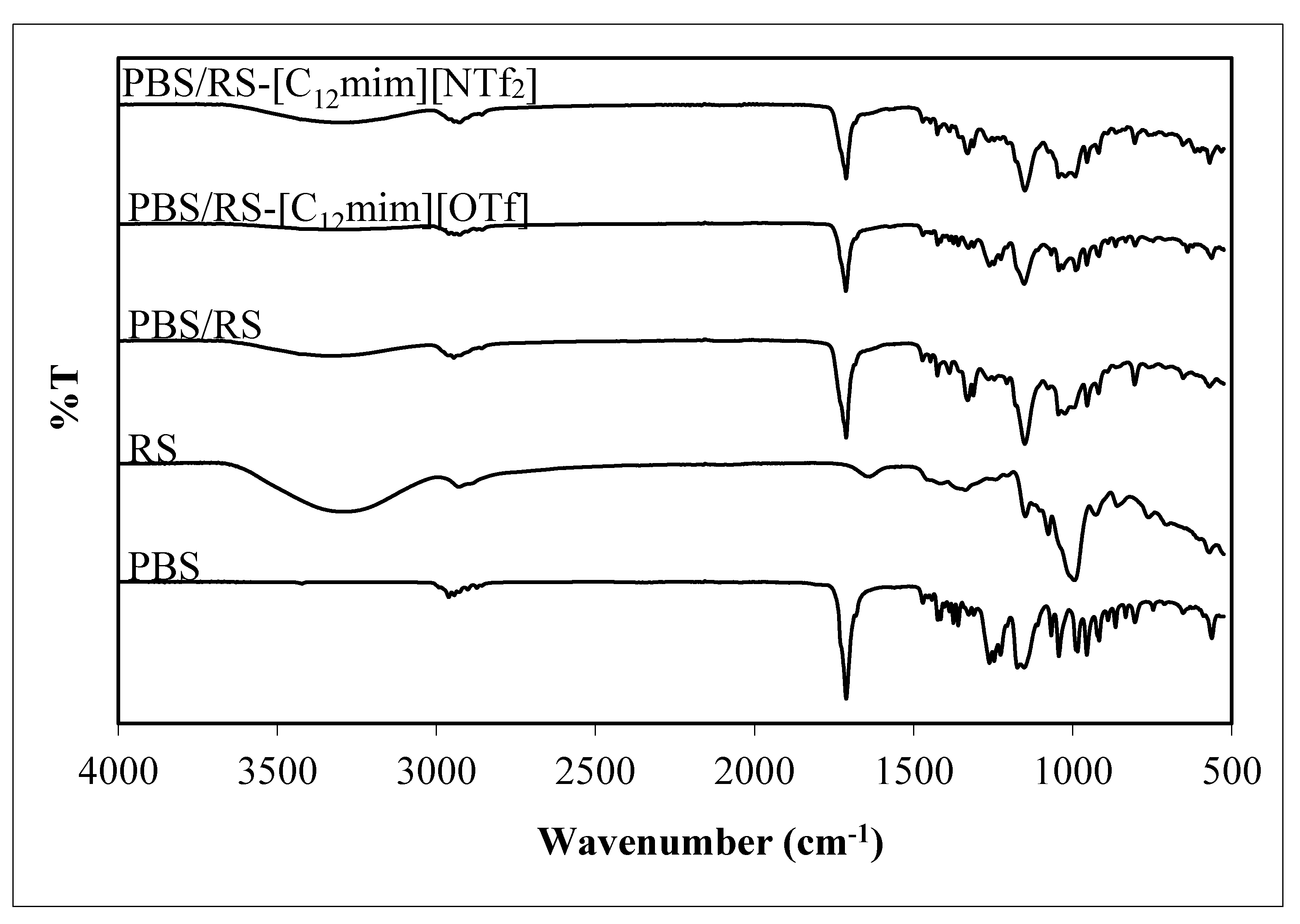
3.2. FTIR Result
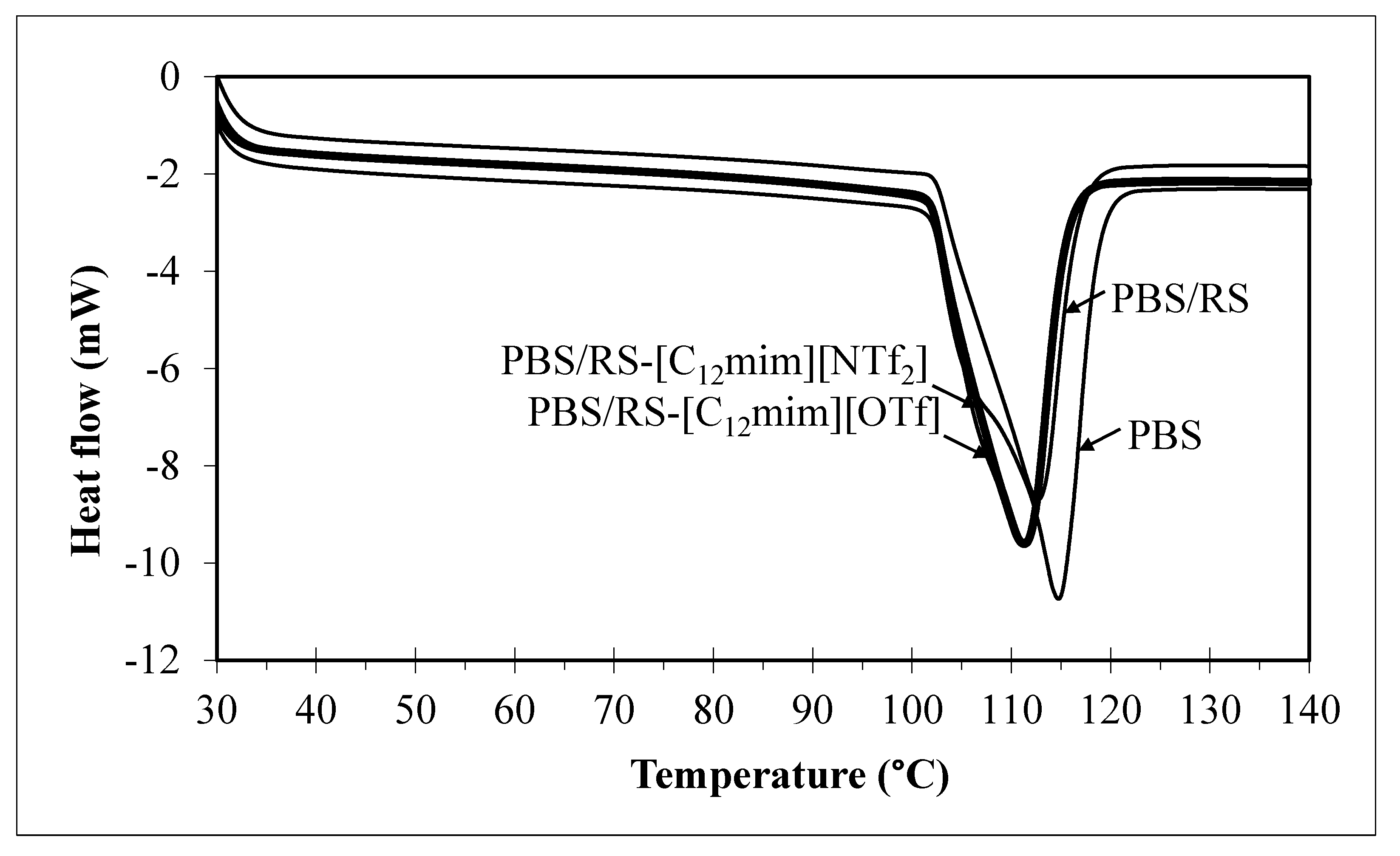
3.3. DSC Result
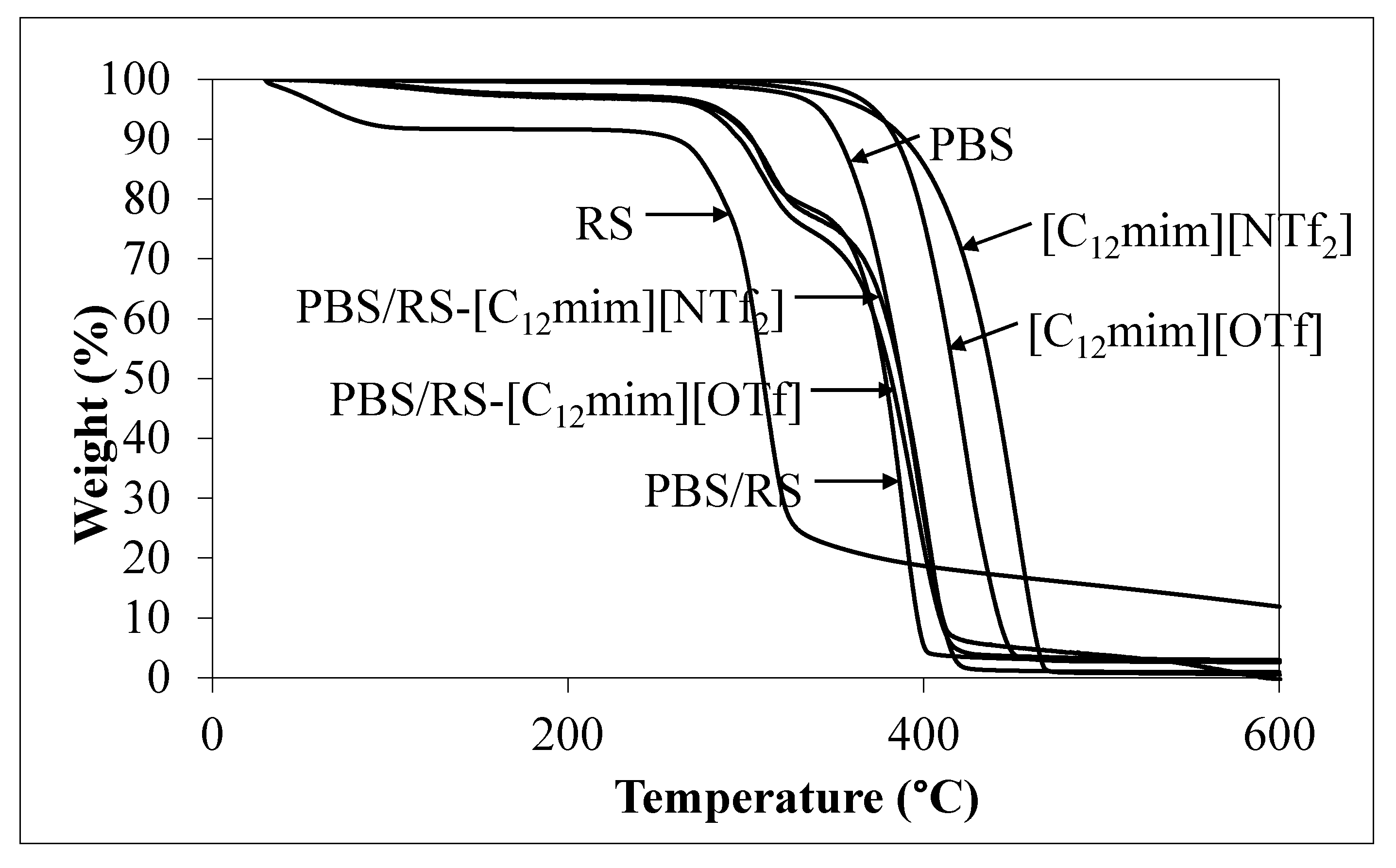
3.4. TGA Result
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zini, E.; Scandola, M. Green composites: An overview. Polym. Compos. 2011, 32, 1905–1915. [Google Scholar] [CrossRef]
- Liu, L.; Huang, G.; Song, P.; Yu, Y.; Fu, S. Converting industrial alkali lignin to biobased functional additives for improving fire behavior and smoke suppression of polybutylene succinate. ACS Sustain. Chem. Eng. 2016, 4, 4732–4742. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Abdullah, D.K.; Daik, R. Fabrication of agar/biopolymer blend aerogels in ionic liquid and co-solvent mixture. Cell. Chem. Technol. 2012, 46, 45–52. [Google Scholar]
- Sudari, A.K.; Shamsuri, A.A.; Zainudin, E.S.; Tahir, P.M. Exploration on compatibilizing effect of nonionic, anionic, and cationic surfactants on mechanical, morphological, and chemical properties of high-density polyethylene/low-density polyethylene/cellulose biocomposites. J. Thermoplast. Compos. 2017, 30, 855–884. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Daik, R.; Ahmad, I.; Jumali, M.H.H. Nylon-6/liquid natural rubber blends prepared via emulsion dispersion. J. Polym. Res. 2009, 16, 381–387. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Azid, M.; Ariff, A.; Sudari, A. Influence of surface treatment on tensile properties of low-density polyethylene/cellulose woven biocomposites: A preliminary study. Polymers 2014, 6, 2345–2356. [Google Scholar] [CrossRef] [Green Version]
- Shamsuri, A.A.; Daik, R.; Zainudin, E.S.; Tahir, P.M. Compatibilization of HDPE/agar biocomposites with eutectic-based ionic liquid containing surfactant. J. Reinf. Plast. Comp. 2014, 33, 440–453. [Google Scholar] [CrossRef] [Green Version]
- Shamsuri, A.A. Compatibilization potential of ionic liquid-based surfactants for polymer blends. Acad. J. Polym. Sci. 2018, 1, 1–2. [Google Scholar]
- Dzulkefly, K.; Khoh, H.F.; Ahmad, F.B.H.; Adlie Ahmad, S.; Lim, W.H. Solvent-free esterification process for the synthesis of glucose bolaform surfactants. Orient. J. Chem. 2010, 26, 747–752. [Google Scholar]
- Cao, Y.; Mu, T. Comprehensive investigation on the thermal stability of 66 ionic liquids by thermogravimetric analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Liu, D.; Qi, Z.; Zhang, Y.; Xu, J.; Guo, B. Poly (butylene succinate) (PBS)/ionic liquid plasticized starch blends: Preparation, characterization, and properties. Starch-Stärke 2015, 67, 802–809. [Google Scholar] [CrossRef]
- Standard Practice for Conditioning Plastics for Testing; ASTM D618-13; American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2013.
- Standard test Method for Tensile Properties of Plastics; ASTM D638-10; American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2010.
- Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials; ASTM D790-10; American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2010.
- Shamsuri, A.A.; Daik, R. Utilization of an ionic liquid/urea mixture as a physical coupling agent for agarose/talc composite films. Materials 2013, 6, 682–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phua, Y.J.; Chow, W.S.; Mohd Ishak, Z.A. Reactive processing of maleic anhydride-grafted poly (butylene succinate) and the compatibilizing effect on poly (butylene succinate) nanocomposites. Express Polym. Lett. 2013, 7, 340–354. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, Y.; Su, T.; Wang, Z. Effect of hydroxyl monomers on the enzymatic degradation of poly (ethylene succinate), poly (butylene succinate), and poly (hexylene succinate). Polymers 2018, 10, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamsuri, A.A.; Daik, R. Plasticizing effect of choline chloride/urea eutectic-based ionic liquid on physicochemical properties of agarose films. BioResources 2012, 7, 4760–4775. [Google Scholar] [CrossRef]
- Phua, Y.J.; Pegoretti, A.; Araujo, T.M.; Ishak, Z.M. Mechanical and thermal properties of poly (butylene succinate)/poly (3-hydroxybutyrate-co-3-hydroxyvalerate) biodegradable blends. J. Appl. Polym. Sci. 2015, 132, 42815–42824. [Google Scholar] [CrossRef]
- Mitsuiki, M.; Mizuno, A.; Motoki, M. Determination of molecular weight of agars and effect of the molecular weight on the glass transition. J. Agric. Food Chem. 1999, 47, 473–478. [Google Scholar] [CrossRef] [PubMed]
| Source of Variation | SS | df | MS | F | P-Value |
|---|---|---|---|---|---|
| BG | 1409.10003 | 2 | 704.5500152 | 285.5294322 | 6.8765 × 10−19 |
| WG | 66.62308072 | 27 | 2.467521508 | - | - |
| Source of Variation | SS | df | MS | F | P-Value |
|---|---|---|---|---|---|
| BG | 738.9567255 | 2 | 369.4783628 | 247.2332543 | 4.37375 × 10−18 |
| WG | 40.35021835 | 27 | 1.494452532 | - | - |
| Source of Variation | SS | df | MS | F | P-Value |
|---|---|---|---|---|---|
| BG | 539,313.715 | 2 | 269,656.8575 | 517.6102415 | 2.9479 × 10−22 |
| WG | 14,066.05698 | 27 | 520.9650735 | - | - |
| - | Wavenumber (cm−1) | ||||||
|---|---|---|---|---|---|---|---|
| Sample | O–H Stretching | C–H Stretching | C=O Stretching | C–O–C Stretching | C–O–C Stretching | O–C–C Stretching | C–O Stretching |
| PBS | - | 2961 | 1713 | 1174 | - | 1044 | - |
| RS | 3285 | 2929 | - | - | 1149 | - | 995 |
| PBS/RS | 3332 | 2946 | 1713 | - | 1151 | 1045 | 997 |
| PBS/RS-[C12mim][OTf] | 3310 | 2927 | 1713 | - | 1153 | 1044 | 990 |
| PBS/RS-[C12mim][NTf2] | 3295 | 2927 | 1713 | - | 1150 | 1045 | 993 |
| Sample | Tm (°C) |
|---|---|
| PBS | 114.7 |
| PBS/RS | 112.7 |
| PBS/RS-[C12mim][OTf] | 111.2 |
| PBS/RS-[C12mim][NTf2] | 111.3 |
| Sample | Td (°C) |
|---|---|
| PBS | 387.4 |
| RS | 309.8 |
| PBS/RS | 378.6 |
| PBS/RS-[C12mim][OTf] | 381.8 |
| PBS/RS-[C12mim][NTf2] | 387.4 |
| [C12mim][OTf] | 417.3 |
| [C12mim][NTf2] | 438.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamsuri, A.A.; Md. Jamil, S.N.A. Compatibilization Effect of Ionic Liquid-Based Surfactants on Physicochemical Properties of PBS/Rice Starch Blends: An Initial Study. Materials 2020, 13, 1885. https://doi.org/10.3390/ma13081885
Shamsuri AA, Md. Jamil SNA. Compatibilization Effect of Ionic Liquid-Based Surfactants on Physicochemical Properties of PBS/Rice Starch Blends: An Initial Study. Materials. 2020; 13(8):1885. https://doi.org/10.3390/ma13081885
Chicago/Turabian StyleShamsuri, Ahmad Adlie, and Siti Nurul Ain Md. Jamil. 2020. "Compatibilization Effect of Ionic Liquid-Based Surfactants on Physicochemical Properties of PBS/Rice Starch Blends: An Initial Study" Materials 13, no. 8: 1885. https://doi.org/10.3390/ma13081885
APA StyleShamsuri, A. A., & Md. Jamil, S. N. A. (2020). Compatibilization Effect of Ionic Liquid-Based Surfactants on Physicochemical Properties of PBS/Rice Starch Blends: An Initial Study. Materials, 13(8), 1885. https://doi.org/10.3390/ma13081885






