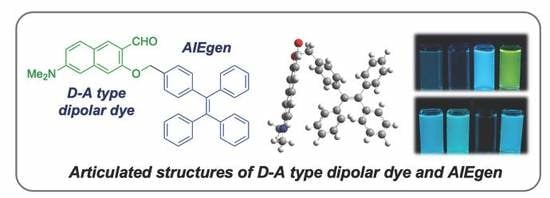
Articulated Structures of D-A Type Dipolar Dye with AIEgen: Synthesis, Photophysical Properties, and Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. UV/Vis Absorption and Fluorescence Assay
2.3. Quantum Chemical Calculations
2.4. Sensing Applications in Real Water Samples
3. Results and Discussion
3.1. Material Design, Synthesis, and Characterization
3.2. Spectroscopic Study
3.3. Quantum Chemical Calculation
3.4. Sensing Applications in Real Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singha, S.; Kim, D.; Roy, B.; Sambasivan, S.; Moon, H.; Rao, A.S.; Kim, J.Y.; Joo, T.; Park, J.W.; Rhee, Y.M.; et al. A structural remedy toward bright dipolar fluorophores in aqueous media. Chem. Sci. 2015, 6, 4335–4342. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ryu, H.G.; Ahn, K.H. Recent development of two-photon fluorescent probes for bioimaging. Org. Biomol. Chem. 2014, 12, 4550–4566. [Google Scholar] [CrossRef] [PubMed]
- Ipuy, M.; Liao, Y.-Y.; Jeanneau, E.; Baldeck, P.L.; Bretonnière, Y.; Andraud, C. Solid state red biphotonic excited emission from small dipolar fluorophores. J. Mater. Chem. C 2016, 4, 766–779. [Google Scholar] [CrossRef]
- Moon, H.; Jung, Y.; Kim, Y.; Kim, B.W.; Choi, J.G.; Kim, N.H.; Oh, M.S.; Park, S.; Kim, B.M.; Kim, D. High Stability of a Donor–Acceptor Type Oxazepine-Containing Fluorophore and Its Applications in Cellular Imaging and Two-Photon Deep Tissue Imaging. Org. Lett. 2019, 21, 3891–3894. [Google Scholar] [CrossRef]
- Kim, H.M.; Cho, B.R. Small-Molecule Two-Photon Probes for Bioimaging Applications. Chem. Rev. 2015, 115, 5014–5055. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, J.; He, Y.; Yang, J.H.; Kim, T.; Peng, X.; Kim, J.S. Macro-/micro-environment-sensitive chemosensing and biological imaging. Chem. Soc. Rev. 2014, 43, 4563–4601. [Google Scholar] [CrossRef]
- Kim, D.; Moon, H.; Baik, S.H.; Singha, S.; Jun, Y.W.; Wang, T.; Kim, K.H.; Park, B.S.; Jung, J.; Mook-Jung, I.; et al. Two-Photon Absorbing Dyes with Minimal Autofluorescence in Tissue Imaging: Application to in Vivo Imaging of Amyloid-β Plaques with a Negligible Background Signal. J. Am. Chem. Soc. 2015, 137, 6781–6789. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Hyun, J.Y.; Wei, T.; Qiang, J.; Ren, X.; Shin, I.; Yoon, J. Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2016, 45, 2976–3016. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, Y.; Yin, J.; Lin, W. Strategies for designing organic fluorescent probes for biological imaging of reactive carbonyl species. Chem. Soc. Rev. 2019, 48, 4036–4048. [Google Scholar] [CrossRef]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef]
- Hu, R.; Leung, N.L.C.; Tang, B.Z. AIE macromolecules: Syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef] [PubMed]
- Shindy, H.A. Fundamentals in the chemistry of cyanine dyes: A review. Dye. Pigment. 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Slama-Schwok, A.; Blanchard-Desce, M.; Lehn, J.M. Intramolecular charge transfer in donor-acceptor molecules. J. Phys. Chem. 1990, 94, 3894–3902. [Google Scholar] [CrossRef]
- Moon, H.; Xuan, Q.P.; Kim, D.; Kim, Y.; Park, J.W.; Lee, C.H.; Kim, H.-J.; Kawamata, A.; Park, S.Y.; Ahn, K.H. Molecular-Shape-Dependent Luminescent Behavior of Dye Aggregates: Bent versus Linear Benzocoumarins. Cryst. Growth Des. 2014, 14, 6613–6619. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Liang, J.; Tang, B.Z.; Liu, B. Specific light-up bioprobes based on AIEgen conjugates. Chem. Soc. Rev. 2015, 44, 2798–2811. [Google Scholar] [CrossRef]
- Wu, W.; Mao, D.; Xu, S.; Ji, S.; Hu, F.; Ding, D.; Kong, D.; Liu, B. High performance photosensitizers with aggregation-induced emission for image-guided photodynamic anticancer therapy. Mater. Horiz. 2017, 4, 1110–1114. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wang, J.; Liang, X.-J. Improved pharmaceutical research and development with AIE-based nanostructures. Mater. Horiz. 2018, 5, 799–812. [Google Scholar] [CrossRef]
- Kim, D.; Xuan, Q.P.; Moon, H.; Jun, Y.W.; Ahn, K.H. Synthesis of Benzocoumarins and Characterization of Their Photophysical Properties. Asian J. Org. Chem. 2014, 3, 1089–1096. [Google Scholar] [CrossRef]
- Frisch, M.J.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Jung, Y.; Kim, Y.; Kim, N.H.; Lee, J.; Kim, K.-H.; Jung, J.; Huh, Y.; Jang, H.-J.; Joo, J.; Park, S.; et al. A wavelength-tunable and facilely functionable D-A type naphthalene core skeleton: Synthesis, photophysical property, and bio-imaging applications for cells and tissues. Dye. Pigment. 2019, 162, 104–111. [Google Scholar] [CrossRef]
- Kim, D.; Baik, S.H.; Kang, S.; Cho, S.W.; Bae, J.; Cha, M.-Y.; Sailor, M.J.; Mook-Jung, I.; Ahn, K.H. Close Correlation of Monoamine Oxidase Activity with Progress of Alzheimer’s Disease in Mice, Observed by in Vivo Two-Photon Imaging. ACS Cent. Sci. 2016, 2, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, N.K.; Kang, S.; Huh, Y.; Jung, J.; Hur, J.K.; Kim, D. Latent turn-on fluorescent probe for the detection of toxic malononitrile in water and its practical applications. Anal. Chim. Acta 2020, 1095, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Ju, I.G.; Choe, Y.H.; Kim, Y.; Park, S.; Hyun, Y.-M.; Oh, M.S.; Kim, D. Hydrazine Exposé: The Next-Generation Fluorescent Probe. ACS Sens. 2019, 4, 441–449. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, D. A Selective Fluorescence Turn-On Probe for the Detection of DCNP (Nerve Agent Tabun Simulant). Materials 2019, 12, 2943. [Google Scholar] [CrossRef]
- Jung, Y.; Park, N.K.; Kang, J.S.; Kim, D. Hydrazine-Selective Fluorescent Turn-On Probe Based on Ortho-Methoxy-Methyl-Ether (o-MOM) Assisted Retro-aza-Henry Type Reaction. Sensors 2019, 19, 4525. [Google Scholar] [CrossRef]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Tetraphenylethylene-Based AIE-Active Probes for Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 12189–12216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, Y.; Zhao, Y.; Ng, K.M. A fluorescent nanoparticle probe based on sugar-substituted tetraphenylethene for label-free detection of galectin-3. J. Mater. Chem. B 2019, 7, 6737–6741. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Ren, W.; Hu, J.; Gao, B.; Yuan, D.; Wang, X.; Fu, J. A novel tetraphenylethene-based fluorescent sensor for uranyl ion detection with aggregation-induced emission character. Dye. Pigment. 2019, 166, 182–188. [Google Scholar] [CrossRef]
- Jones, G.A.; Bradshaw, D.S. Resonance Energy Transfer: From Fundamental Theory to Recent Applications. Front. Phys. 2019, 7. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Hao, Y.; Chen, W.; Zhang, Y.; Xu, M.; Yang, M.; Liu, Y.-N. Recent progress in the development of fluorescent probes for hydrazine. Luminescence 2018, 33, 816–836. [Google Scholar] [CrossRef] [PubMed]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.H.; Kim, B.W.; Kim, Y.; Hur, J.K.; Jung, J.; Oh, Y.; Park, S.; Kim, B.M.; Kim, D. Articulated Structures of D-A Type Dipolar Dye with AIEgen: Synthesis, Photophysical Properties, and Applications. Materials 2020, 13, 1939. https://doi.org/10.3390/ma13081939
Kim NH, Kim BW, Kim Y, Hur JK, Jung J, Oh Y, Park S, Kim BM, Kim D. Articulated Structures of D-A Type Dipolar Dye with AIEgen: Synthesis, Photophysical Properties, and Applications. Materials. 2020; 13(8):1939. https://doi.org/10.3390/ma13081939
Chicago/Turabian StyleKim, Na Hee, Byeong Wook Kim, Youngseo Kim, Junho K. Hur, Junyang Jung, Yohan Oh, Sungnam Park, B. Moon Kim, and Dokyoung Kim. 2020. "Articulated Structures of D-A Type Dipolar Dye with AIEgen: Synthesis, Photophysical Properties, and Applications" Materials 13, no. 8: 1939. https://doi.org/10.3390/ma13081939
APA StyleKim, N. H., Kim, B. W., Kim, Y., Hur, J. K., Jung, J., Oh, Y., Park, S., Kim, B. M., & Kim, D. (2020). Articulated Structures of D-A Type Dipolar Dye with AIEgen: Synthesis, Photophysical Properties, and Applications. Materials, 13(8), 1939. https://doi.org/10.3390/ma13081939