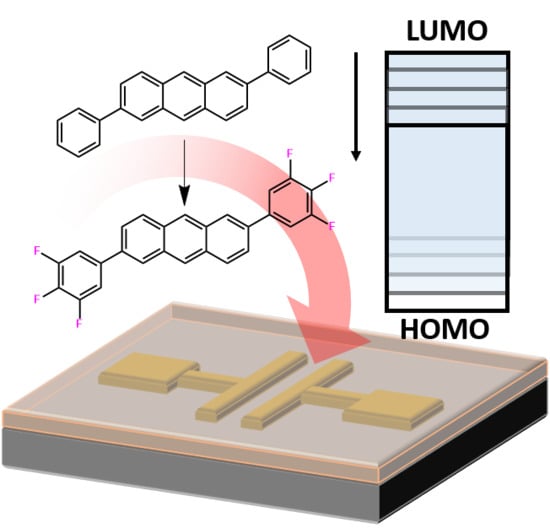
Developing and Comparing 2,6-Anthracene Derivatives: Optical, Electrochemical, Thermal, and Their Use in Organic Thin Film Transistors
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods and Procedures
2.1.1. Preparation of 2,6-bis(2-fluorophenyl)anthracene (o-FPh)
2.1.2. Preparation of 2,6-(3-fluorophenyl)anthracene (m-FPh)
2.1.3. Preparation of 2,6-bis(4-fluorophenyl)anthracene (p-FPh)
2.1.4. Preparation of 2,6-bis(3-(trifluoromethyl)phenyl)anthracene (m-CF3Ph)
2.1.5. Preparation of 2,6-bis(4-(trifluoromethyl)phenyl)anthracene (p-CF3Ph)
2.1.6. Preparation of 2,6-bis(3,4,5-trifluorophenyl)anthracene (3,4,5-F3Ph)
2.2. Electrochemistry
2.3. Thermogravimetric Analysis
2.4. Crystallographic Characterization
2.5. Electrical Characterization
3. Results and Discussion
3.1. Synthesis and Purification of 2,6-Anthracene Derivatives
3.2. Optical and Electrochemical Properties
3.3. Thermogravimetric Analysis
3.4. Single Crystal X-Ray Diffraction
3.5. OTFT Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chang, Y.L.; Song, Y.; Wang, Z.; Helander, M.G.; Qiu, J.; Chai, L.; Lu, Z. Highly efficient warm white organic light-emitting diodes by triplet exciton conversion. Adv. Funct. Mater. 2013, 23, 705–712. [Google Scholar] [CrossRef]
- Qiu, J.; Greiner, M.; Liu, Z.; Helander, M.G. Unlocking the full potential of organic light-emitting diodes on flexible plastic. Nat. Photonics 2015, 5, 753–757. [Google Scholar]
- Grant, T.M.; Josey, D.S.; Sampson, K.L.; Mudigonda, T.; Lessard, B.H.; Bender, T.P. Boron Subphthalocyanines and Silicon Phthalocyanines for Use as Active Materials in Organic Photovoltaics. Chem. Rec. 2019, 19, 1093–1112. [Google Scholar] [CrossRef] [PubMed]
- Melville, O.A.; Lessard, B.H.; Bender, T.P. Phthalocyanine-Based Organic Thin-Film Transistors: A Review of Recent Advances. ACS Appl. Mater. Interfaces 2015, 7, 13105–13118. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Becerril, H.A.; Kim, D.H.; Lee, B.L.; Lee, S.; Bao, Z. Microfluidic arrays for rapid characterization of organic thin-film transistor performance. Adv. Mater. 2011, 23, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Sun, F.; Goldfinger, M.B.; Gao, F.; Londono, D.J.; Marshal, W.J.; Keys, D.E. 2,6-Bis[2-(4-pentylphenyl)vinyl]anthracene: A stable and high charge mobility organic semiconductor with densely packed crystal structure. J. Am. Chem. Soc. 2006, 128, 9304–9305. [Google Scholar] [CrossRef]
- Chen, H.Z.; Shi, M.M.; Aernouts, T.; Wang, M.; Borghs, G.; Heremans, P. A novel organic n-type material: Fluorinated perylene diimide. Sol. Energy Mater. Sol. Cells 2005, 87, 521–527. [Google Scholar] [CrossRef]
- Tian, H.; Han, Y.; Bao, C.; Yan, D.; Geng, Y.; Wang, F. An asymmetric oligomer based on thienoacene for solution processed crystal organic thin-film transistors. Chem. Commun. 2012, 48, 3557–3559. [Google Scholar] [CrossRef]
- Piliego, C.; Jarzab, D.; Gigli, G.; Chen, Z.; Facchetti, A.; Loi, M.A. High Electron Mobility and Ambient Stability in Solution-Processed Perylene-Based Organic Field-Effect Transistors. Adv. Mater. 2009, 21, 1573–1576. [Google Scholar] [CrossRef]
- Ma, R.-Q.; Hewitt, R.; Rajan, K.; Silvernail, J.; Urbanik, K.; Hack, M.; Brown, J.J. Flexible active-matrix OLED displays: Challenges and progress. J. Soc. Inf. Disp. 2007, 16, 169. [Google Scholar] [CrossRef]
- Glawe, A.; Eggerath, D.; Schäfer, F. Printing versus coating—What will be the future production technology for printed electronics? AIP Conf. Proc. 2015, 1646, 87–90. [Google Scholar]
- Boileau, N.T.; Melville, O.A.; Mirka, B.; Cranston, R.; Lessard, B.H. P and N type copper phthalocyanines as effective semiconductors in organic thin-film transistor based DNA biosensors at elevated temperatures. RSC Adv. 2019, 9, 2133–2142. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xiao, W.; Wu, W.; Liu, B. Research Progress on Flexible Oxide-Based Thin Film Transistors. Appl. Sci. 2019, 9, 773. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Yan, L.; Zhao, Y.; Murtaza, I.; Meng, H.; Huang, W. Anthracene-based semiconductors for organic field-effect transistors. J. Mater. Chem. C 2018, 6, 7416–7444. [Google Scholar] [CrossRef]
- Xie, H.; Cheng, C.Y.; Li, L.; Deng, X.Y.; Yang, K.K.; Wang, Y.Z. Integrating shape-memory technology and photo-imaging on a polymer platform for a high-security information storage medium. J. Mater. Chem. C 2018, 6, 10422–10427. [Google Scholar] [CrossRef]
- Kommandeur, J. Photoconductivity in organic single crystals. J. Phys. Chem. Solids 1961, 22, 339–349. [Google Scholar] [CrossRef]
- LeBlanc, O.H. Band Structure and Transport of Holes and Electrons in Anthracene. J. Chem. Phys. 1961, 35, 1275–1280. [Google Scholar] [CrossRef]
- Quinn, J.T.E.; Zhu, J.; Li, X.; Wang, J.; Li, Y. Recent progress in the development of n-type organic semiconductors for organic field effect transistors. J. Mater. Chem. C 2017, 5, 8654–8681. [Google Scholar] [CrossRef]
- Chaari, M.; Kelemen, Z.; Planas, J.G.; Teixidor, F.; Choquesillo-Lazarte, D.; Salah, A.B.; Vinas, C.; Nunez, R. Photoluminescence in: M-carborane-anthracene triads: A combined experimental and computational study. J. Mater. Chem. C 2018, 6, 11336–11347. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Dong, H.; Wang, Z.; Ji, D.; Cheng, C.; Geng, H.; Bo, Z. Thin film field-effect transistors of 2,6-diphenyl anthracene (DPA). Chem. Commun. 2015, 51, 11777–11779. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Dong, H.; Meng, L.; Jiang, L.; Jiang, L.; Heeger, A.J. High mobility emissive organic semiconductor. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Zhao, Y.; Yu, H.; Hu, Z.; He, Y.; Li, A.; Loo, Y.L. Influence of heteroatoms on the charge mobility of anthracene derivatives. J. Mater. Chem. C 2016, 4, 3517–3522. [Google Scholar] [CrossRef]
- Li, A.; Yan, L.; Liu, M.; Murtaza, I.; He, C.; Zhang, D.; Meng, H. Highly responsive phototransistors based on 2,6-bis (4-methoxyphenyl) anthracene single crystal. J. Mater. Chem. C 2017, 5, 5304–5309. [Google Scholar] [CrossRef]
- Brixi, S.; Melville, O.A.; Boileau, N.T.; Lessard, B.H. The influence of air and temperature on the performance of PBDB-T and P3HT in organic thin film transistors. J. Mater. Chem. C 2018, 6, 11972–11979. [Google Scholar] [CrossRef]
- Mai, J.; Tang, N.; He, W.; Zou, Z.; Luo, C.; Zhang, A.; Zhou, G. Effects of Ambient Gases on the Electrical Performance of Solution-Processed C8-BTBT Thin-Film Transistors. Nanoscale Res. Lett. 2019, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brixi, S.; Melville, O.A.; Mirka, B.; He, Y.; Hendsbee, A.D.; Meng, H.; Li, Y.; Lessard, B.H. Air and temperature sensitivity of n-type polymer materials to meet and exceed the standard of N2200. Sci. Rep. 2020, 10, 4014. [Google Scholar] [PubMed] [Green Version]
- Liu, J.; Zhang, Z.; Xu, C.; Li, Q.; Zhou, K.; Hu, W. Enhancing field-effect mobility and maintaining solid-state emission by incorporating 2,6-diphenyl substitution to 9,10-bis(phenylethynyl)anthracene. J. Mater. Chem. C 2017, 5, 2519–2523. [Google Scholar] [CrossRef]
- Payne, M.M.; Parkin, S.R.; Anthony, J.E.; Kuo, C.C.; Jackson, T.N. Organic field-effect transistors from solution-deposited functionalized acenes with mobilities as high as 1 cm2/V.·s. J. Am. Chem. Soc. 2005, 127, 4986–4987. [Google Scholar] [CrossRef]
- Vorona, M.Y.; Yutronkie, N.J.; Melville, O.; Daszczynski, A.J.; Agyei, K.T.; Brusso, J.L.; Lessard, B.H. Developing 9,10-anthracene Derivatives: Optical, Electrochemical, Thermal, and Electrical Characterization. Materials 2019, 12, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ando, S.; Nishida, J.I.; Fujiwara, E.; Tada, H.; Inoue, Y.; Tokito, S.; Yamashita, Y. Novel p- and n-Type Organic Semiconductors with an Anthracene Unit. Chem. Mater. 2009, 17, 2–5. [Google Scholar] [CrossRef]
- Diffraction, X. APEX2 Release 2010. 2010. [Google Scholar]
- Blessing, B.Y.R.H. An Empirical Correction for Absorption Anisotropy. Acta Crystallogr. Sect. A Found. Crystallogr. 1995, 51, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 46, 1281–1284. [Google Scholar]
- Melville, O.A.; Grant, T.M.; Rice, N.A.; Wang, B.; Josse, P.; Lessard, B.H. Functionalization of commercial pigment Hostasol Red GG for incorporation into organic thin-film transistors. New J. Chem. 2020, 44, 845–851. [Google Scholar] [CrossRef]
- Zafar, M.N.; Mohsin, M.A.; Danish, M.; Nazar, M.F.; Murtaza, S. Palladium Catalyzed Heck—Mizoroki and Suzuki—Miyaura Coupling Reactions (Review). Russ. J. Coord. Chem. 2014, 40, 781–800. [Google Scholar] [CrossRef]
- Smith, G.B.; Dezeny, G.C.; Hughes, D.L.; King, A.O.; Verhoeven, T.R. Mechanistic Studies of the Suzuki Cross-Coupling Reaction. J. Org. Chem. 1994, 59, 8151–8156. [Google Scholar] [CrossRef]
- Pizzoferrato, R.; Tagliatesta, P.; Schillaci, C.; Prosposito, P.; De Angelis, R. Synthesis and Photophysical Properties of 9,10 Disubstituted Anthracenes. Mater. Sci. Appl. 2015, 6, 943–952. [Google Scholar]
- Wang, Z.; Xu, C.; Wang, W.; Duan, L.; Li, Z. High-color-purity and high-efficiency non-doped deep-blue electroluminescent devices based on novel anthracene derivatives. New J. Chem. 2012, 36, 662–667. [Google Scholar] [CrossRef]
- Wan, Z.; Qi, W.; Xing, F.; Li, Y. Anthracene-based Derivatives: Synthesis, Photophysical Properties and Electrochemical Properties. Chem. Res. Chin. Univ. 2017, 33, 603–610. [Google Scholar]
- Kim, R.; Lee, S.; Kim, K.H.; Lee, Y.J.; Kwon, S.K.; Kim, J.J.; Kim, Y.H. Extremely deep blue and highly efficient non-doped organic light emitting diodes using an asymmetric anthracene derivative with a xylene unit. Chem. Comm. 2013, 49, 4664–4666. [Google Scholar] [CrossRef]
- Gidron, O.; Dadvand, A.; Sun, E.W.H.; Chung, I.; Shimon, L.J.; Bendikov, M.; Perepichka, D.F. Oligofuran-containing molecules for organic electronics. J. Mater. Chem. C 2013, 1, 4358–4367. [Google Scholar] [CrossRef]
- Usta, H.; Kim, C.; Wang, Z.; Lu, S.; Huang, H.; Facchetti, A.; Marks, T.J. Anthracenedicarboximide-based semiconductors for air-stable, n-channel organic thin-film transistors: Materials design, synthesis, and structural characterization. J. Mater. Chem. 2012, 22, 4459–4472. [Google Scholar] [CrossRef]
- Dadvand, A.; Sun, W.H.; Moiseev, A.G.; Bélanger-Gariépy, F.; Rosei, F.; Meng, H.; Perepichka, D.F. 1,5-, 2,6- and 9,10-Distyrylanthracenes As Luminescent Organic Semiconductors. J. Mater. Chem. C 2013, 1, 2817–2825. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Zhao, Y.; Yan, L.; Yang, S.; Zhu, Y.; Murtaza, I.; Huang, W.A. Unique Blend of 2-Fluorenyl-2-anthracene and 2-Anthryl-2-anthracence Showing White Emission and High Charge Mobility. Angew. Chem. Int. Ed. 2017, 56, 722–727. [Google Scholar] [CrossRef]
- Dadvand, A.; Moiseev, A.G.; Sawabe, K.; Sun, W.H.; Djukic, B.; Chung, I.; Perepichka, D.F. Maximizing field-effect mobility and solid-state luminescence in organic semiconductors. Angew. Chem. Int. Ed. 2012, 51, 3837–3841. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.I.; Hsiang, J.; Khalil, A.M.; Hull, N.P.; Dickson, R.M. FRET-Enabled Optical Modulation for High Sensitivity Fluorescence Imaging. J. Am. Chem. Soc. 2010, 18, 6318–6323. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.G.; Su, C.Y.; Wang, W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef]
- Aranzaes, J.R.; Daniel, M.-C.; Astruc, D. Metallocenes as references for the determination of redox potentials by cyclic voltammetry—Permethylated iron and cobalt sandwich complexes, inhibition by polyamine dendrimers, and the role of hydroxy-containing ferrocenes. Can. J. Chem. 2006, 84, 288–299. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Gao, J.; Wang, D.; Yu, G.; Heeger, A.J. Electrochemical properties of luminescent polymers and polymer light-emitting electrochemical cells. Synth. Met. 2002, 99, 243–248. [Google Scholar]
- Dang, M.T.; Grant, T.M.; Yan, H.; Seferos, D.S.; Lessard, B.H.; Bender, T.P. Bis (tri-n-alkylsilyl oxide) silicon phthalocyanines: A start to establishing a structure property relationship as both ternary additives and non-fullerene electron acceptors in bulk heterojunction organic photovoltaic devices. J. Mater. Chem. A 2017, 5, 12168–12182. [Google Scholar] [CrossRef]
- Jeannin, O.; Barrière, F.; Fourmigué, M. Trifluoromethyl-substituted tetrathiafulvalenes. Beilstein J. Org. Chem. 2015, 6226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikoofard, H. Theoretical study of fl uorinated phenylthiophenes as candidate materials for charge-storage applications. J. Fluor. Chem. 2016, 185, 181–186. [Google Scholar] [CrossRef]
- Yudi, L.M.; Monzo, L.M.A. Effect of electron acceptor groups on partition coefficient of phenothiazine derivatives at the water j 1, 2-dichloroethane interface. J. Electroanal. Chem. 2006, 591, 46–52. [Google Scholar]
- Shiwaku, R.; Yoshimura, Y.; Takeda, Y.; Fukuda, K.; Kumaki, D.; Tokito, S. Control of threshold voltage in organic thin-film transistors by modifying gate electrode surface with MoOX aqueous solution and inverter circuit applications. Appl. Phys. Lett. 2017, 053301. [Google Scholar] [CrossRef] [Green Version]
- Wang, S. The interface state assisted charge transport at the/metal interface. J. Chem. Phys. 2009, 130. [Google Scholar]
- Melville, O.A.; Grant, T.M.; Lochhead, K.; King, B.; Ambrose, R.; Rice, N.A.; Lessard, B.H. Contact Engineering Using Manganese, Chromium, and 2 Bathocuproine in Group 14 Phthalocyanine Organic Thin-Film 3 Transistors 1. Appl. Electron. Mater. 2020, 10. [Google Scholar] [CrossRef]
- Comeau, Z.J.; Boileau, N.T.; Lee, T.; Melville, O.A.; Rice, N.A.; Troung, Y.; Harris, C.S.; Lessard, B.H.; Shuhendler, A.J. On-the-Spot Detection and Speciation of Cannabinoids Using Organic Thin-Film Transistors. ACS Sens. 2019, 10, 2706–2715. [Google Scholar] [CrossRef]
- Inoue, Y.; Sakamoto, Y.; Suzuki, T.; Kobayashi, M.; Gao, Y.; Tokito, S. Organic Thin-Film Transistors with High Electron Mobility Based on Perfluoropentacene. Jpn. J. Appl. Phys. 2005, 44, 3663. [Google Scholar] [CrossRef]





| (V) a | (eV) b | (nm) | (eV) c | (nm) | Stokes Shift (nm) | |
|---|---|---|---|---|---|---|
| 2,6-DPA d | – | −5.60 | 360, 379, 400 | 2.95 | 411, 436 | 33 |
| o-FPh | 0.86 | −5.69 | 351, 368, 388 | 3.00 | 407, 431 | 40 |
| m-FPh | 0.89 | −5.69 | 365, 380, 398 | 3.04 | 411, 436 | 46 |
| p-FPh | 0.91 | −5.70 | 345, 366, 387 | 3.00 | 412, 436 | 46 |
| m-CF3Ph | 0.92 | −5.72 | 359, 377, 399 | 2.97 | 412, 436 | 35 |
| p-CF3Ph | 0.99 | −5.80 | 361, 380, 401 | 2.96 | 412, 441 | 36 |
| 3,4,5-F3Ph | 0.99 | −5.79 | 357, 369, 389 | 2.96 | 413, 436 | 44 |
| ϕ1, ϕ2 (°) | Herringbone Angle (°) | Centroid Distances (Å) | Plane Distances (Å) | |
|---|---|---|---|---|
| 2,6-DPA a | 20.6, 20.6 | 41.5 | 6.24 | 2.21 |
| o-FPh | 48.3, 48.3 | 78.0 | 6.37, 7.00, 7.40 | 2.43, 4.24, 1.81 |
| m-FPh b | 24.0, 24.0 27.3, 27.3 | 49.9 | 5.91 | 2.48, 2.50 |
| p-FPh c | 13.2, 13.2 14.7, 14.7 | 45.2 | 6.07 | 2.33 |
| m-CF3Ph | 26.2, 26.2 | 51.8 | 6.14 | 2.68 |
| p-CF3Ph | 7.2, 7.2 | 47.9 | 6.13 | 2.48 |
| 3,4,5-F3Ph | 39.3, 39.3 | 23.5 | 6.99 | 6.77 |
| Compound | δplaneb (Å) | Testing Atmosphere | μavg,h (p-type) (cm2 V−1 s−1) | μavg,e (n-type) (cm2 V−1 s−1) | VT, avg (V) | n | Ion/off |
|---|---|---|---|---|---|---|---|
| 2,6-DPA | 2.850 | N2 | 2.71 ± 1.04 | – | −51.0 ± 4.9 | 57 | 107 |
| Air | 0.145 ± 0.079 | – | −49.9 ± 10.8 | 58 | 106 | ||
| o-FPh | 2.430, 4.344, 1.813 | N2 | 7.43 ± 2.78 × 10−5 | – | −64.8 ± 4.0 | 32 | 101 |
| Air | 2.34 ± 1.42 × 10−5 | – | −87.0 ± 12.3 | 28 | 101 | ||
| m-FPh | 2.486, 2.503 | N2 | 1.74 ± 2.02 × 10−6 | – | −65.9 ± 22.7 | 28 | 101 |
| Air | 8.08 ± 3.45 × 10−8 | – | −122.2 ± 11.3 | 35 | 101 | ||
| p-FPh | 2.330 | N2 | 5.89 ± 0.41 × 10−6 | – | −87.3 ± 30.4 | 31 | 102 |
| Air | 3.52 ± 6.96 × 10−5 | – | −75.0 ± 25.9 | 23 | 101 | ||
| m-CF3Ph | 2.549 | N2 | – | 5.48 ± 3.48 × 10−6 | 79.8 ± 21.2 | 20 | 101 |
| Air | – | – | – | – | – | ||
| p-CF3Ph | 2.482 | N2 | – | 3.11 ± 1.27 × 10−3 | 54.7 ± 1.1 | 35 | 103 |
| Air | – | – | – | – | – | ||
| 3,4,5-F3Ph | 3.602 | N2 | – | – | – | – | – |
| Air | – | – | – | – | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorona, M.Y.; Yutronkie, N.J.; Melville, O.A.; Daszczynski, A.J.; Ovens, J.S.; Brusso, J.L.; Lessard, B.H. Developing and Comparing 2,6-Anthracene Derivatives: Optical, Electrochemical, Thermal, and Their Use in Organic Thin Film Transistors. Materials 2020, 13, 1961. https://doi.org/10.3390/ma13081961
Vorona MY, Yutronkie NJ, Melville OA, Daszczynski AJ, Ovens JS, Brusso JL, Lessard BH. Developing and Comparing 2,6-Anthracene Derivatives: Optical, Electrochemical, Thermal, and Their Use in Organic Thin Film Transistors. Materials. 2020; 13(8):1961. https://doi.org/10.3390/ma13081961
Chicago/Turabian StyleVorona, Mikhail Y., Nathan J. Yutronkie, Owen A. Melville, Andrew J. Daszczynski, Jeffrey S. Ovens, Jaclyn L. Brusso, and Benoît H. Lessard. 2020. "Developing and Comparing 2,6-Anthracene Derivatives: Optical, Electrochemical, Thermal, and Their Use in Organic Thin Film Transistors" Materials 13, no. 8: 1961. https://doi.org/10.3390/ma13081961