Versatile Movements of Liquid Metal Droplet under Electrostatic Actuation in Alkaline Solutions
Abstract
1. Introduction
2. Materials and Theory
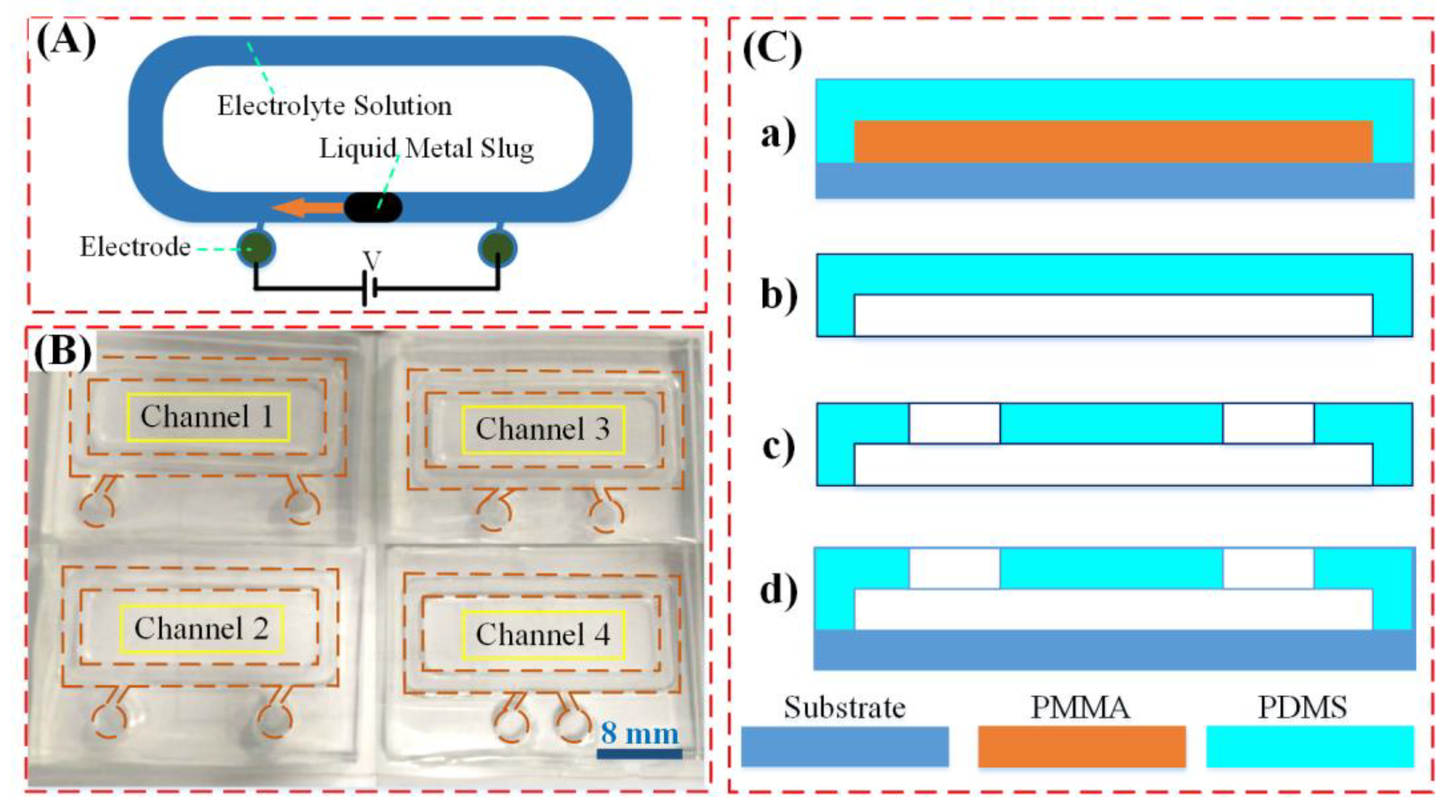
2.1. Experimental Design and Chip Fabrication
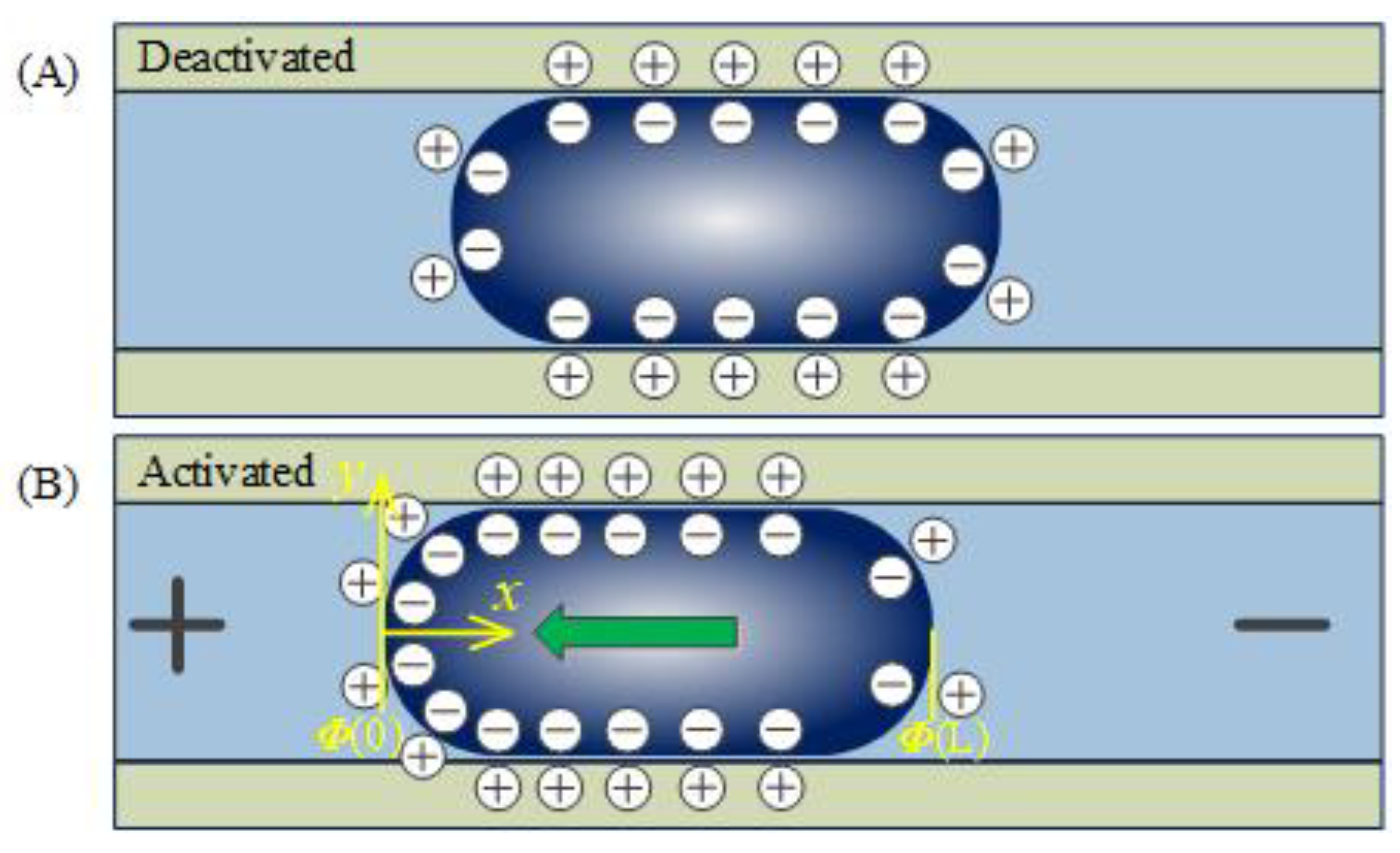
2.2. Continuous Electrowetting Actuation
3. Results and Discussion
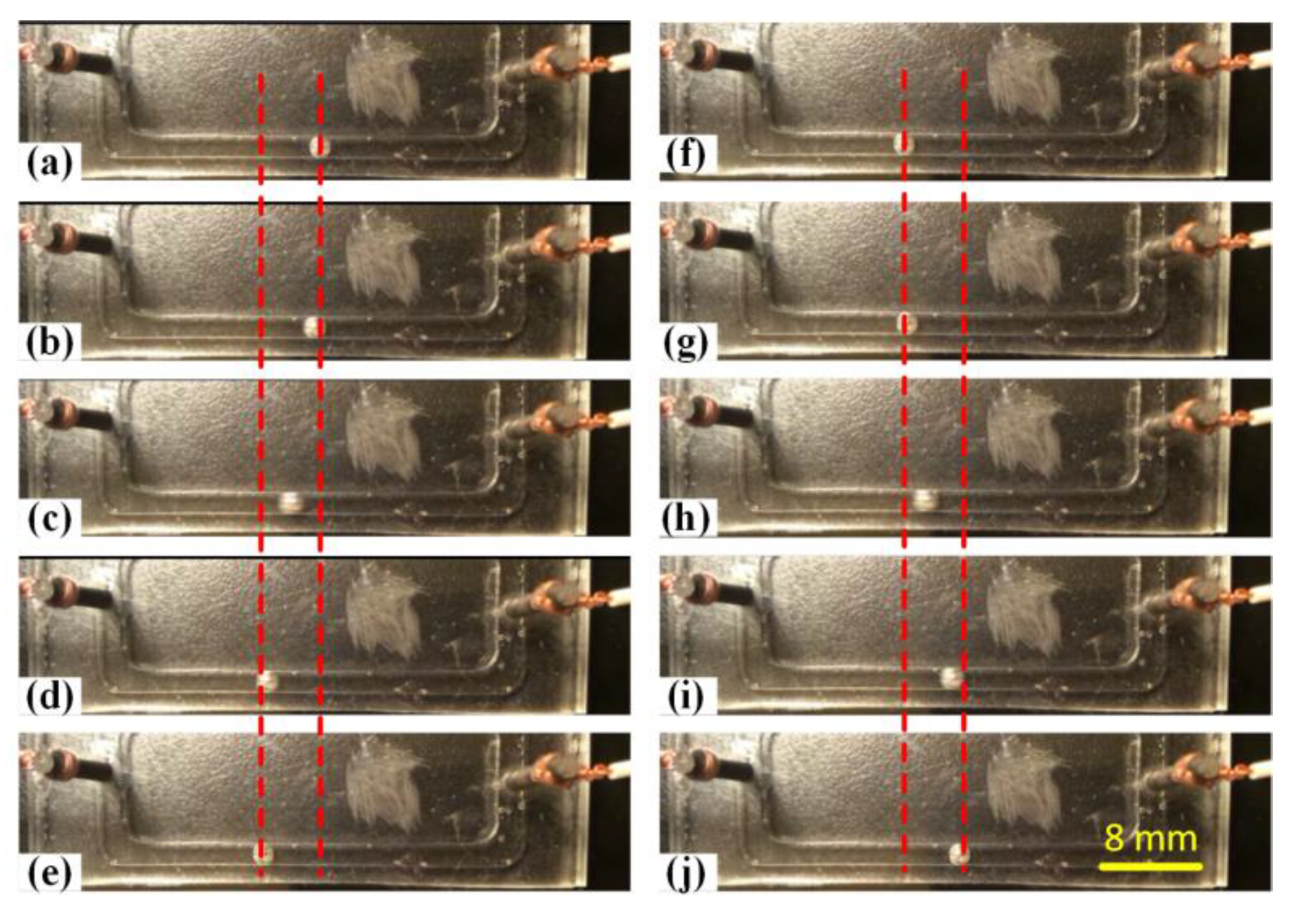
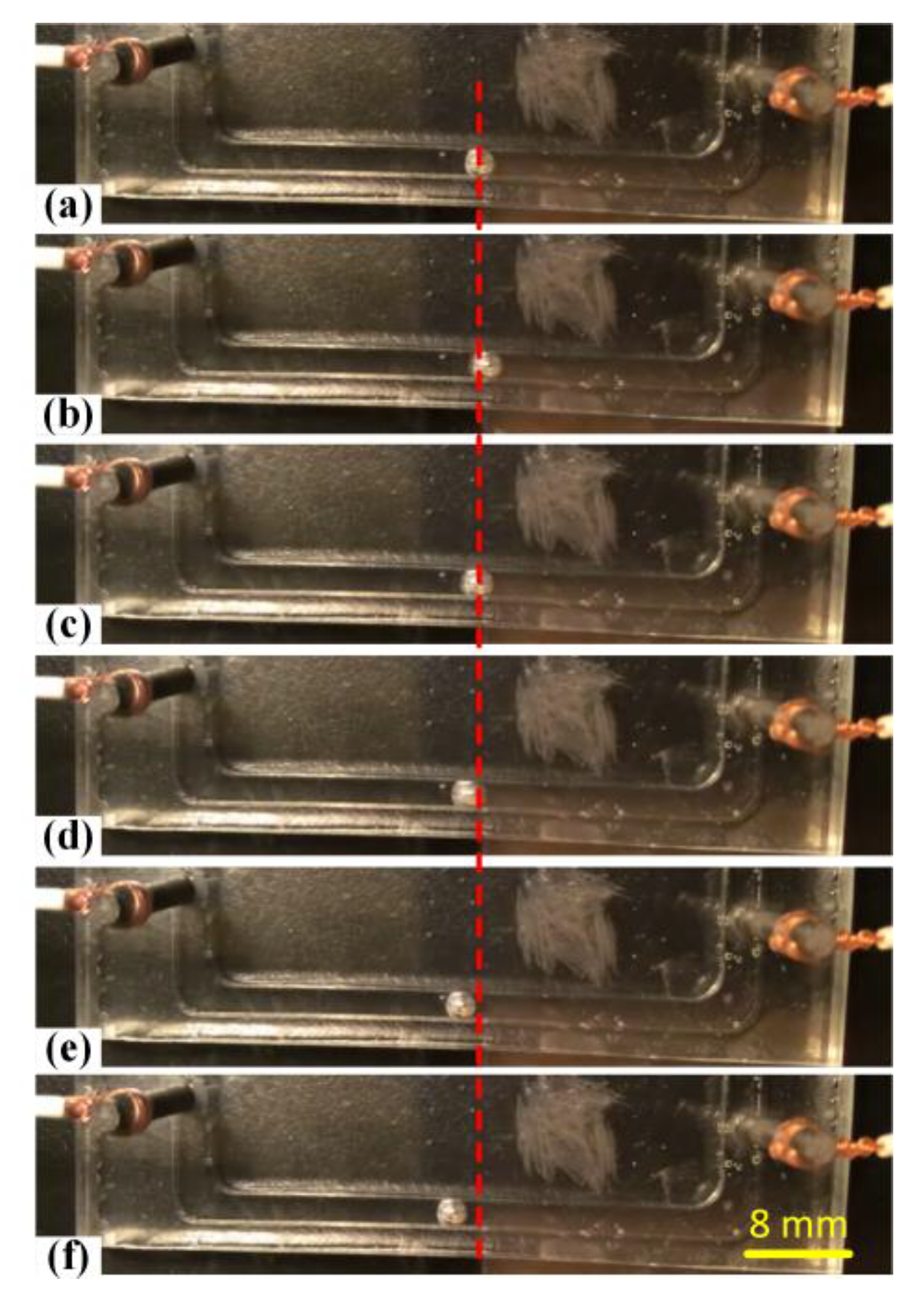
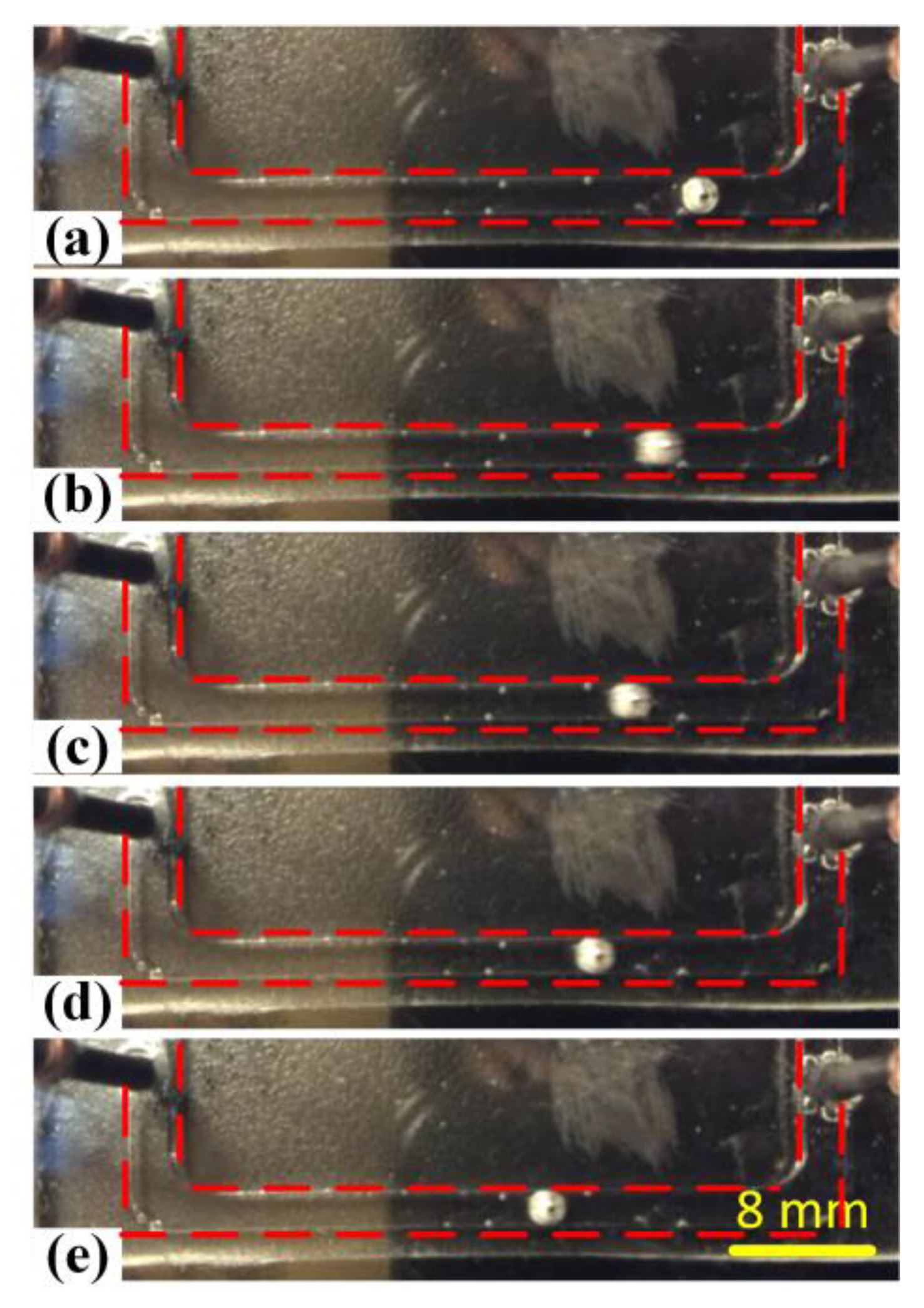
3.1. Effect of Electrolyte Concentration
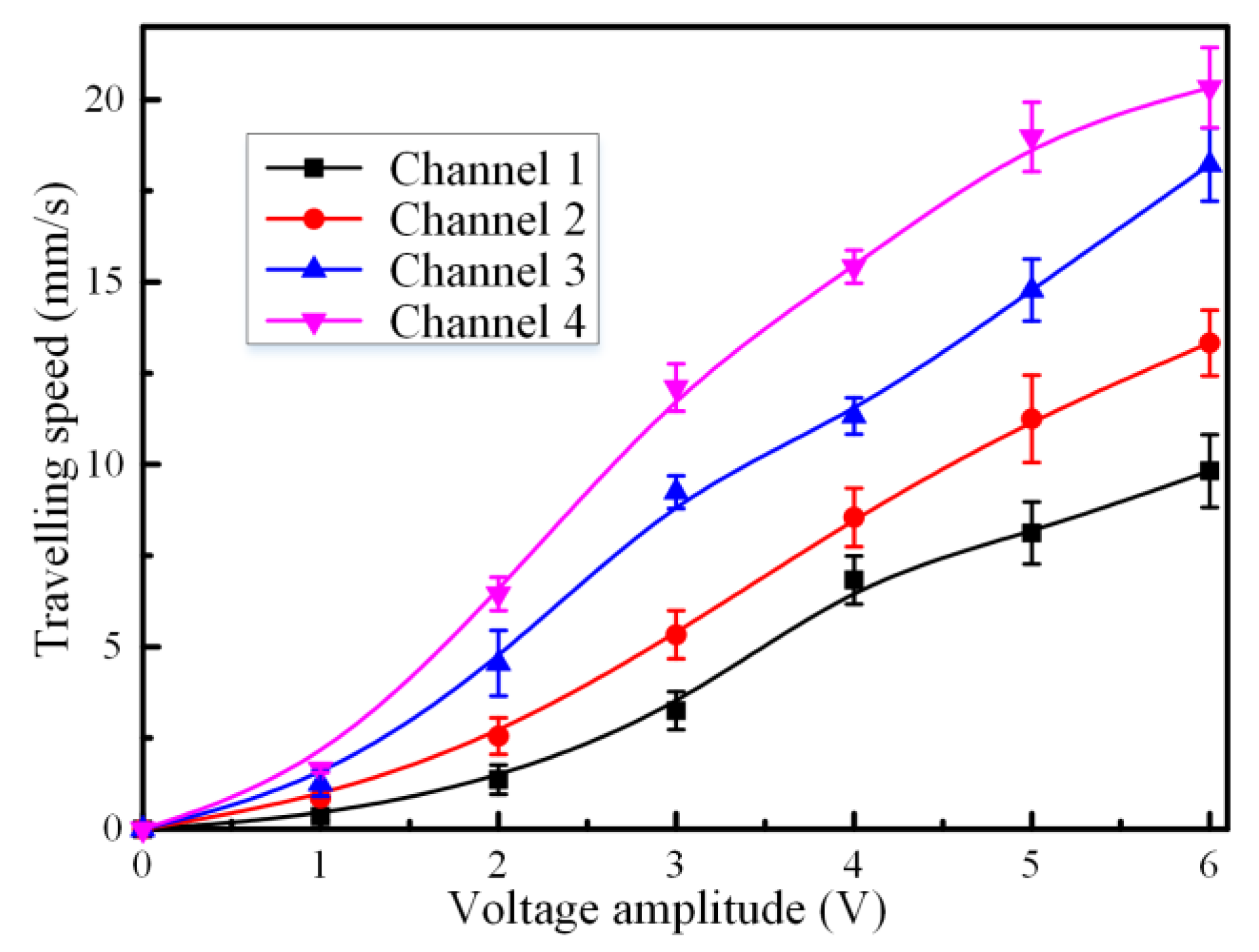
3.2. Effect of Voltage Amplitude of Applied Electric Field
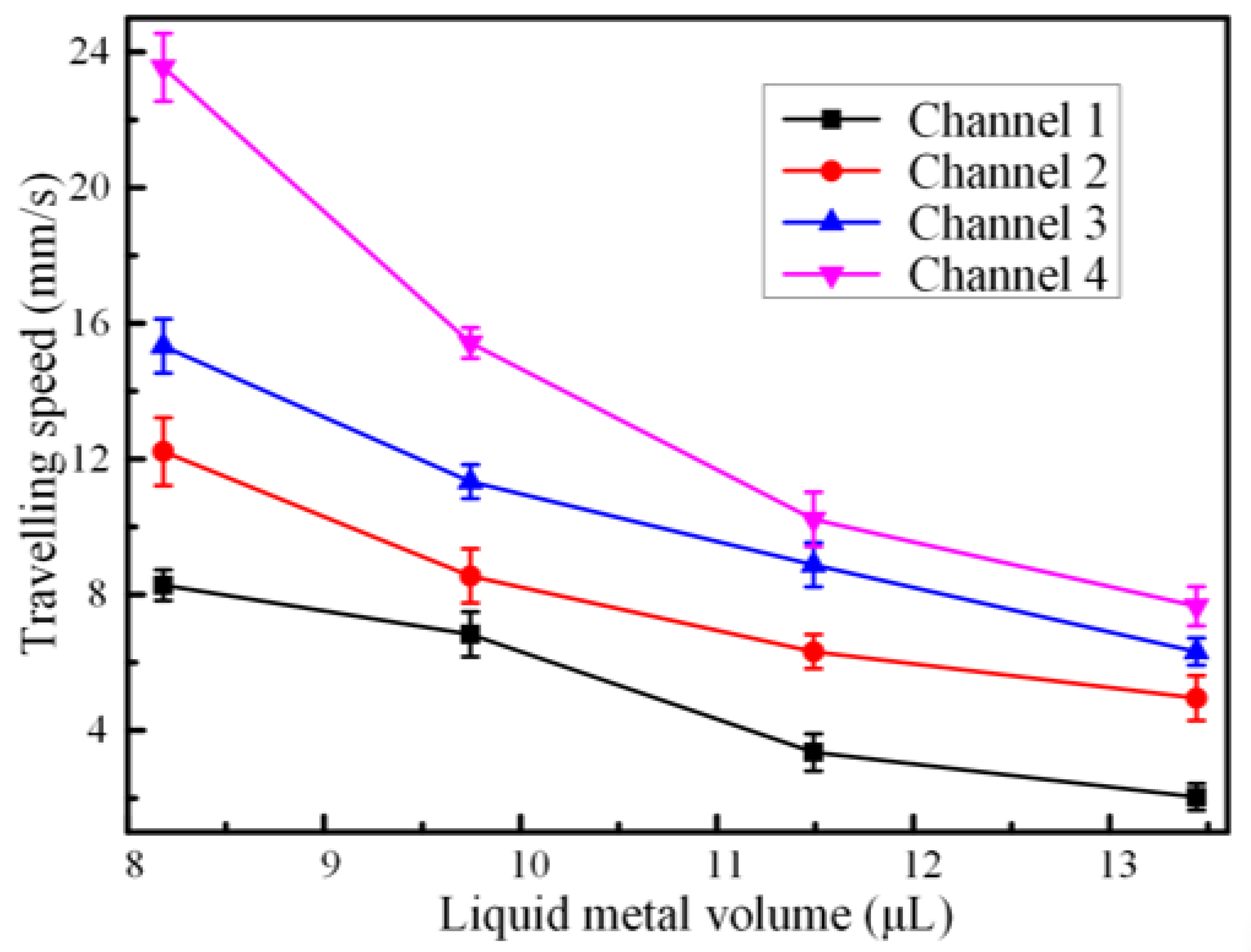
3.3. Effect of Galinstan Liquid Metal Volume
3.4. Effect of the Frequency of Alternating Current Electrical Field
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abalde-Cela, S.; Taladriz-Blanco, P.; Oliveira, M.G.D.; Abell, C. Droplet microfluidics for the highly controlled synthesis of branched gold nanoparticles. Sci. Rep. 2018, 8, 2440. [Google Scholar] [CrossRef]
- Moon, H.S.; Je, K.; Min, J.W.; Park, D.; Han, K.-Y.; Shin, S.-H.; Park, W.-Y.; Chang, E.Y.; Kim, S.H. Inertial-ordering-assisted droplet microfluidics for high-throughput single-cell RNA-sequencing. Lab Chip 2018, 18, 775–784. [Google Scholar] [CrossRef]
- Thomas, T.; Unni, H.N. LED-based opto-wetting and fluidic transport for droplet mixing. Microfluid. Nanofluid. 2019, 23. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, P.; Tian, Y.; Xu, M.; Wang, L. Engineering micromotors with droplet microfluidics. ACS Nano 2019, 13, 6319–6329. [Google Scholar] [CrossRef]
- Bhushan, B.; Ling, X. Manipulating microobject by using liquid droplet as a transporting vehicle. J. Colloid Interface Sci. 2009, 329, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhou, Y.; Yuan, F.; Mo, X.; Zhou, J. Self-Powered Multimodal Temperature and Force Sensor Based-On a Liquid Droplet. Angew. Chem. Int. Ed. 2016, 55, 15864–15868. [Google Scholar] [CrossRef]
- Angiulli, G.; Jannelli, A.; Morabito, F.C.; Versaci, M. Reconstructing the membrane detection of a 1D electrostatic-driven MEMS device by the shooting method: Convergence analysis and ghost solutions identification. Comput. Appl. Math. 2018, 37, 4484–4498. [Google Scholar] [CrossRef]
- Di Barba, P.F.L.; Versaci, M. Electrostatic field in terms of geometric curvature in membrane MEMS devices. Commun. Appl. Ind. Math. 2017, 8, 165–184. [Google Scholar] [CrossRef]
- Joshipura, I.D.; Ayers, H.R.; Majidi, C.; Dickey, M.D. Methods to pattern liquid metals. J. Mater. Chem. C 2015, 3, 3834–3841. [Google Scholar] [CrossRef]
- Cho, H.J.; Sresht, V.; Wang, E.N. Predicting Surface Tensions of Surfactant Solutions from Statistical Mechanics. Langmuir 2018, 34, 2386–2395. [Google Scholar] [CrossRef]
- Shin, J.Y.; Abbott, N.L. Using light to control dynamic surface tensions of aqueous solutions of water soluble surfactants. Langmuir 1999, 15, 4404–4410. [Google Scholar] [CrossRef]
- Eastoe, J.; Dalton, J. Dynamic surface tension and adsorption mechanisms of surfactants at the air–water interface. Adv. Colloid Interface Sci. 2000, 85, 103–144. [Google Scholar] [CrossRef]
- Chen, J.Z.; Troian, S.M.; Darhuber, A.A.; Wagner, S. Effect of contact angle hysteresis on thermocapillary droplet actuation. J. Appl. Phys. 2005, 97, 014906. [Google Scholar] [CrossRef]
- Wu, Z.-B.; Hu, W.-R. Thermocapillary migration of a planar droplet at moderate and large Marangoni numbers. Acta Mech. 2012, 223, 609–626. [Google Scholar] [CrossRef][Green Version]
- Karapetsas, G.; Chamakos, N.T.; Papathanasiou, A.G. Thermocapillary droplet actuation: Effect of solid structure and wettability. Langmuir 2017, 33, 10838–10850. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Feng, L.; Jiang, L. Tunable adhesive superhydrophobic surfaces for superparamagnetic microdroplets. Adv. Funct. Mater. 2008, 18, 3219–3225. [Google Scholar] [CrossRef]
- Wauer, T.; Gerlach, H.; Mantri, S.; Hill, J.; Bayley, H.; Sapra, K.T. Construction and manipulation of functional three-dimensional droplet networks. ACS Nano 2014, 8, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Zhang, N.; Zheng, X.; Hou, G.; Tian, Y.; Du, Y.; Jiang, L.; Dou, S.X. Fast responsive and controllable liquid transport on a magnetic fluid/nanoarray composite interface. ACS Nano 2016, 10, 6220–6226. [Google Scholar] [CrossRef] [PubMed]
- Velev, O.D.; Prevo, B.G.; Bhatt, K.H. On-chip manipulation of free droplets. Nature 2003, 426, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Millman, J.R.; Bhatt, K.H.; Prevo, B.G.; Velev, O.D. Anisotropic particle synthesis in dielectrophoretically controlled microdroplet reactors. Nat. Mater. 2005, 4, 98–102. [Google Scholar] [CrossRef]
- Gong, J. All-electronic droplet generation on-chip with real-time feedback control for EWOD digital microfluidics. Lab Chip 2008, 8, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Im, D.J.; Noh, J.; Moon, D.; Kang, I.S. Electrophoresis of a charged droplet in a dielectric liquid for droplet actuation. Anal. Chem. 2011, 83, 5168–5174. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Lu, Y.; Chen, G.; Yang, M.; Gu, Z. Advances in liquid metals for biomedical applications. Chem. Soc. Rev. 2018, 47, 2518–2533. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tang, S.Y.; Fang, T.; Li, W.; Li, X.; Zhang, S. A Wheeled Robot Driven by a Liquid-Metal Droplet. Adv. Mater. 2018, 30, e1805039. [Google Scholar] [CrossRef] [PubMed]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-Zadeh, K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Deng, W.; Jin, L.; Chun, F.; Pan, H.; Gu, B.; Zhang, H.; Lv, Z.; Yang, W.; et al. Self-Powered Acceleration Sensor Based on Liquid Metal Triboelectric Nanogenerator for Vibration Monitoring. ACS Nano 2017, 11, 7440–7446. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Khoshmanesh, K.; Sivan, V.; Petersen, P.; O’Mullane, A.P.; Abbott, D.; Mitchell, A.; Kalantar-zadeh, K. Liquid metal enabled pump. Proc. Natl. Acad. Sci. USA 2014, 111, 3304–3309. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Sivan, V.; Petersen, P.; Zhang, W.; Morrison, P.D.; Kalantar-zadeh, K.; Mitchell, A.; Khoshmanesh, K. Liquid Metal Actuator for Inducing Chaotic Advection. Adv. Funct. Mater. 2014, 24, 5851–5858. [Google Scholar] [CrossRef]
- Yao, Y.-Y.; Liu, J. A polarized liquid metal worm squeezing across a localized irregular gap. RSC Adv. 2017, 7, 11049–11056. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, R.; Liu, J. Self-propelled liquid metal motors steered by a magnetic or electrical field for drug delivery. J. Mater. Chem. B 2016, 4, 5349–5357. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, J. A liquid metal cooling system for the thermal management of high power LEDs. Int. Commun. Heat Mass Transf. 2010, 37, 788–791. [Google Scholar] [CrossRef]
- Je, J.; Lee, J. Design, fabrication, and characterization of liquid metal microheaters. J. Microelectromech. Syst. 2014, 23, 1156–1163. [Google Scholar]
- Jin, C.; Zhang, J.; Li, X.; Yang, X.; Li, J.; Liu, J. InjecTable 3-D fabrication of medical electronics at the target biological tissues. Sci. Rep. 2013, 3, 3442. [Google Scholar] [CrossRef] [PubMed]
- Shay, T.; Velev, O.D.; Dickey, M.D. Soft electrodes combining hydrogel and liquid metal. Soft Matter 2018, 14, 3296–3303. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yang, X.; Jin, C.; Fu, J.; Chen, W.; Liu, J. Development of three-dimension microelectrode array for bioelectric measurement using the liquidmetal-micromolding technique. Appl. Phys. Lett. 2013, 103, 193701. [Google Scholar] [CrossRef]
- Li, F.; Kuang, S.; Li, X.; Shu, J.; Li, W.; Tang, S.-Y.; Zhang, S. Magnetically- and Electrically-Controllable Functional Liquid Metal Droplets. Adv. Mater. Technol. 2019, 4, 1800694. [Google Scholar] [CrossRef]
- Wang, M.; Trlica, C.; Khan, M.R.; Dickey, M.D.; Adams, J.J. A reconfigurable liquid metal antenna driven by electrochemically controlled capillarity. J. Appl. Phys. 2015, 117, 194901. [Google Scholar] [CrossRef]
- Rashed Khan, M.; Hayes, G.J.; So, J.-H.; Lazzi, G.; Dickey, M.D. A frequency shifting liquid metal antenna with pressure responsiveness. Appl. Phys. Lett. 2011, 99, 013501. [Google Scholar] [CrossRef]
- Kubo, M.; Li, X.; Kim, C.; Hashimoto, M.; Wiley, B.J.; Ham, D.; Whitesides, G.M. Stretchable microfluidic radiofrequency antennas. Adv. Mater. 2010, 22, 2749–2752. [Google Scholar] [CrossRef]
- Cheng, S.; Rydberg, A.; Hjort, K.; Wu, Z. Liquid metal stretchable unbalanced loop antenna. Appl. Phys. Lett. 2009, 94, 144103. [Google Scholar] [CrossRef]
- Wan, Z.; Zeng, H.; Feinerman, A. Area-tunable micromirror based on electrowetting actuation of liquid-metal droplets. Appl. Phys. Lett. 2006, 89, 201107. [Google Scholar] [CrossRef]
- Junghoon, L.; Chang-Jin, K. Surface-tension-driven microactuation based on continuous electrowetting. J. Microelectromech. Syst. 2000, 9, 171–180. [Google Scholar] [CrossRef]
- Wan, Z.; Zeng, H.; Feinerman, A. Reversible Electrowetting of Liquid-Metal Droplet. J. Fluids Eng. 2007, 129, 388. [Google Scholar] [CrossRef]
- Hong, F.J.; Jiang, D.D.; Cheng, P. Frequency-dependent resonance and asymmetric droplet oscillation under ac electrowetting on coplanar electrodes. J. Micromech. Microeng. 2012, 22, 85024. [Google Scholar] [CrossRef]
- Gough, R.C.; Morishita, A.M.; Dang, J.H.; Hu, W.; Shiroma, W.A.; Ohta, A.T. Continuous electrowetting of non-toxic liquid metal for RF applications. IEEE Access 2014, 2, 874–882. [Google Scholar] [CrossRef]
- Beni, G. Continuous electrowetting effect. Appl. Phys. Lett. 1982, 40, 912. [Google Scholar] [CrossRef]
- Gough, R.C.; Morishita, A.M.; Dang, J.H.; Moorefield, M.R.; Shiroma, W.A.; Ohta, A.T. Rapid electrocapillary deformation of liquid metal with reversible shape retention. Micro Nano Syst. Lett. 2015, 3, 1796. [Google Scholar] [CrossRef]
- Gough, R.C.; Dang, J.H.; Morishita, A.M.; Ohta, A.T.; Shiroma, W.A. Frequency-tunable slot antenna using continuous electrowetting of liquid metal. In Proceedings of the 2014 IEEE MTT-S International Microwave Symposium (IMS2014), Tampa, FL, USA, 1–6 June 2014. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Jiang, T.; Jiang, H. Versatile Movements of Liquid Metal Droplet under Electrostatic Actuation in Alkaline Solutions. Materials 2020, 13, 2122. https://doi.org/10.3390/ma13092122
Hu Q, Jiang T, Jiang H. Versatile Movements of Liquid Metal Droplet under Electrostatic Actuation in Alkaline Solutions. Materials. 2020; 13(9):2122. https://doi.org/10.3390/ma13092122
Chicago/Turabian StyleHu, Qingming, Tianyi Jiang, and Hongyuan Jiang. 2020. "Versatile Movements of Liquid Metal Droplet under Electrostatic Actuation in Alkaline Solutions" Materials 13, no. 9: 2122. https://doi.org/10.3390/ma13092122
APA StyleHu, Q., Jiang, T., & Jiang, H. (2020). Versatile Movements of Liquid Metal Droplet under Electrostatic Actuation in Alkaline Solutions. Materials, 13(9), 2122. https://doi.org/10.3390/ma13092122



