Hot Corrosion and Mechanical Performance of Repaired Inconel 718 Components via Laser Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
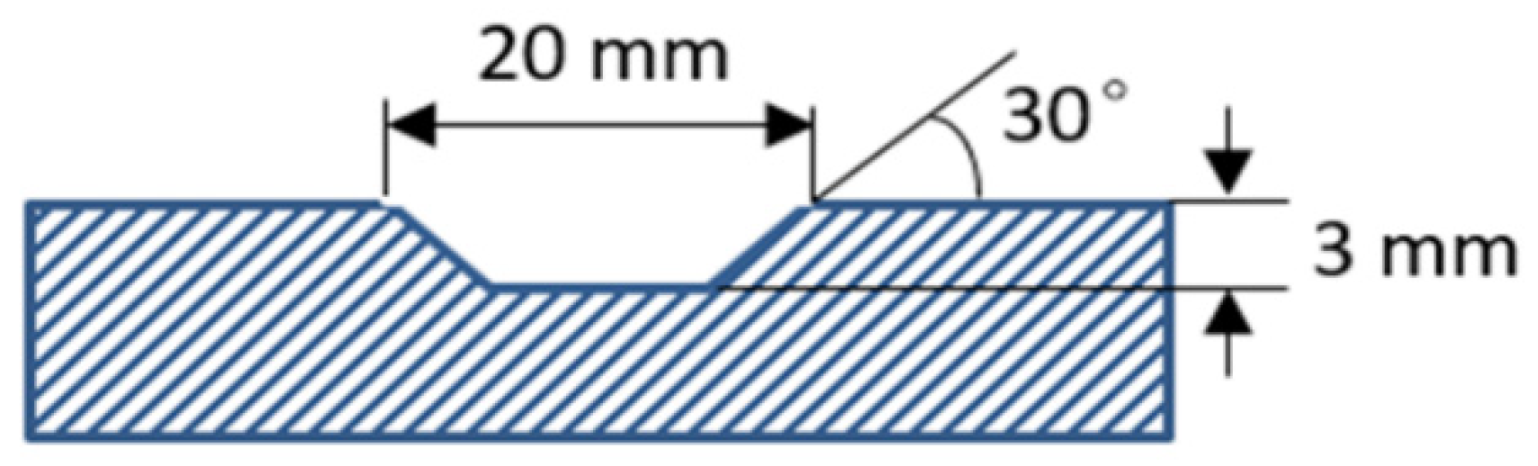
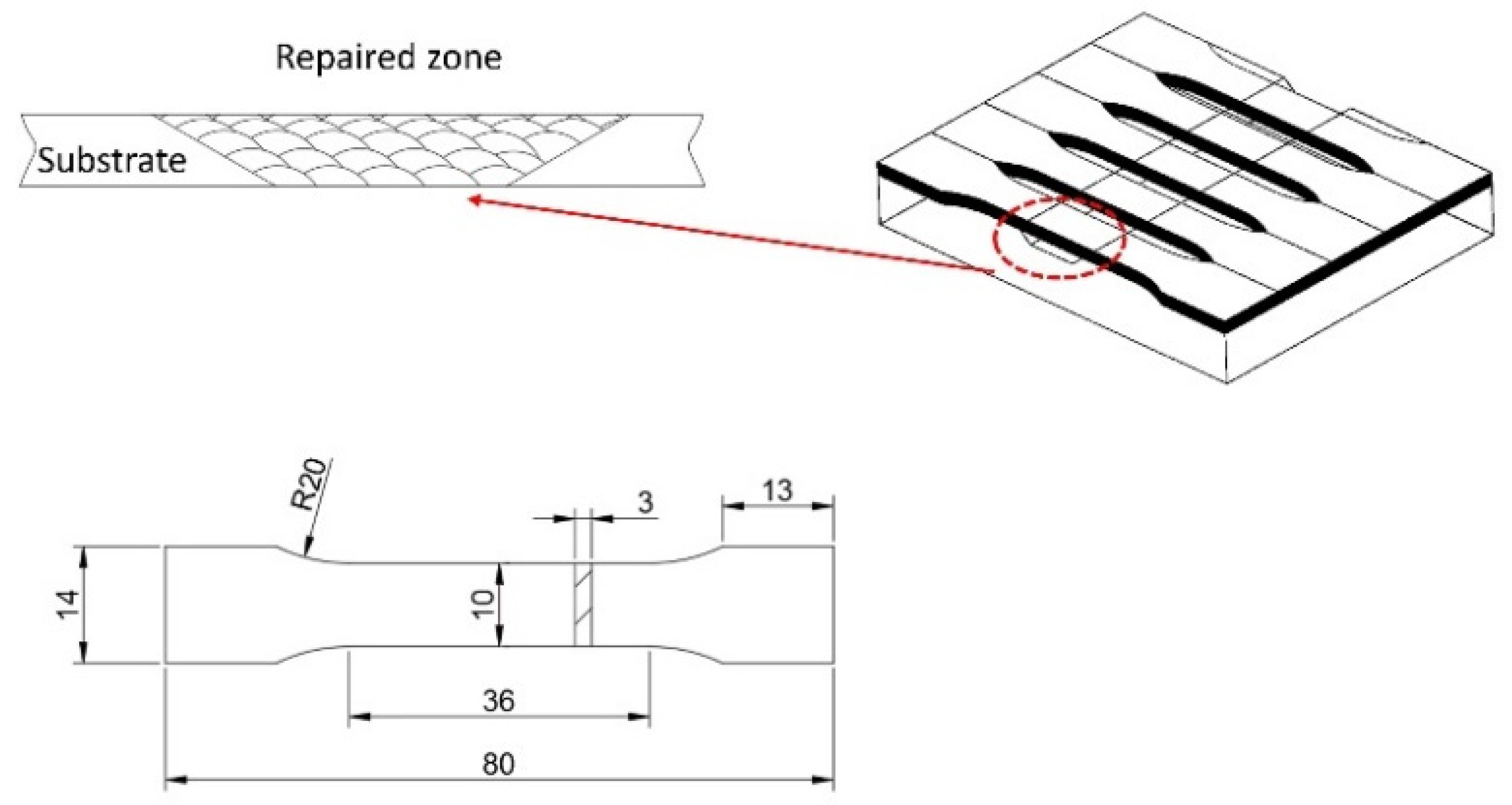
2.1. Materials and Laser Repair Experiment
2.2. Hot Corrosion Testing
2.3. Performance Characterization
3. Results
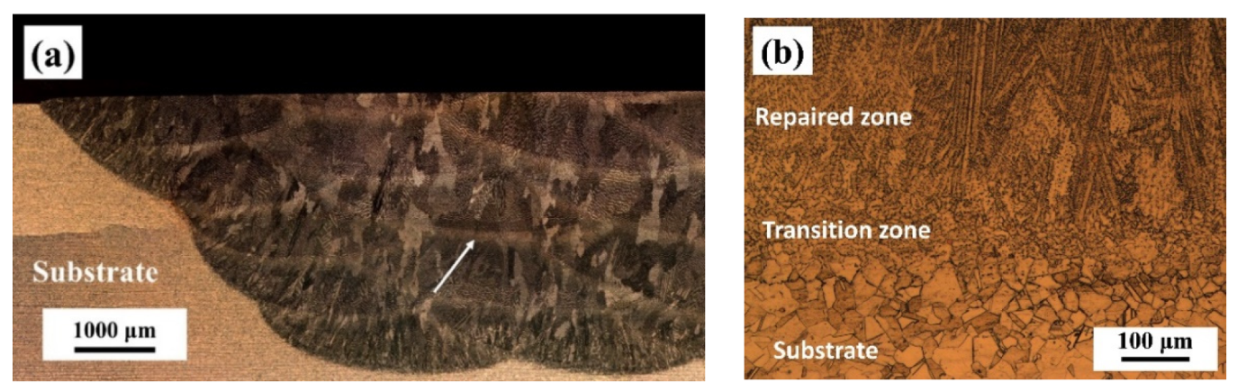
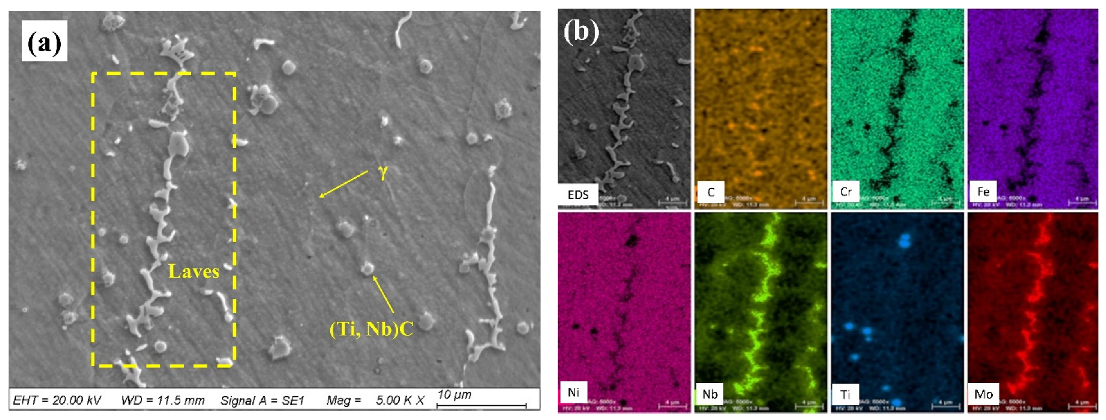
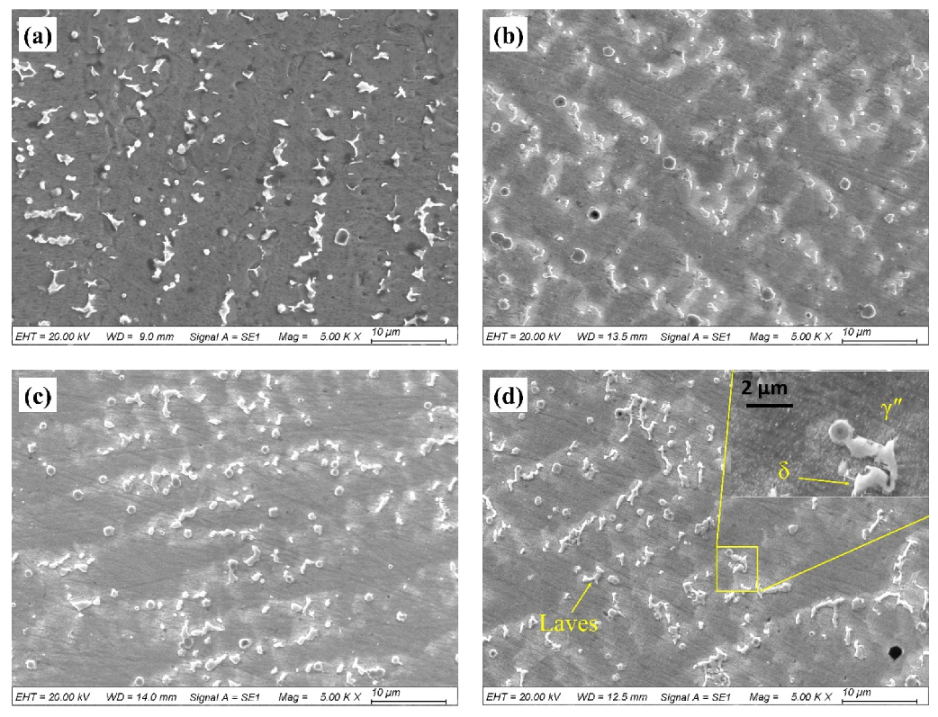
3.1. Microstructures and Phases
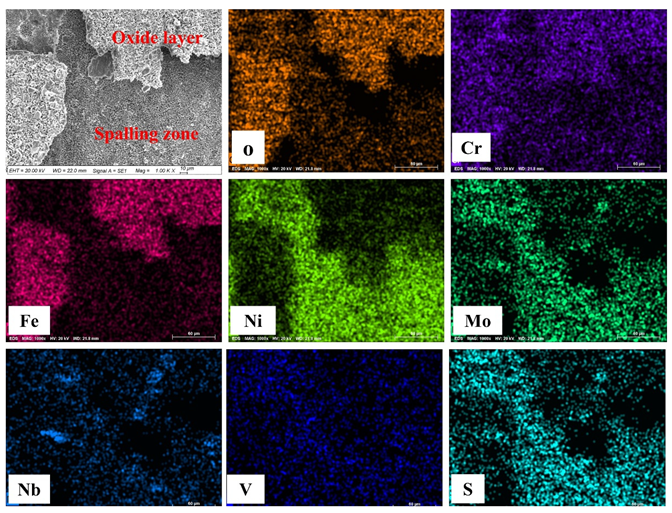
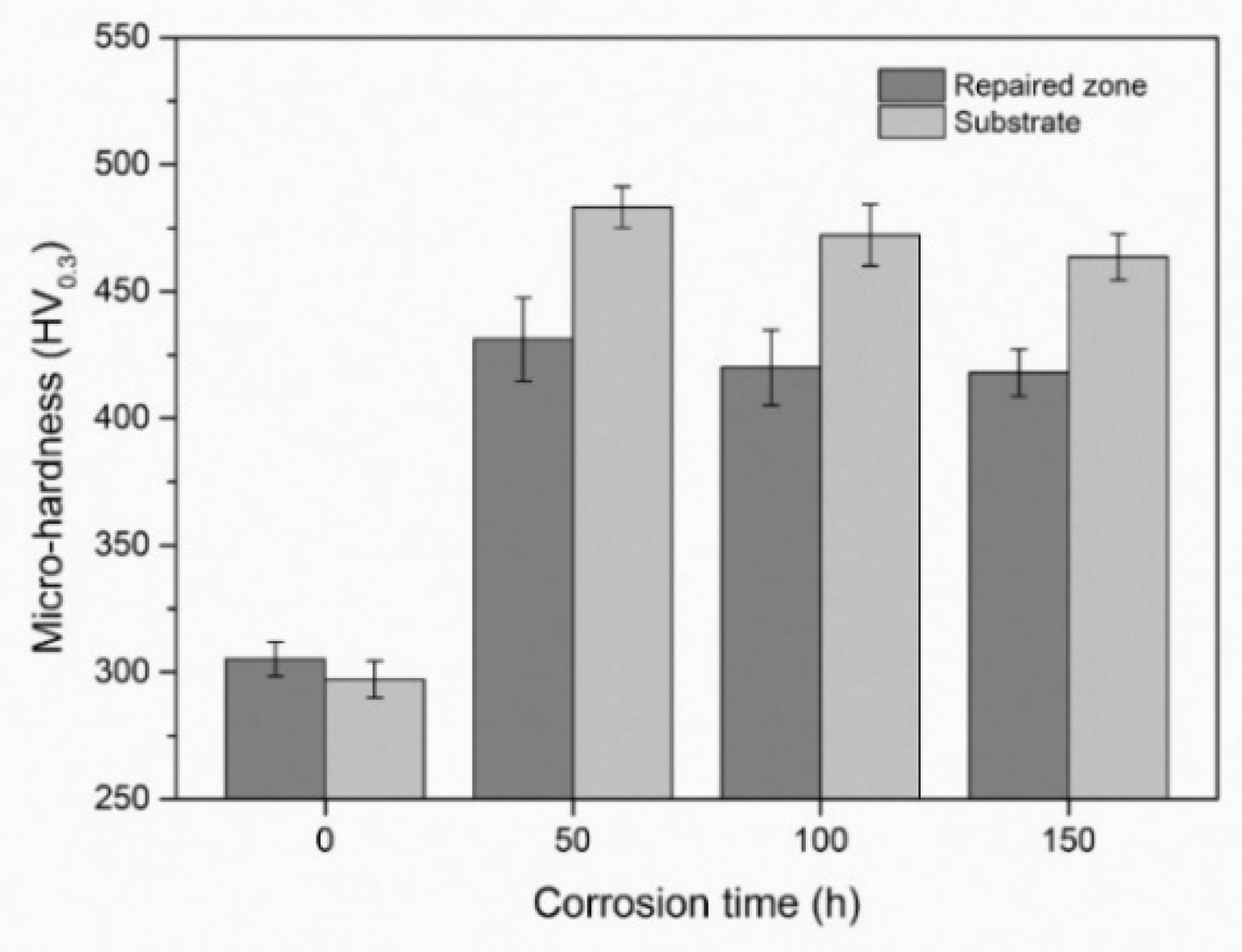
3.2. Hot Corrosion Performance
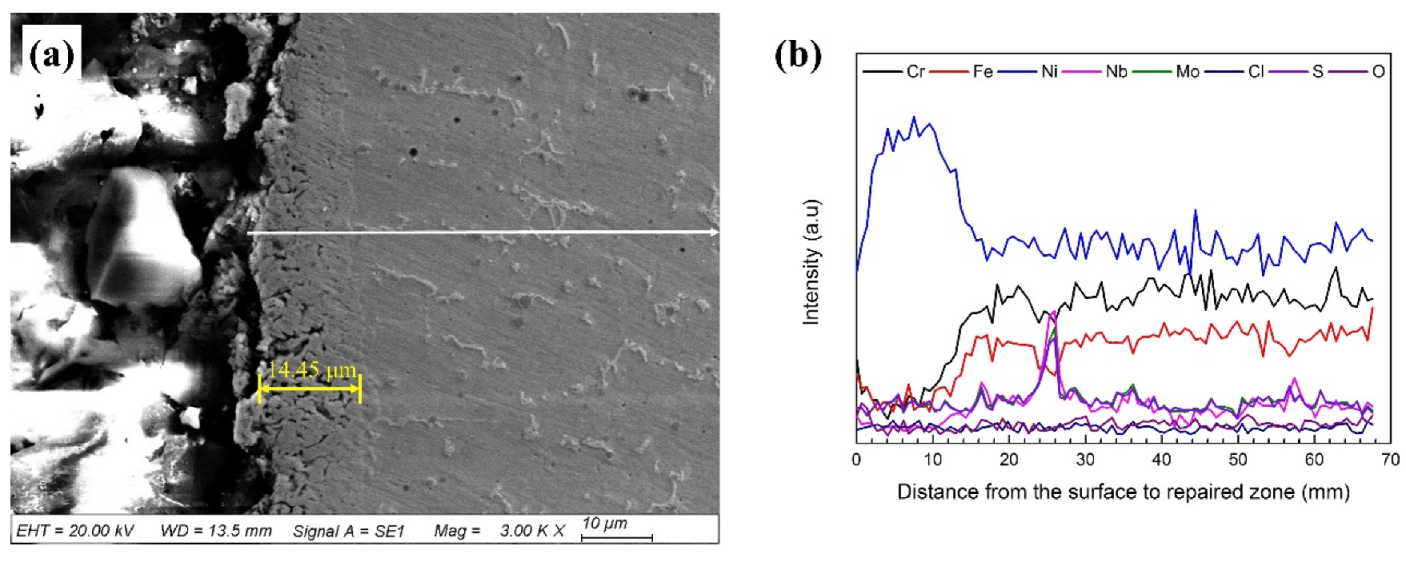
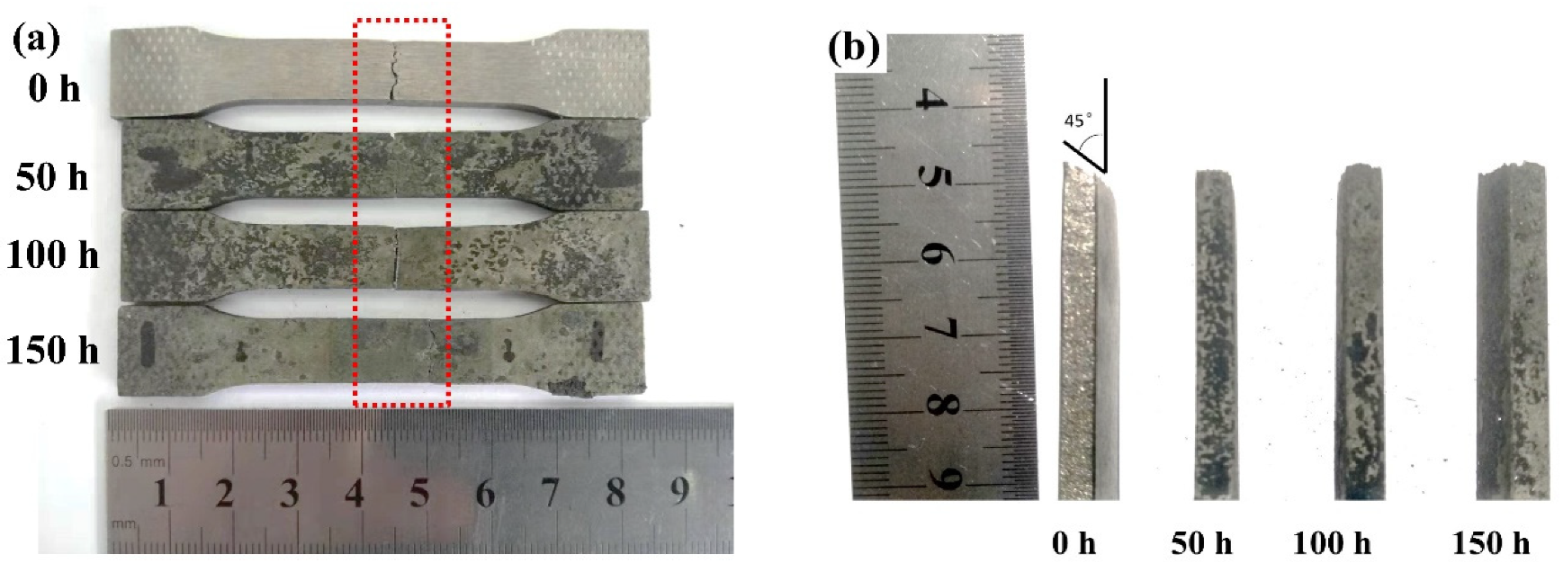
3.3. Microstructural Change Due to Hot Corrosion
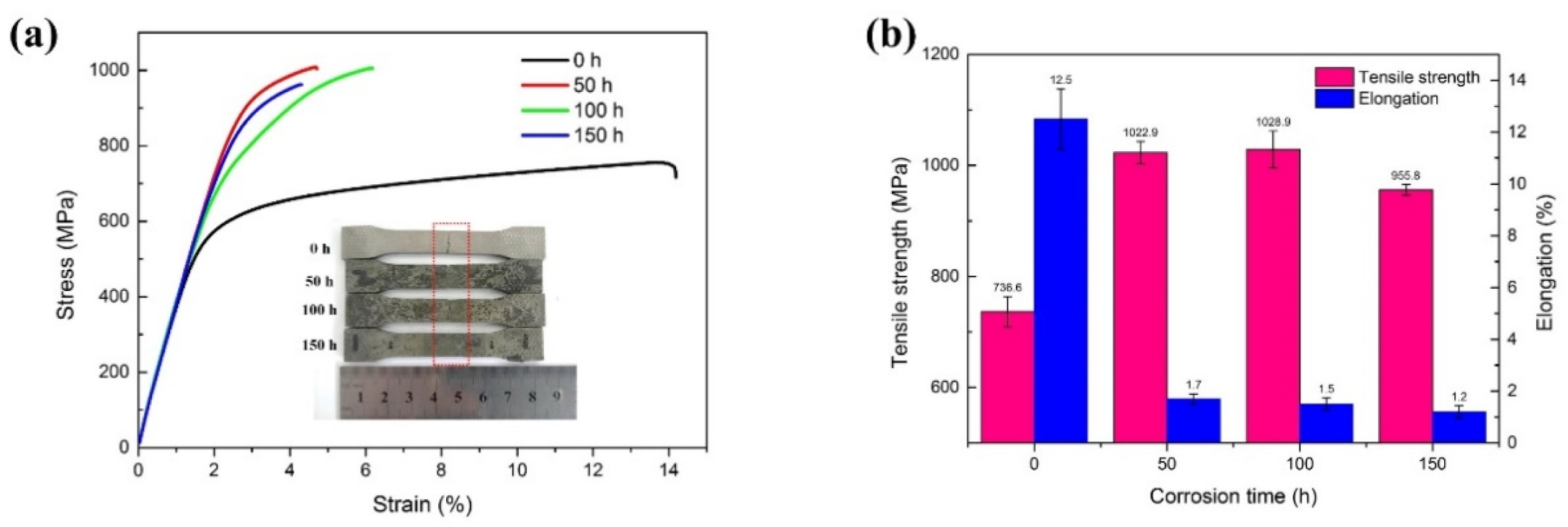
3.4. Mechanical Properties
3.4.1. Micro-Hardness
3.4.2. Tensile Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vakili-Tahami, F.; Adibeig, M.R. Investigating the possibility of replacing IN 738LC gas turbine blades with IN 718. Mech. Sci. Technol. 2015, 29, 4167–4178. [Google Scholar] [CrossRef]
- Huang, L.; Qi, F.; Yu, L.; Xin, X.; Liu, F.; Sun, W.; Hu, Z. Necking behavior and microstructural evolution during high strain rate superplastic deformation of IN718 superalloy. Mater. Sci. Eng. A Struct. 2015, 634, 71–76. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, K.; Gao, M.; Li, X.; Chen, X.; Zeng, X. The microstructure and mechanical properties of deposited-IN718 by selective laser melting. J. Alloy. Compd. 2012, 513, 518–523. [Google Scholar] [CrossRef]
- Salehnasab, B.; Poursaeidi, E.; Mortazavi, S.A.; Farokhian, G.H. Hot corrosion failure in the first stage nozzle of a gas turbine engine. Eng. Fail. Anal. 2016, 60, 316–325. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, M.; Handa, A. Combating hot corrosion of boiler tubes—A study. Eng. Fail. Anal. 2018, 94, 379–395. [Google Scholar] [CrossRef]
- Kanesund, J.; Brodin, H.; Johansson, S. Hot corrosion influence on deformation and damage mechanisms in turbine blades made of IN-792 during service. Eng. Fail. Anal. 2019, 96, 118–129. [Google Scholar] [CrossRef]
- Sahraeian, R.; Omidvar, H.; Hadavi, S.M.M.; Shakerin, S.; Maleki, V. An Investigation on High-Temperature Oxidation and Hot Corrosion Resistance Behavior of Coated TLP (Transient Liquid Phase)-Bonded IN738-LC. Trans. Indian Inst. Met. 2018, 71, 2903–2918. [Google Scholar] [CrossRef]
- Liu, W.; Hou, Y.; Liu, C.; Wang, Y.; Jiang, R.; Xu, G. Hot corrosion behavior of a centimeter Fe-based amorphous composite coating prepared by laser cladding in molten Na2SO4+K2SO4 salts. Surf. Coat. Technol. 2015, 270, 33–38. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Bai, P.; Qu, H.; Liu, B.; Li, L.; Wu, L.; Guan, R.; Liu, H.; Guo, Z. Microstructural evolution and mechanical properties of IN718 alloy fabricated by selective laser melting following different heat treatments. J. Alloy. Compd. 2019, 772, 861–870. [Google Scholar] [CrossRef]
- Jia, Q.; Gu, D. Selective laser melting additive manufacturing of Inconel 718 superalloy parts: Densification, microstructure and properties. J. Alloy. Compd. 2014, 585, 713–721. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.L.; Chen, Z.J.; Li, D.; Yao, J.H.; Liu, R. Effects of solution temperature on microstructure and properties of Inconel 718 alloy fabricated via laser additive manufacturing. Surf. Technol. 2019, 48, 47–53. [Google Scholar]
- Chen, Y.; Lu, F.; Zhang, K.; Nie, P.; Elmi Hosseini, S.R.; Feng, K.; Li, Z. Laser powder deposition of carbon nanotube reinforced nickel-based superalloy Inconel 718. Carbon 2016, 107, 361–370. [Google Scholar] [CrossRef]
- Chenna Krishna, S.; Agilan, M.; Sudarshan Rao, G.; Singh, S.K.; Narayana Murty, S.V.S.; Venkata Narayana, G.; Beena, A.P.; Rajesh, L.; Jha, A.K.; Pant, B.; et al. Design, development and testing of Inconel alloy IN718 spherical gas bottle for oxygen storage. J. Mater. Eng. Perform. 2017, 26, 5355–5365. [Google Scholar] [CrossRef]
- Lu, J.; Yang, Z.; Zhou, Y.; Huang, J.; Yan, J.; Dang, Y.; Yuan, Y.; Gu, Y. Failure analysis for a dry-pulverized coal gasifier burner made up of Inconel 718 superalloy. Eng. Fail. Anal. 2019, 97, 227–233. [Google Scholar] [CrossRef]
- Graf, B.; Ammer, S.; Gumenyuk, A.; Rethmeier, M. Design of Experiments for Laser Metal Deposition in Maintenance, Repair and Overhaul Applications. Procedia CIRP 2013, 11, 245–248. [Google Scholar] [CrossRef]
- Pei, R.; Wang, Q.Y.; Tang, M.; Jiang, Y.M.; Xi, Y. Research status of erosion-corrosion of laser cladding layer. Surf. Technol. 2019, 48, 179–187. [Google Scholar]
- Lin, C.-M. Parameter optimization of laser cladding process and resulting microstructure for the repair of tenon on steam turbine blade. Vacuum 2019, 115, 117–123. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Zheng, H.; Tang, K.; Li, H.; Gong, S. TC17 titanium alloy laser melting deposition repair process and properties. Opt. Laser Technol. 2016, 82, 1–9. [Google Scholar] [CrossRef]
- Vilar, R.; Almeida, A. Repair and manufacturing of single crystal Ni-based superalloys components by laser powder deposition—A review. J. Laser Appl. 2015, 27, S17004. [Google Scholar] [CrossRef]
- Onuike, B.; Bandyopadhyay, A. Additive manufacturing in repair: Influence of processing parameters on properties of Inconel 718. Mater. Lett. 2019, 252, 256–259. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Y.; Xu, M.; Ma, C.; Zhang, Q.; Wang, L.; Yao, J.; Li, Z. Study on the element segregation and Laves phase formation in the laser metal deposited IN718 superalloy by flat top laser and gaussian distribution laser. Mater. Sci. Eng. A 2019, 754, 339–347. [Google Scholar] [CrossRef]
- Zhang, Q.-l.; Yao, J.-H.; Mazumder, J. Laser Direct metal deposition technology and microstructure and composition segregation of Inconel 718 superalloy. J. Iron Steel Res. Int. 2011, 18, 73–78. [Google Scholar] [CrossRef]
- Wang, X.; Gong, X.; Chou, K. Review on powder-bed laser additive manufacturing of Inconel 718 parts. Proc. Inst. Mech. Eng. B J. Eng. 2016, 231, 1890–1903. [Google Scholar] [CrossRef]
- Jia, Q.; Gu, D. Selective laser melting additive manufactured Inconel 718 superalloy parts: High-temperature oxidation property and its mechanisms. Opt. Laser Technol. 2014, 62, 161–171. [Google Scholar] [CrossRef]
- Sadeghi, E.; Karimi, P.; Momeni, S.; Seifi, M.; Eklund, A.; Andersson, J. Influence of thermal post treatments on microstructure and oxidation behavior of EB-PBF manufactured Alloy 718. Mater. Charact. 2019, 150, 236–251. [Google Scholar] [CrossRef]
- Kang, J.; Yang, S.; Kim, Y.; AlMangour, B.; Lee, K. Effect of post-treatment on the microstructure and high-temperature oxidation behaviour of additively manufactured Inconel 718 alloy. Corros. Sci. 2019, 158, 108082. [Google Scholar] [CrossRef]
- Mahobia, G.S.; Paulose, N.; Singh, V. Hot Corrosion behavior of superalloy IN718 at 550 and 650 °C. J. Mater. Eng. Perform. 2013, 22, 2418–2435. [Google Scholar] [CrossRef]
- Mahobia, G.S.; Paulose, N.; Sreekanth, K.; Mannan, S.L.; Sudhakar Rao, G.; Singh, V. Effect of saline environment on LCF behavior of Inconel 718 at 550 °C. J. Mater. Eng. Perform. 2014, 24, 338–344. [Google Scholar] [CrossRef]
- Liu, G.; Li, M.; Zhou, Y.; Zhang, Y. Corrosion behavior and strength degradation of Ti3SiC2 exposed to a eutectic K2CO3 and Li2CO3 mixture. J. Eur. Ceram. Soc. 2003, 23, 1957–1962. [Google Scholar] [CrossRef]
- Pradhan, D.; Mahobia, G.S.; Chattopadhyay, K.; Singh, V. Effect of pre hot corrosion on high cycle fatigue behavior of the superalloy IN718 at 600 °C. Int. J. Fatigue 2018, 114, 120–129. [Google Scholar] [CrossRef]
- Mannava, V.; Rao, A.S.; Paulose, N.; Kamaraj, M.; Kottada, R.S. Hot corrosion studies on Ni-base superalloy at 650°C under marine-like environment conditions using three salt mixture (Na2SO4 + NaCl + NaVO3). Corros. Sci. 2016, 105, 109–119. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, J.; Wu, G.; Wang, L.; Zhang, Q.; Liu, R. Microstructure and wear resistance of laser cladded composite coatings prepared from pre-alloyed WC-NiCrMo powder with different laser spots. Opt. Laser Technol. 2018, 101, 520–530. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Chen, Z.; Li, D.; Tong, W.; Yao, J.; Kovalenko, V. Experimental and statistical analyses of geometry characteristics of Inconel 718 laser clad layer with response surface methodology. J. Laser Appl. 2019, 31, 032016. [Google Scholar] [CrossRef]
- Mannava, V.; Sambasiva Rao, A.; Kamaraj, M.; Kottada, R.S. Influence of two different salt mixture combinations of Na2SO4-NaCl-NaVO3 on Hot Corrosion behavior of Ni-Base superalloy Nimonic263 at 800 °C. J. Mater. Eng. Perform. 2019, 28, 1077–1093. [Google Scholar] [CrossRef]
- AlMangour, B.; Kim, Y.; Grzesiak, D.; Lee, K. Novel TiB2-reinforced 316L stainless steel nanocomposites with excellent room- and high-temperature yield strength developed by additive manufacturing. Compos. Part. B Eng. 2019, 156, 51–63. [Google Scholar] [CrossRef]
- Sui, S.; Chen, J.; Zhang, R.; Ming, X.; Liu, F.; Lin, X. The tensile deformation behavior of laser repaired Inconel 718 with a non-uniform microstructure. Mater. Sci. Eng. A 2017, 688, 480–487. [Google Scholar] [CrossRef]
- He, D.-G.; Lin, Y.C.; Tang, Y.; Li, L.; Chen, J.; Chen, M.-S.; Chen, X.-M. Influences of solution cooling on microstructures, mechanical properties and hot corrosion resistance of a nickel-based superalloy. Mater. Sci. Eng. A 2019, 746, 372–383. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Q.; Li, C.; Mei, Y.; Zhou, X.; Huan, Y.; Li, H. Recent progress on evolution of precipitates in Inconel 718 superalloy. Acta Metall. Sin. 2016, 52, 1259–1266. [Google Scholar]
- Anderson, M.; Thielin, A.L.; Bridier, F.; Bocher, P.; Savoie, J. δ Phase precipitation in Inconel 718 and associated mechanical properties. Mater. Sci. Eng. A 2017, 679, 48–55. [Google Scholar] [CrossRef]
- Bian, H.; Zhao, X.; Yang, G.; Qin, L.; Wang, W.; Wang, S.; Ren, Y. Microstructure and properties of heat treatment GH4169 alloy by laser deposition repair. Chinese J. Lasers 2015, 42(12), 1206001. [Google Scholar] [CrossRef]
- Xi, M.; Zhou, W.; Shang, J.; Lv, C.; Wu, Z.; Gao, S. Effect of Heat Treatment on microstructure and mechanical properties of consecutive point-mode forging and laser rapid forming GH4169 alloy. Acta Metall. Sin. 2017, 53, 239–247. [Google Scholar]
- Lu, J.; Lu, H.; Xu, X.; Yao, J.; Cai, J.; Luo, K. High-performance integrated additive manufacturing with laser shock peening—Induced microstructural evolution and improvement in mechanical properties of Ti6Al4V alloy components. Int. J. Mach. Tool. Manuf. 2020, 148, 103475. [Google Scholar] [CrossRef]
- Mahobia, G.S.; Paulose, N.; Mannan, S.L.; Chattopadhyay, K.; Santhi Srinivas, N.C.; Singh, V. Tensile Behavior of Alloy 718 in Hot Corrosive Environment. J. Mater. Eng. Perform. 2013, 22, 3810–3817. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Horikawa, S.; Kakehi, K. The effect of interdendritic δ phase on the mechanical properties of Alloy 718 built up by additive manufacturing. Mater. Des. 2017, 116, 411–418. [Google Scholar] [CrossRef]
- Cai, J.; Gao, C.; Lv, P.; Zhang, C.; Guan, Q.; Lu, J.; Xu, X. Hot corrosion behaviour of thermally sprayed CoCrAlY coating irradiated by high-current pulsed electron beam. J. Alloy. Compd. 2019, 784, 1221–1233. [Google Scholar] [CrossRef]
- Asala, G.; Andersson, J.; Ojo, O.A. Hot corrosion behavior of wire-arc additive manufactured Ni-based superalloy ATI719Plus. Corros. Sci. 2019, 158, 108086. [Google Scholar] [CrossRef]
- Lyu, F.; Liu, F.; Hu, X.; Yang, X.; Huang, C.; Shi, D. The δ Phase precipitation of an Inconel 718 superalloy fabricated by electromagnetic stirring assisted laser solid forming. Materials 2019, 12, 2604. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Azer, M.; Ritter, A. Studies of standard heat treatment effects on microstructure and mechanical properties of laser net shape manufactured INCONEL 718. Metall. Mater. Trans. A 2009, 40, 2410–2422. [Google Scholar] [CrossRef]
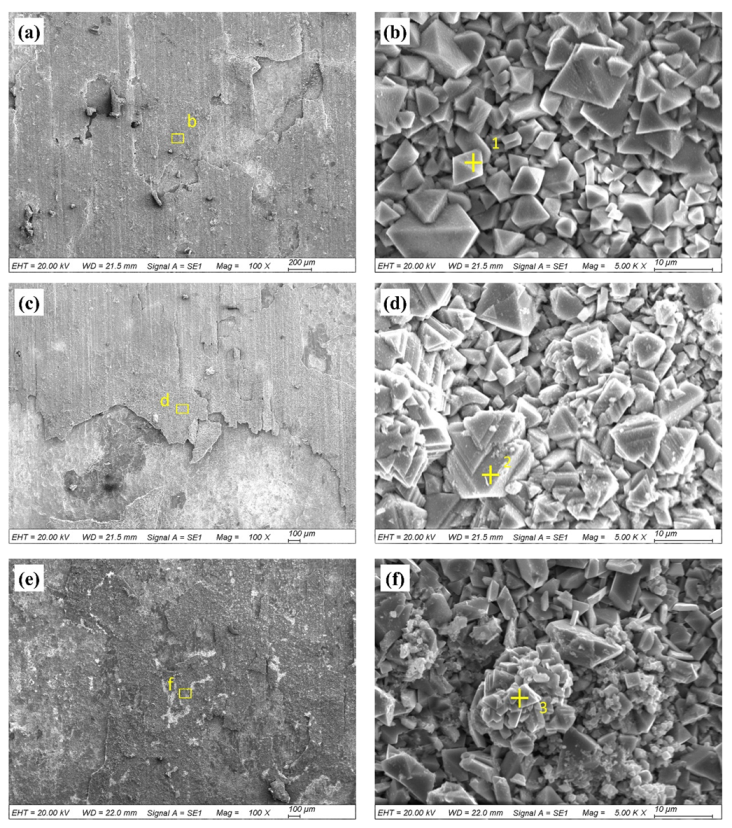

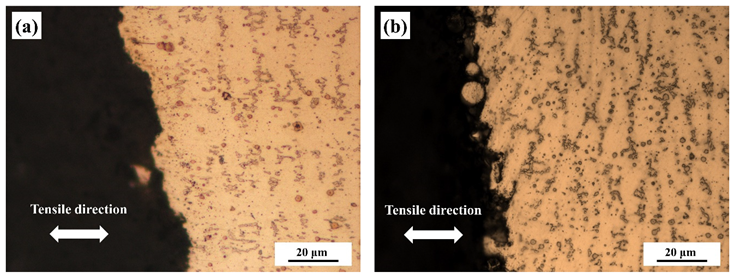

| Area | O | Fe | Cr | Ni |
|---|---|---|---|---|
| 1 | 32.85 | 42.52 | 1.79 | 27.50 |
| 2 | 32.33 | 34.56 | 10.71 | 22.40 |
| 3 | 29.32 | 33.26 | 13.31 | 24.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhang, J.; Zhuang, Y.; Lu, J.; Yao, J. Hot Corrosion and Mechanical Performance of Repaired Inconel 718 Components via Laser Additive Manufacturing. Materials 2020, 13, 2128. https://doi.org/10.3390/ma13092128
Zhang Q, Zhang J, Zhuang Y, Lu J, Yao J. Hot Corrosion and Mechanical Performance of Repaired Inconel 718 Components via Laser Additive Manufacturing. Materials. 2020; 13(9):2128. https://doi.org/10.3390/ma13092128
Chicago/Turabian StyleZhang, Qunli, Jie Zhang, Yifan Zhuang, Jinzhong Lu, and Jianhua Yao. 2020. "Hot Corrosion and Mechanical Performance of Repaired Inconel 718 Components via Laser Additive Manufacturing" Materials 13, no. 9: 2128. https://doi.org/10.3390/ma13092128





