Crystal Structure and Thermoelectric Transport Properties of As-Doped Layered Pnictogen Oxyselenides NdO0.8F0.2Sb1−xAsxSe2
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
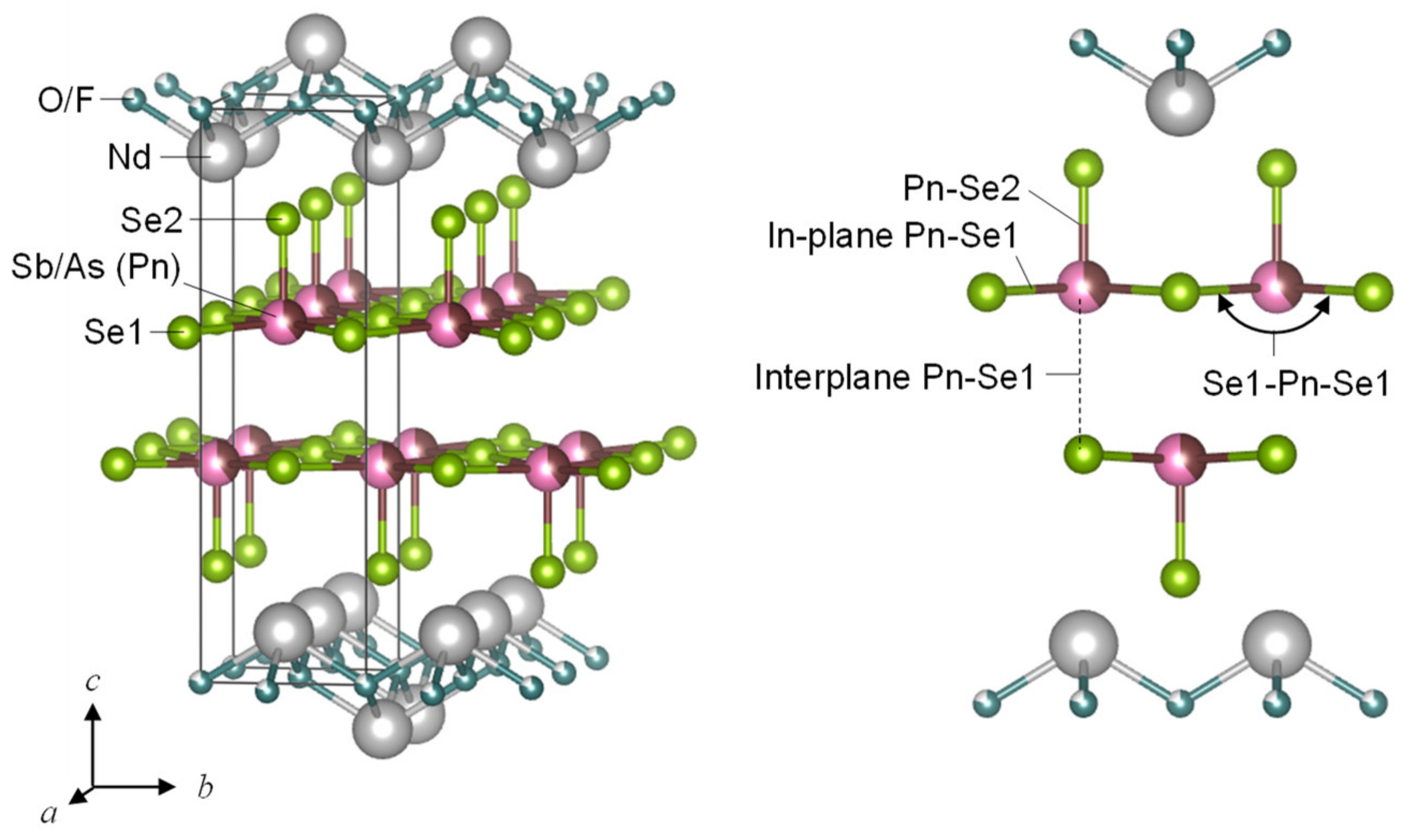
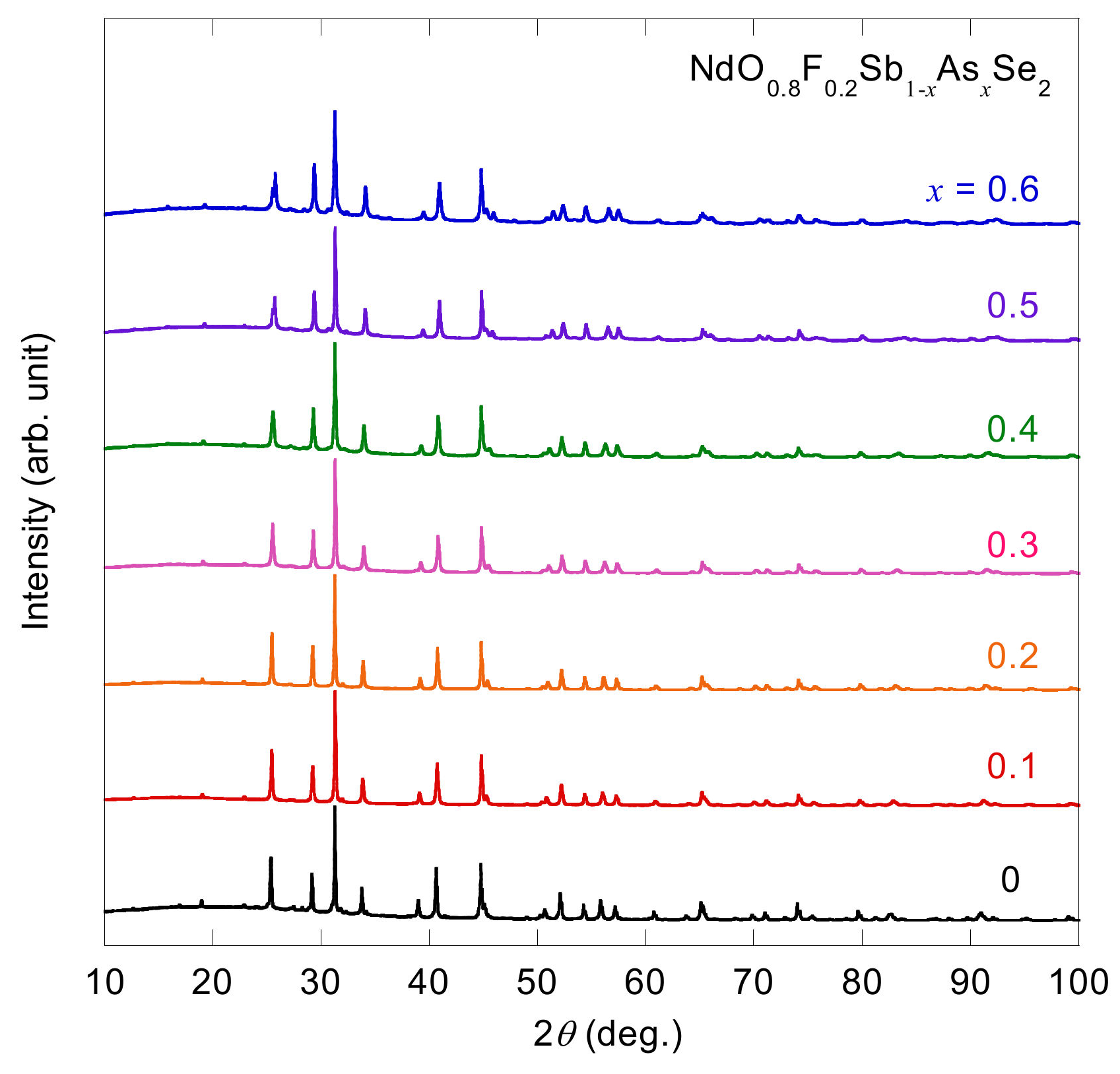
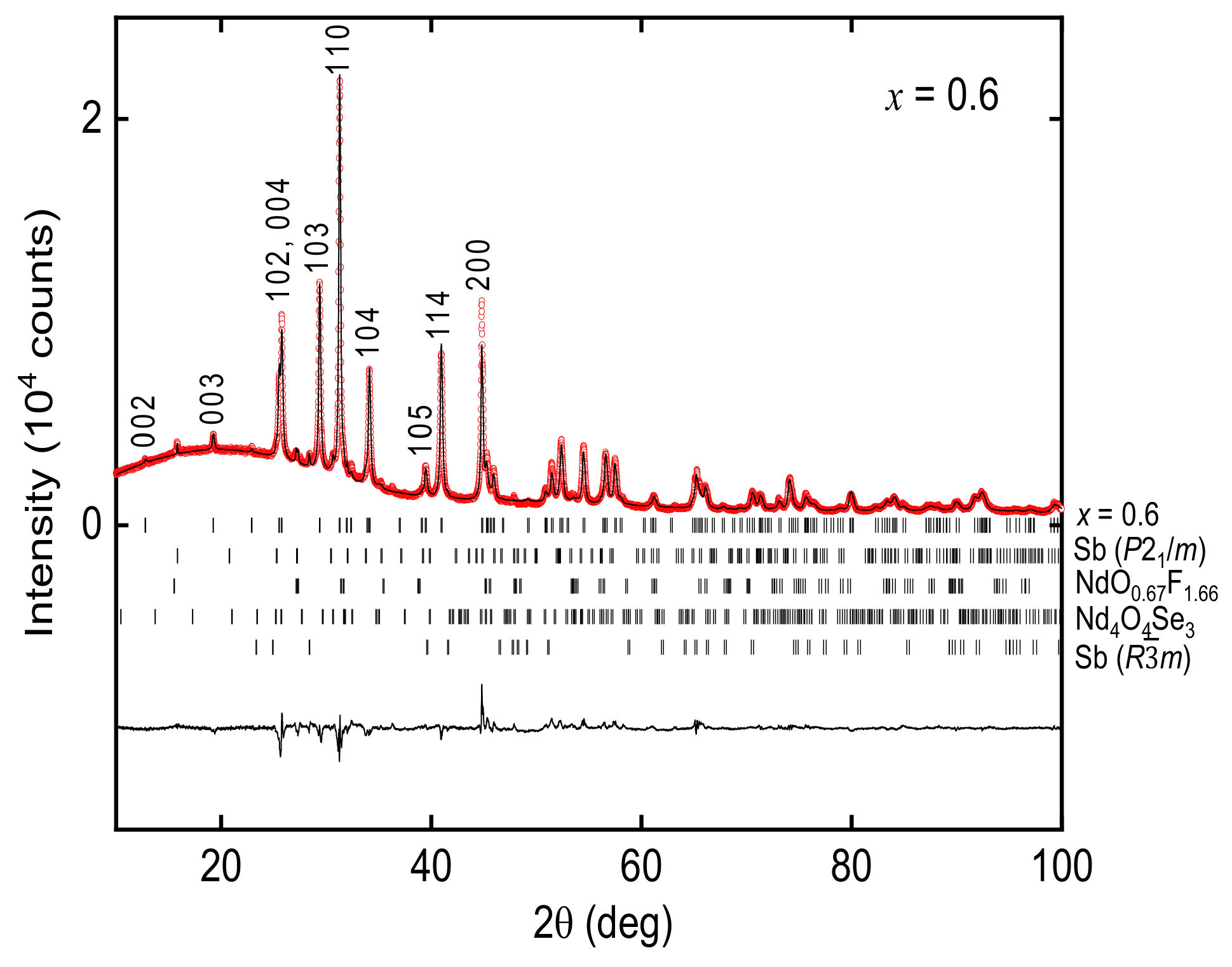
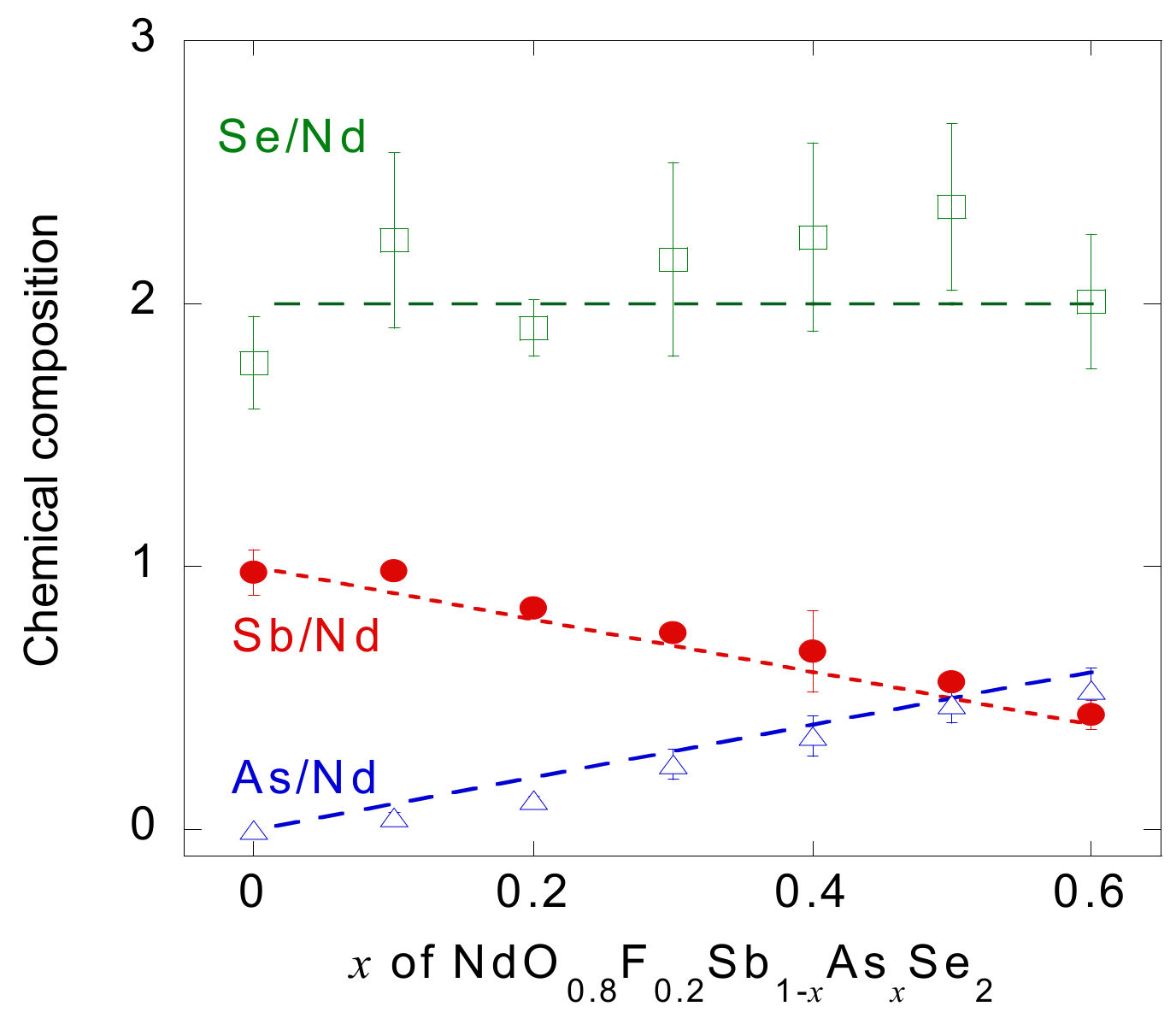
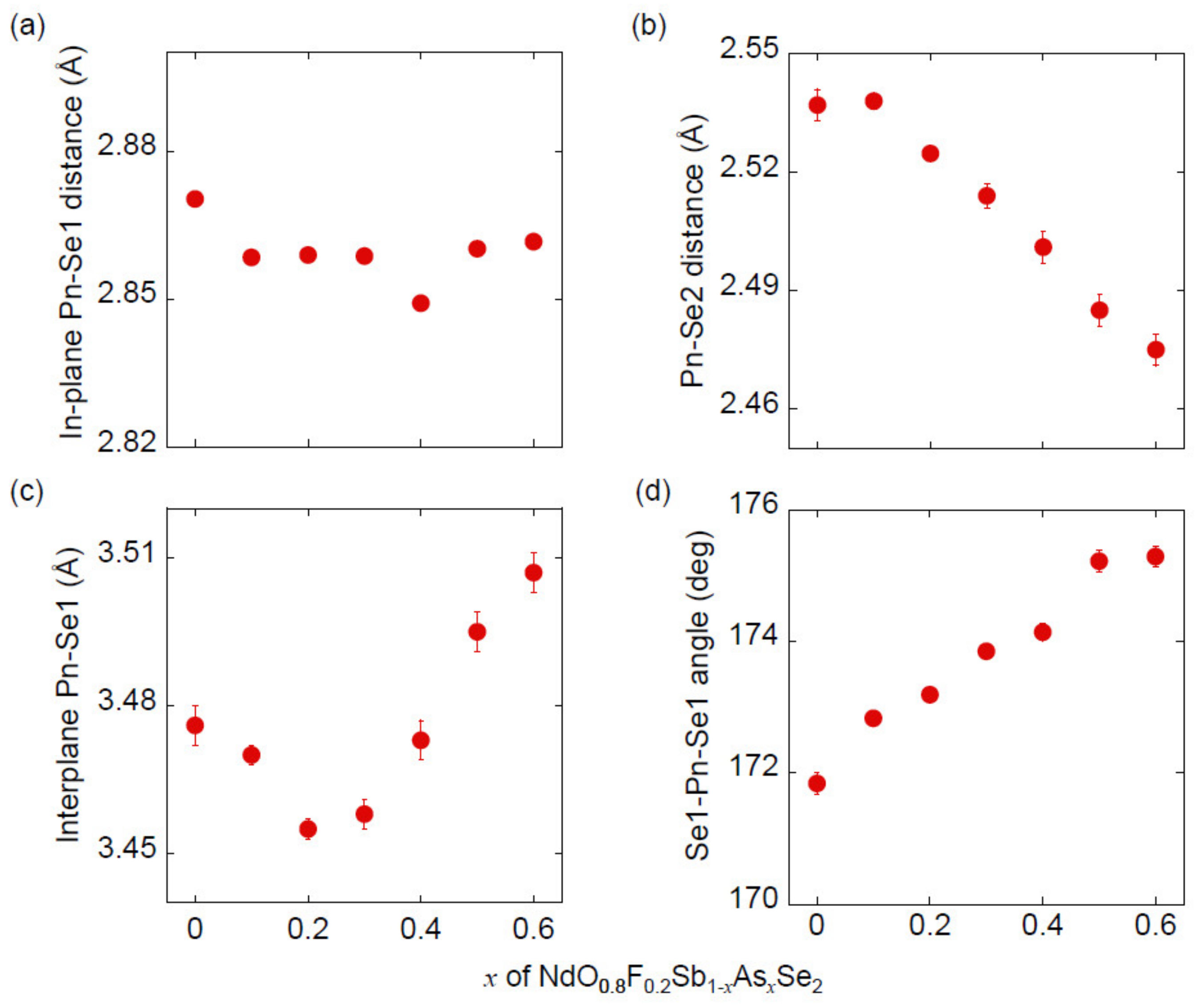
3.1. Sample Characterization and Crystal Structure Analysis
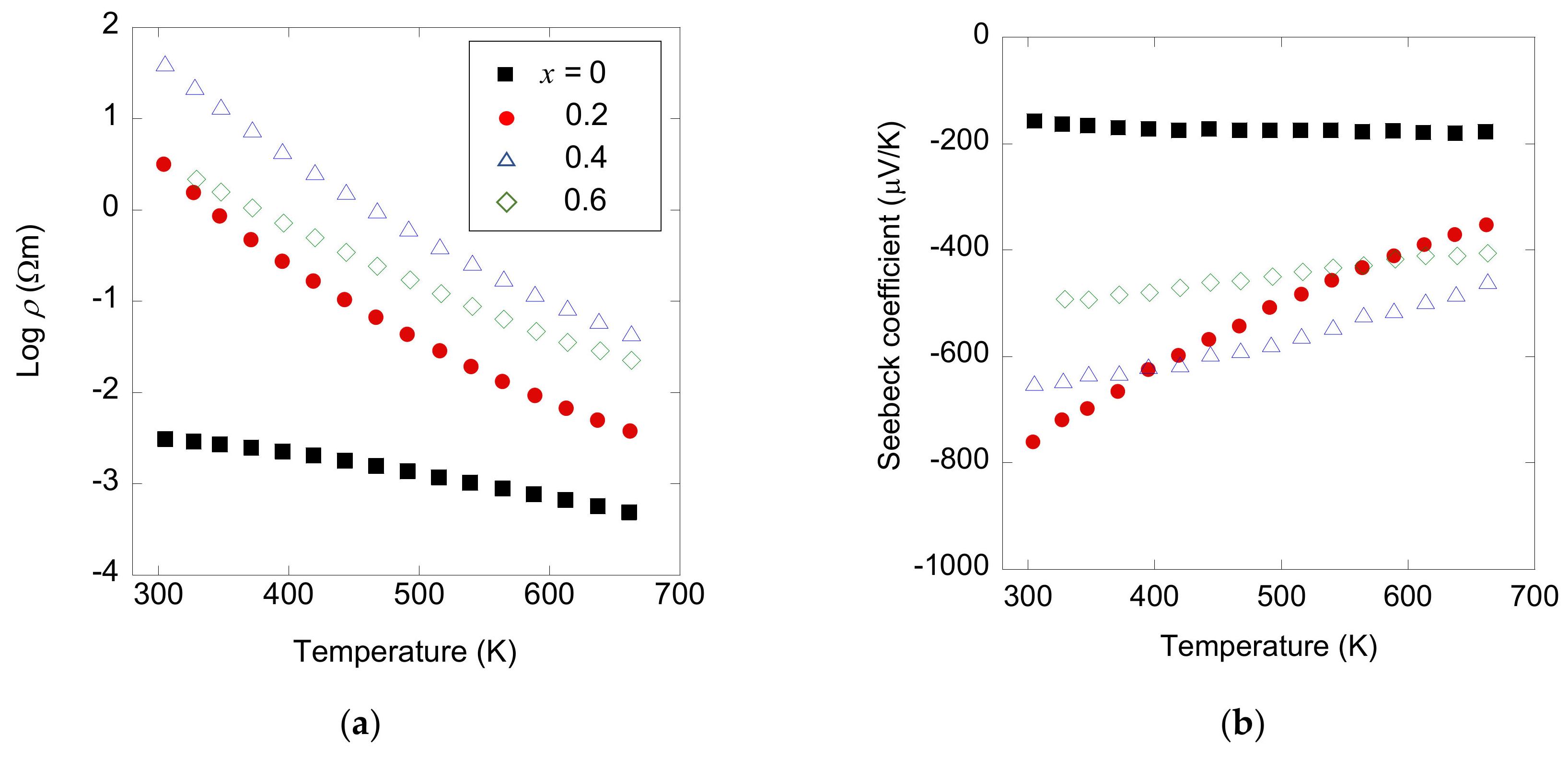
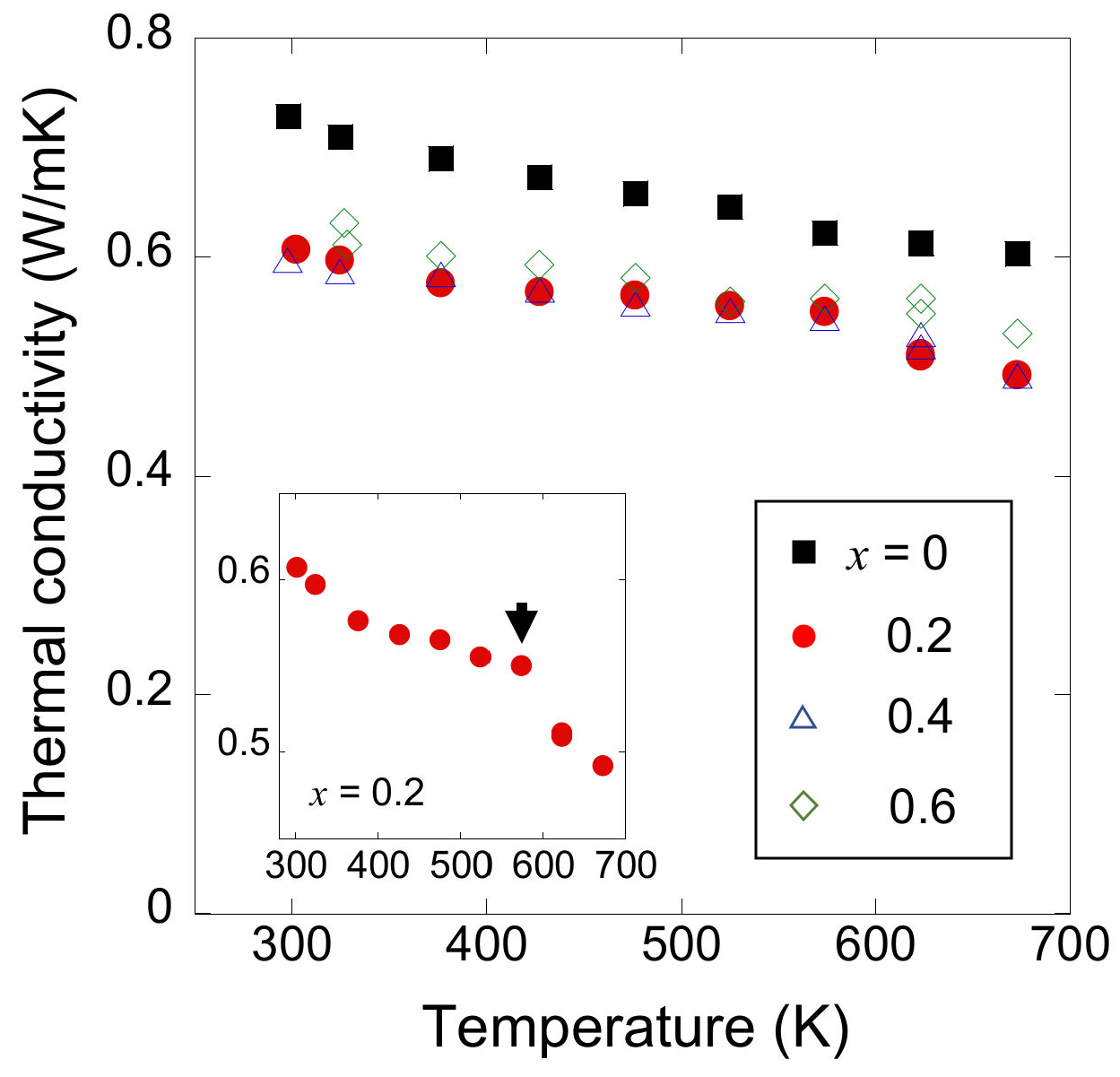
3.2. Thermoelectric Transport Properties
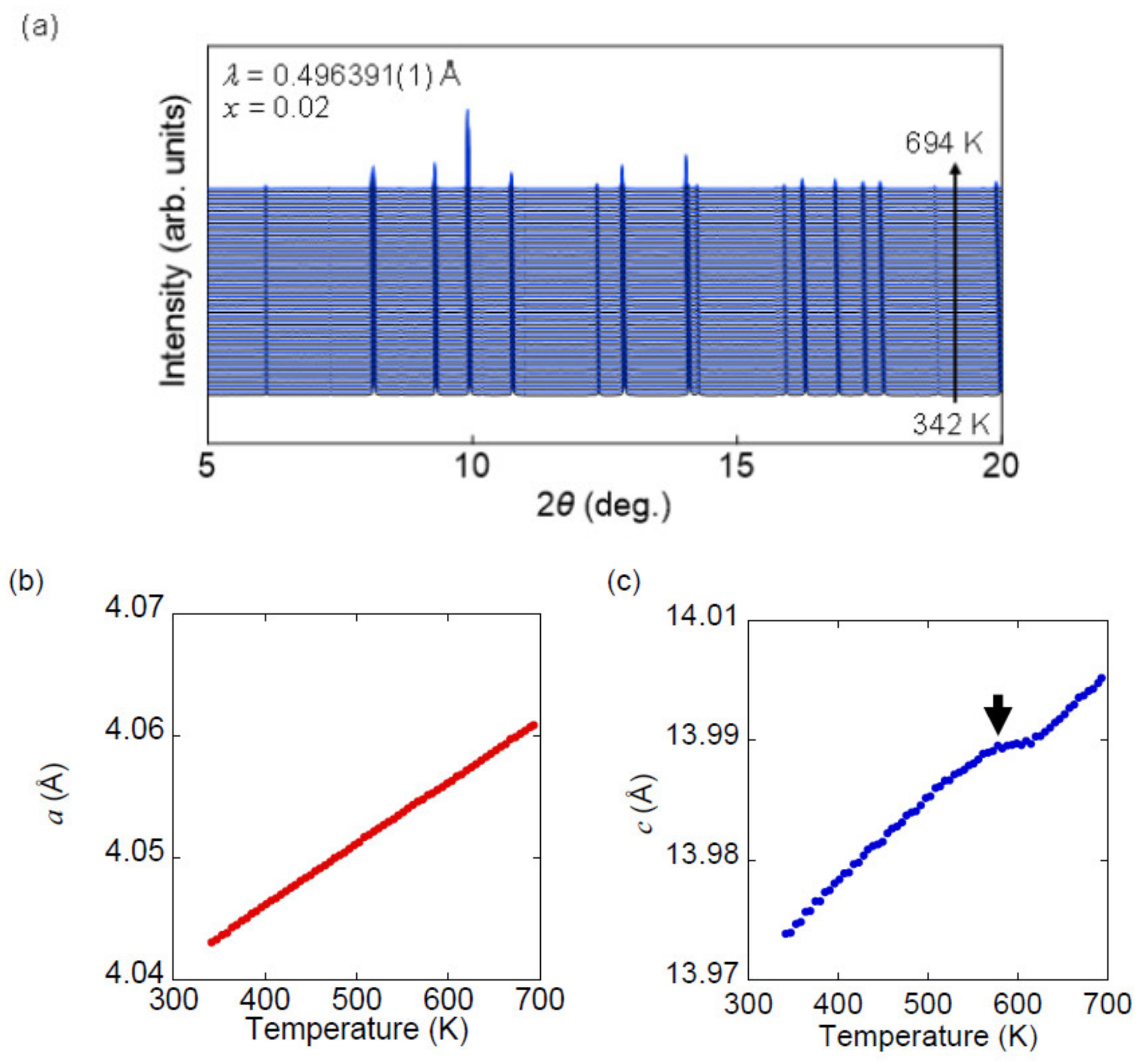
3.3. High-Temperature Synchrotron X-ray Diffraction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Zeier, W.G.; Zevalkink, A.; Gibbs, Z.M.; Hautier, G.; Kanatzidis, M.G.; Snyder, G.J. Thinking like a chemist: Intuition in thermoelectric materials. Angew. Chem. Int. Ed. 2016, 55, 6826–6841. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.D.; Dravid, V.P.; Kanatzidis, M.G. The panoscopic approach to high performance thermoelectrics. Energy Environ. Sci. 2014, 7, 251–268. [Google Scholar] [CrossRef]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and old concepts in thermoelectric materials. Angew. Chem. Int. Ed. 2009, 48, 8616–8639. [Google Scholar] [CrossRef] [PubMed]
- Toberer, E.S.; Zevalkink, A.; Snyder, G.J. Phonon engineering through crystal chemistry. J. Mater. Chem. 2011, 21, 15843–15852. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, Z.; Setyawan, W.; Mingo, N.; Curtarolo, S. Assessing the thermoelectric properties of sintered compounds via high-throughput ab-initio calculations. Phys. Rev. X 2011, 1, 0210122–0210129. [Google Scholar]
- Gaultois, M.W.; Sparks, T.D.; Borg, C.K.H.; Seshadri, R.; Boni, W.D.; Clarke, D.R. Data-driven review of thermoelectric materials: Performance and resource considerations. Chem. Mater. 2013, 25, 2911–2920. [Google Scholar] [CrossRef]
- Carrete, J.; Li, W.; Mingo, N.; Martyrs, R. Finding unprecedentedly low-thermal-conductivity half-heusler semiconductors via high-throughput materials modeling. Phys. Rev. X 2014, 4, 011019–011027. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Shin, Y.; Persson, K.A. Computational predictions of energy materials using density functional theory. Nat. Rev. Mater. 2016, 1, 15004–15016. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The materials project: A materials genome approach to accelerating materials innovation. Appl. Mater. 2013, 1, 011002–011012. [Google Scholar] [CrossRef] [Green Version]
- Pöhls, J.H.; Faghaninia, A.; Petretto, G.; Aydemir, U.; Ricci, F.; Li, G.; Wood, M.; Ohno, S.; Hautier, G.; Snyder, G.J.; et al. Metal phosphides as potential thermoelectric materials. J. Mater. Chem. C 2017, 5, 12441–12456. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.A.; Gorai, P.; Aydemir, U.; Mason, T.O.; Stevanovic, V.; Toberer, E.S.; Snyder, G.J. SnO as a potential oxide thermoelectric candidate. J. Mater. Chem. C 2017, 5, 8854–8861. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Pedersen, S.H.; Yin, H.; Hung, L.T.; Iversen, B.B. Designing high-performance layered thermoelectric materials through orbital engineering. Nat. Commun. 2017, 8, 13901–13908. [Google Scholar] [CrossRef] [Green Version]
- Gorai, P.; Goyal, A.; Toberer, E.S.; Stevanovic, V. A simple chemical guide for finding novel n-type dopable Zintl pnictide thermoelectric materials. J. Mater. Chem. A 2019, 7, 19385–19395. [Google Scholar] [CrossRef] [Green Version]
- Tamaki, H.; Sato, H.K.; Kanno, T. Isotropic conduction network and defect chemistry in Mg3+dSb2-based layered zintl compounds with high thermoelectric performance. Adv. Mater. 2016, 28, 10182–10187. [Google Scholar] [CrossRef]
- Ohno, S.; Imasato, K.; Anand, S.; Tamaki, H.; Kang, S.D.; Gorai, P.; Sato, H.K.; Toberer, E.S.; Kanno, T.; Snyder, G.J. Phase boundary mapping to Obtain n-type Mg3Sb2-based thermoelectrics. Joule 2018, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ochi, M.; Usui, H.; Kuroki, K. Prediction of the high thermoelectric performance of pnictogen dichalcogenide layered compounds with quasi-one-dimensional gapped dirac-like band dispersion. Phys. Revire Appl. 2017, 8, 064020–064031. [Google Scholar] [CrossRef] [Green Version]
- Ochi, M.; Usui, H.; Kuroki, K. Theoretical aspects of the study on the thermoelectric properties of pnictogen-dichalcogenide layered compounds. J. Phys. Soc. Jpn. 2019, 88, 041010–041018. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Fujihisa, H.; Gotoh, Y.; Suzuki, K.; Usui, H.; Kuroki, K.; Demura, S.; Takano, Y.; Izawa, H.; Miura, O. BiS2-based layered superconductor Bi4O4S3. Phys. Rev. B 2012, 86, 220510–220514. [Google Scholar] [CrossRef] [Green Version]
- Mizuguchi, Y.; Izawa, H.; Miura, O.; Demura, S.; Deguchi, K.; Takano, Y. Superconductivity in novel BiS2-based layered superconductor LaO1−xFxBiS2. J. Phys. Soc. Jpn. 2012, 81, 114725–114729. [Google Scholar] [CrossRef] [Green Version]
- Mizuguchi, Y. Material development and physical properties of BiS2-based layered compounds. J. Phys. Soc. Jpn. 2019, 88, 041001–041017. [Google Scholar] [CrossRef]
- Nishida, A.; Miura, O.; Lee, C.; Mizuguchi, Y. High thermoelectric performance and low thermal conductivity of densified LaOBiSSe. Appl. Phys. Express 2015, 8, 111801–111804. [Google Scholar] [CrossRef] [Green Version]
- Mizuguchi, Y.; Nishida, A.; Omachi, A.; Miura, O. Thermoelectric properties of new Bi-chalcogenide layered compounds. Cogent Phys. 2016, 287, 1–24. [Google Scholar] [CrossRef]
- Nishida, A.; Nishiate, H.; Lee, C.; Miura, O.; Mizuguchi, Y. Electronic origins of large thermoelectric power factor of LaOBiS2-xSex. J. Phys. Soc. Jpn. 2016, 85, 074702–074704. [Google Scholar] [CrossRef]
- Goto, Y.; Kajitani, J.; Mizuguchi, Y.; Kamihara, Y.; Matoba, M. Electrical and thermal transport of layered bismuth-sulfide EuBiS2F at temperatures between 300 and 623 K. J. Phys. Soc. Jpn. 2015, 84, 085003–085004. [Google Scholar] [CrossRef] [Green Version]
- Mizuguchi, Y.; Omachi, A.; Goto, Y.; Kamihara, Y.; Matoba, M.; Hiroi, T.; Kajitani, J.; Miura, O. Enhancement of thermoelectric properties by Se substitution in layered bismuth-chalcogenide LaOBiS2-xSex. J. Appl. Phys. 2014, 116, 163915–163918. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Nishida, A.; Hasegawa, T.; Nishiate, H.; Kunioka, H.; Ohira-Kawamura, S.; Nakamura, M.; Nakajima, K.; Mizuguchi, Y. Effect of rattling motion without cage on lattice thermal conductivity in LaOBiS2-xSex. Appl. Phys. Lett. 2018, 112, 023903–023906. [Google Scholar] [CrossRef]
- Lee, C.H. Effect of planar rattling on suppression of thermal conductivity in thermoelectric materials. J. Phys. Soc. Jpn. 2019, 88, 041009–041011. [Google Scholar] [CrossRef]
- Nagao, M.; Tanaka, M.; Matsumoto, R.; Tanaka, H.; Watauchi, S.; Takano, Y.; Tanaka, I. Growth and structure of Ce (O,F) SbS2 single crystals. Cryst. Growth Des. 2016, 16, 3037–3042. [Google Scholar] [CrossRef]
- Goto, Y.; Miura, A.; Sakagami, R.; Kamihara, Y.; Moriyoshi, C.; Kuroiwa, Y.; Mizuguchi, Y. Synthesis, crystal structure, and thermoelectric properties of layered antimony selenides REOSbSe2 (RE = La, Ce). J. Phys. Soc. Jpn. 2018, 87, 074703–074708. [Google Scholar] [CrossRef] [Green Version]
- Goto, Y.; Miura, A.; Moriyoshi, C.; Kuroiwa, Y.; Mizuguchi, Y. Effect of Bi substitution on thermoelectric properties of SbSe2-based layered compounds NdO0.8F0.2Sb1−xBixSe2. J. Phys. Soc. Jpn. 2019, 88, 024705–024709. [Google Scholar] [CrossRef]
- Nagao, M.; Tanaka, M.; Miura, A.; Kitamura, M.; Horiba, K.; Watauchi, S.; Takano, Y.; Kumigashira, H.; Tanaka, I. Growth and physical properties of Ce (O,F) Sb(S,Se)2 single crystals with site-selected chalcogen atoms. Solid State Commun. 2019, 289, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, R.M.; Nagao, M.; Ochi, H.; Tanaka, H.; Hara, S.; Adachi, K.; Nakamura, R.; Murakami, S.; Yamamoto, T.; Irifune, H.; et al. Pressure-induced insulator to metal transition of mixed valence compound Ce(O,F)SbS2. J. Appl. Phys. 2019, 125, 075102. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, R.; Goto, Y.; Yamamoto, S.; Sudo, K.; Usui, H.; Miura, A.; Moriyoshi, C.; Kuroiwa, Y.; Adachi, S.; Irifune, T.; et al. Pressure-induced superconductivity in the layered pnictogen diselenide NdO0.8F0.2Sb1−xBixSe2 (x = 0.3 and 0.7). Phys. Rev. B 2019, 100, 094528–094537. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, S.; Takemoto, M.; Osaka, K.; Nishibori, E.; Moriyoshi, C.; Kubota, Y.; Kuroiwa, Y.; Sugimoto, K. High-throughput powder diffraction measurement system consisting of multiple MYTHEN detectors at beamline BL02B2 of SPring-8. Rev. Sci. Instrum. 2017, 88, 085111–085119. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Cryst. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta. Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Miura, A.; Kajitani, J.; Hiroi, T.; Miura, O. In-plane chemical pressure essential for superconductivity in BiCh2-based (Ch: S, Se) layered structure. Sci. Rep. 2015, 5, 14968–14975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paris, E.; Joseph, B.; Iadecola, A.; Sugimoto, T.; Olivi, L.; Demura, S.; Mizuguchi, Y.; Takano, Y.; Mizokawa, T.; Saini, N.L. Determination of local atomic displacements in CeO1–xFxBiS2 system. J. Phys. Condens. Matter. 2014, 26, 435701–435706. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Paris, E.; Sugimoto, T.; Iadecola, A.; Kajitani, J.; Miura, O.; Mizokawa, T.; Saini, N.L. The effect of RE substitution in layered REO0.5F0.5BiS2: Chemical pressure, local disorder and superconductivity. Phys. Chem. Chem. Phys. 2015, 17, 22090–22096. [Google Scholar] [CrossRef] [PubMed]
- Paris, E.; Mizuguchi, Y.; Hacisalihoglu, M.Y.; Hiroi, T.; Joseph, B.; Aquilanti, G.; Miura, O.; Mizokawa, T.; Saini, N.L. Role of the local structure in superconductivity of LaO0.5F0.5BiS2–xSex system. J. Phys. Condens. Matter. 2017, 29, 145603. [Google Scholar] [CrossRef] [PubMed]
- Athauda, A.; Yang, J.; Lee, S.; Mizuguchi, Y.; Deguchi, K.; Takano, Y.; Miura, O.; Louca, D. In-plane charge fluctuations in bismuth-sulfide superconductors. Phys. Rev. B 2015, 91, 144112–144117. [Google Scholar] [CrossRef] [Green Version]
- Athauda, A.; Mizuguchi, Y.; Nagao, M.; Neuefeind, J.; Louca, D. Charge fluctuations in the NdO1−xFxBiS2 superconductors. J. Phys. Soc. Jpn. 2017, 86, 124718–124722. [Google Scholar] [CrossRef]
- Athauda, A.; Hoffman, C.; Aswartham, S.; Terzic, J.; Cao, G.; Zhu, X.; Ren, Y.; Louca, D. Ferro-lattice-distortions and charge fluctuations in superconducting LaO1−xFxBiS2. J. Phys. Soc. Jpn. 2017, 86, 054701–054705. [Google Scholar] [CrossRef] [Green Version]
- Athauda, A.; Louca, D. Nanoscale atomic distortions in the BiS2 superconductors: Ferrodistortive sulfur modes. J. Phys. Soc. Jpn. 2019, 88, 041004–041013. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Hoshi, K.; Goto, Y.; Miura, A.; Tadanaga, K.; Moriyoshi, C.; Kuroiwa, Y. Evolution of anisotropic displacement parameters and superconductivity in BiS2-based REO0.5F0.5BiS2 (RE = La, Ce, Pr, and Nd). J. Phys. Soc. Jpn. 2018, 87, 023704–023707. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Ding, H.-C.; Savrasov, S.; Duan, C.-G. Electron-phonon superconductivity near charge-density-wave instability in LaO0.5F0.5BiS2. Phys. Rev. B 2013, 87, 115124–115129. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Xing, Z.W.; Huang, G.Q. Phonon spectra and superconductivity of the BiS2-based compounds LaO1-xFxBiS2. EPL 2013, 101, 47002–47006. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Zunger, A. Polytypism in LaOBi S2-type compounds based on different three-dimensional stacking sequences of two-dimensional BiS2 layers. Phys. Rev. B 2016, 93, 174119–174124. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, T. Ferroelectric soft phonons, charge density wave instability, and strong electron-phonon coupling in BiS2 layered superconductors: A first-principles study. Phys. Rev. B 2013, 87, 020506–020509. [Google Scholar] [CrossRef] [Green Version]
- Callaway, J.; Baeyer, H.C. Effect of point imperfections on lattice thermal conductivity. Phys. Rev. 1960, 120, 1149–1154. [Google Scholar] [CrossRef]
- Klemens, P.G. Thermal resistance due to point defects at high temperatures. Phys. Rev. 1960, 119, 507–509. [Google Scholar] [CrossRef]
- Abeles, B. Lattice thermal conductivity of disordered semiconductor alloys at high temperatures. Phys. Rev. 1963, 131, 1906–1911. [Google Scholar] [CrossRef]
- Yang, J.; Meisner, G.P.; Chen, L. Strain field fluctuation effects on lattice thermal conductivity of ZrNiSn-based thermoelectric compounds. Appl. Phys. Lett. 2004, 85, 1140–1142. [Google Scholar] [CrossRef]
- Skoug, E.J.; Cain, J.D.; Morelli, D.T.; Kirkham, M.; Majsztrik, P.; Lara-Curzio, E. Lattice thermal conductivity of the Cu3SbSe4-Cu3SbS4 solid solution. J. Appl. Phys. 2011, 110, 023501–023505. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Cao, X.; Snyder, G.J. Thermoelectric alloys between PbSe and PbS with effective thermal conductivity reduction and high figure of merit. J. Mater. Chem. A 2014, 2, 3169–3174. [Google Scholar] [CrossRef] [Green Version]
- Goto, Y.; Nishida, A.; Nishiate, H.; Murata, M.; Lee, C.H.; Miura, A.; Moriyoshi, C.; Kuroiwa, Y.; Mizuguchi, Y. Effect of Te substitution on crystal structure and transport properties of AgBiSe2 thermoelectric material. Dalt. Trans. 2018, 47, 2575–2580. [Google Scholar] [CrossRef]
- Kreyssig, A.; Green, M.A.; Lee, Y.; Samolyuk, G.D.; Zajdel, P.; Lynn, J.W.; Bud’ko, S.L.; Torikachvili, M.S.; Ni, N.; Nandi, S.; et al. Pressure-induced volume-collapsed tetragonal phase of CaFe2As2 as seen via neutron scattering. Phys. Rev. B 2008, 78, 184517–184522. [Google Scholar] [CrossRef] [Green Version]
- Drachuck, G.; Sapkota, A.; Jayasekara, W.T.; Kothapalli, K.; Bud’Ko, S.L.; Goldman, A.I.; Kreyssig, A.; Canfield, P.C. Collapsed tetragonal phase transition in LaRu2P2. Phys. Rev. B 2017, 96, 184509–184514. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morino, K.; Goto, Y.; Miura, A.; Moriyoshi, C.; Kuroiwa, Y.; Mizuguchi, Y. Crystal Structure and Thermoelectric Transport Properties of As-Doped Layered Pnictogen Oxyselenides NdO0.8F0.2Sb1−xAsxSe2. Materials 2020, 13, 2164. https://doi.org/10.3390/ma13092164
Morino K, Goto Y, Miura A, Moriyoshi C, Kuroiwa Y, Mizuguchi Y. Crystal Structure and Thermoelectric Transport Properties of As-Doped Layered Pnictogen Oxyselenides NdO0.8F0.2Sb1−xAsxSe2. Materials. 2020; 13(9):2164. https://doi.org/10.3390/ma13092164
Chicago/Turabian StyleMorino, Kota, Yosuke Goto, Akira Miura, Chikako Moriyoshi, Yoshihiro Kuroiwa, and Yoshikazu Mizuguchi. 2020. "Crystal Structure and Thermoelectric Transport Properties of As-Doped Layered Pnictogen Oxyselenides NdO0.8F0.2Sb1−xAsxSe2" Materials 13, no. 9: 2164. https://doi.org/10.3390/ma13092164




