Effect of Physiological Saline Solution Contamination on Selected Mechanical Properties of Seasoned Acrylic Bone Cements of Medium and High Viscosity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Mechanical Testing
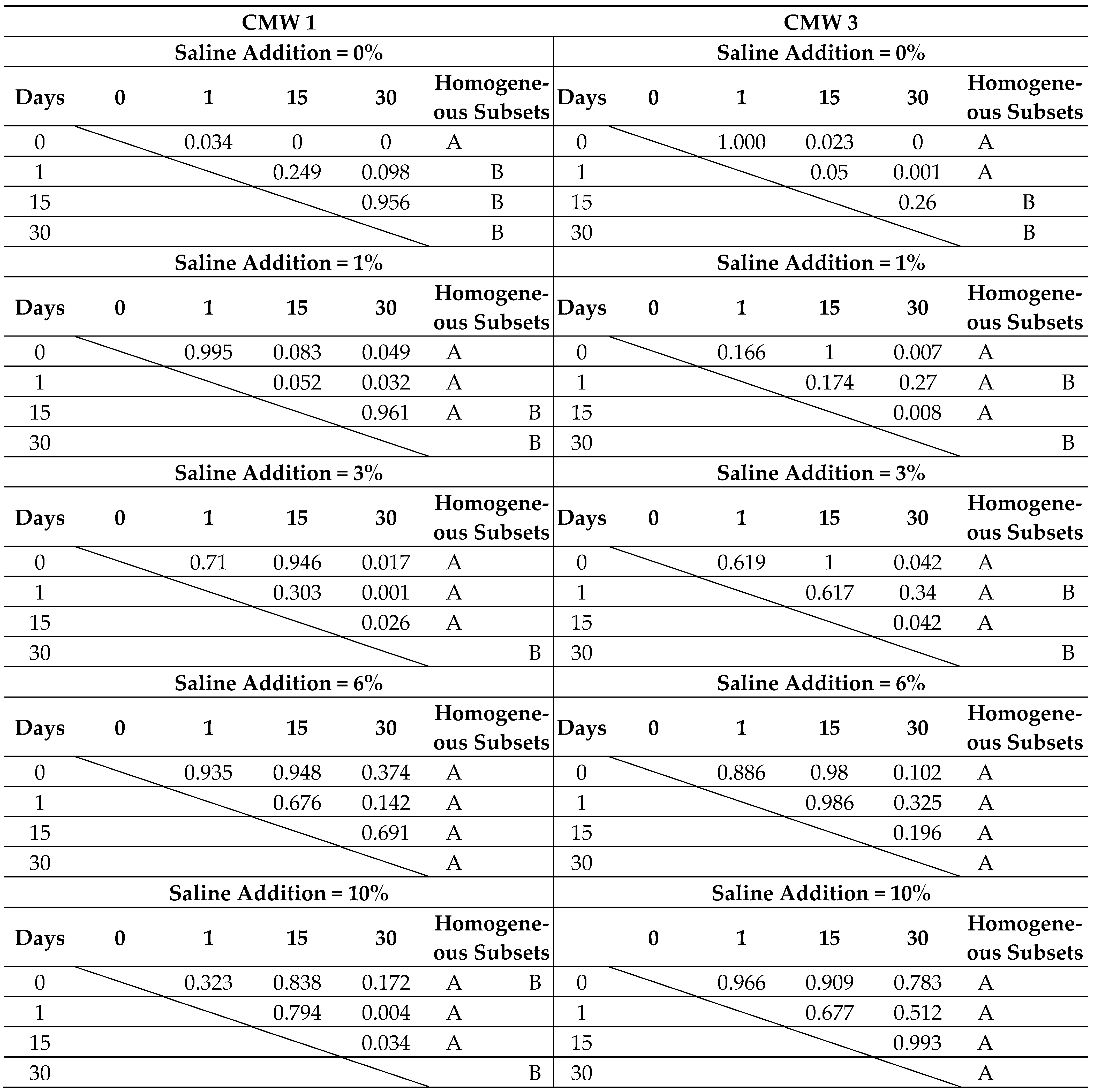
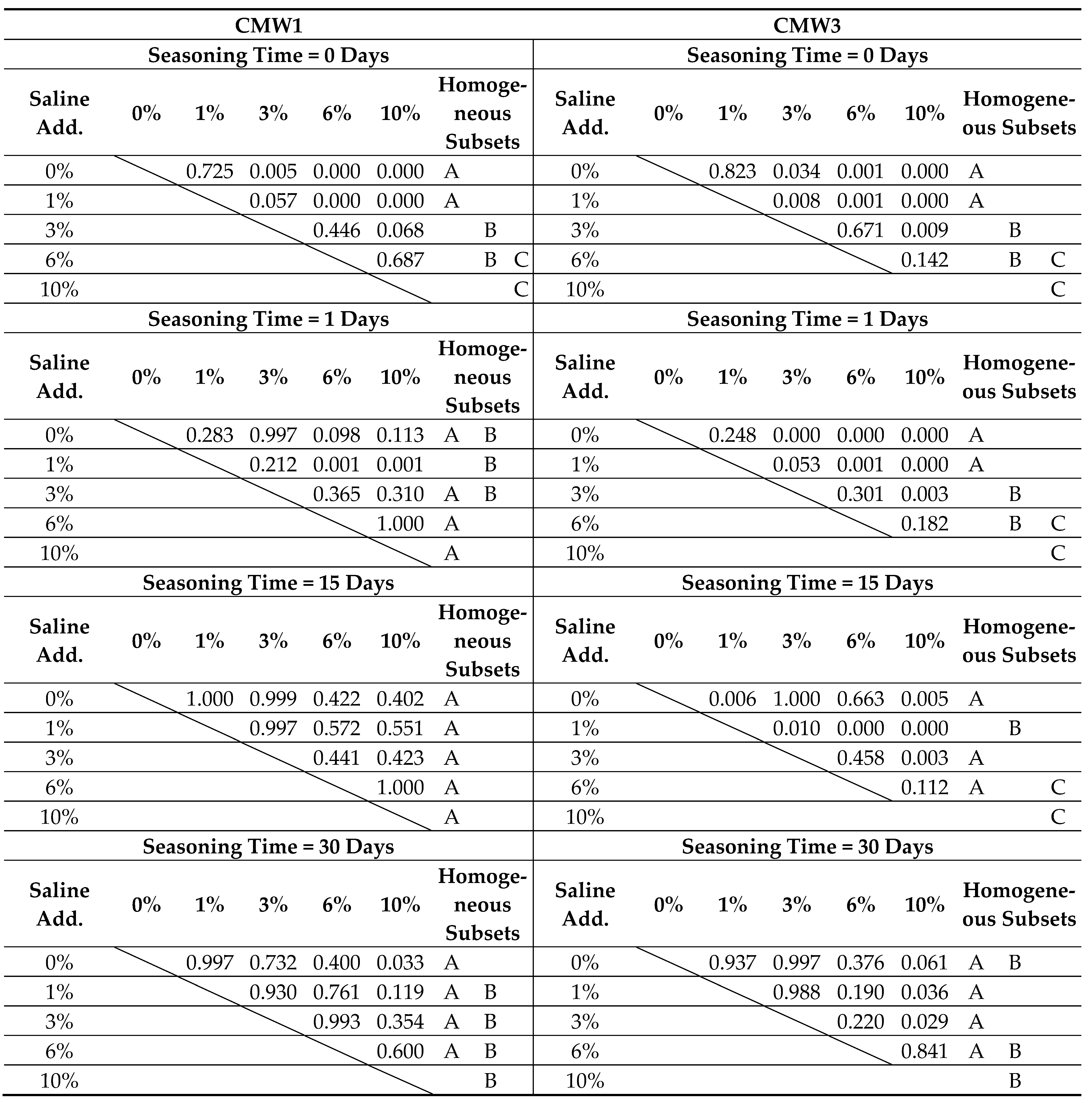
2.4. Statistical Analysis
3. Results
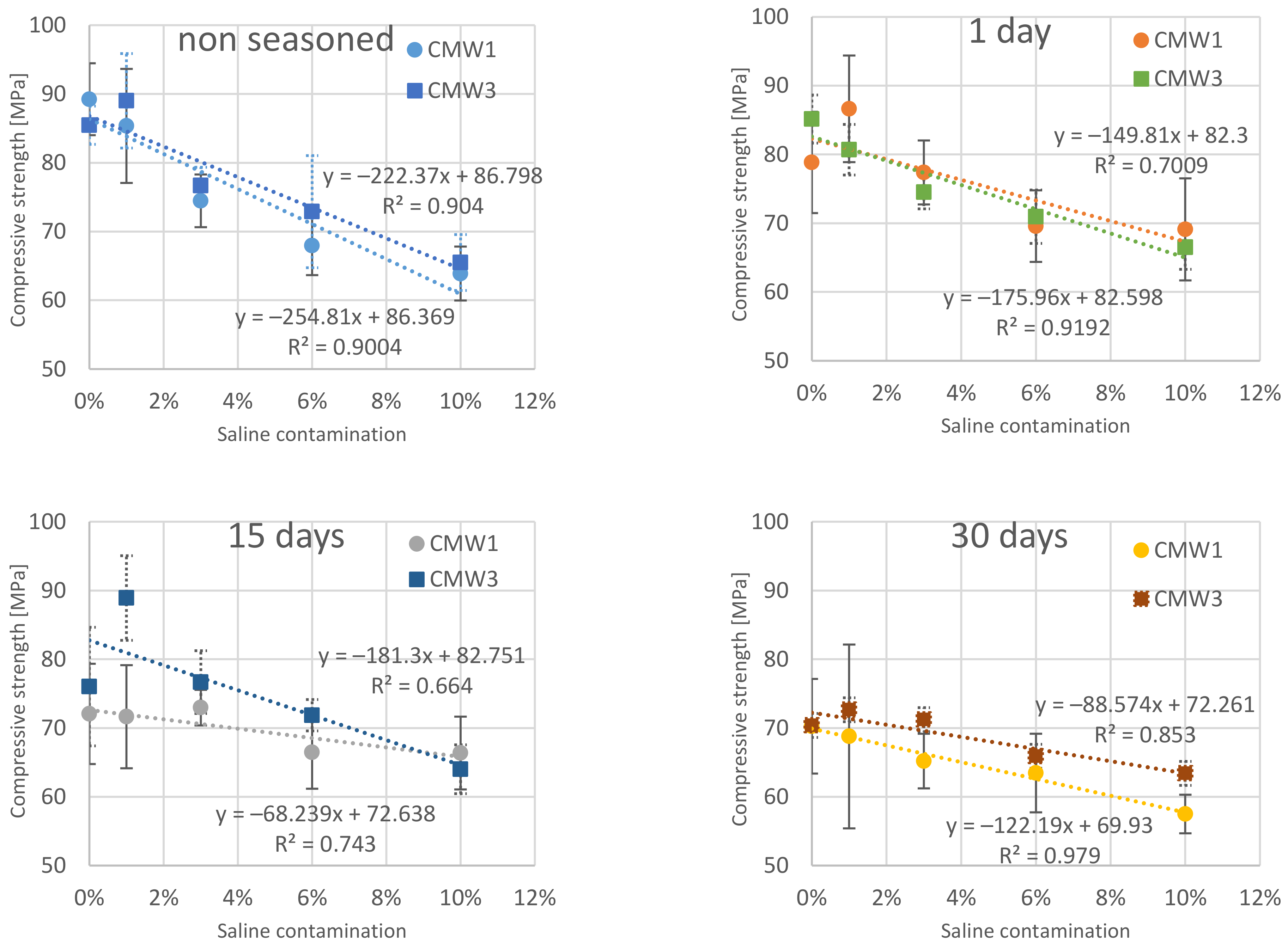
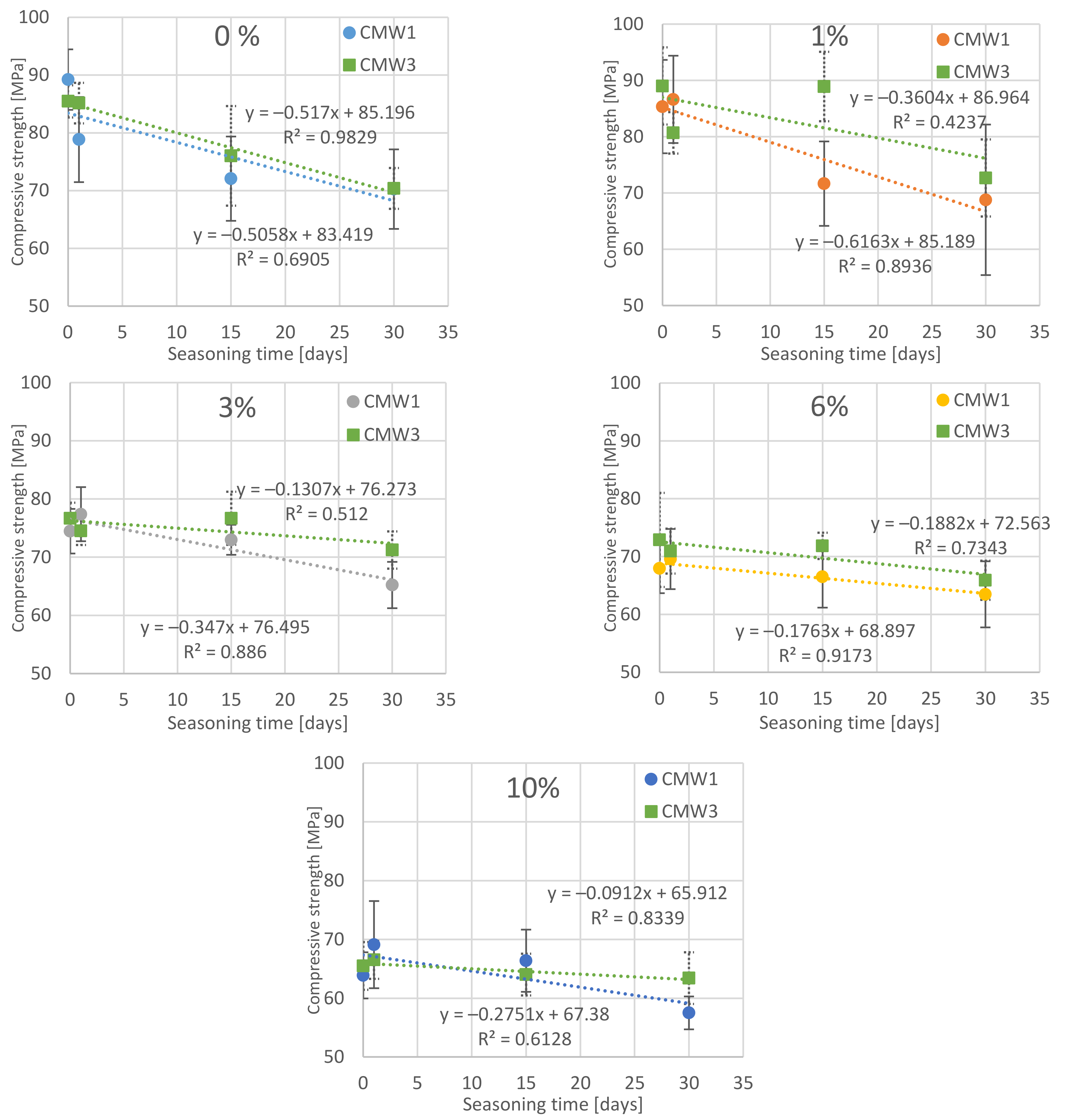
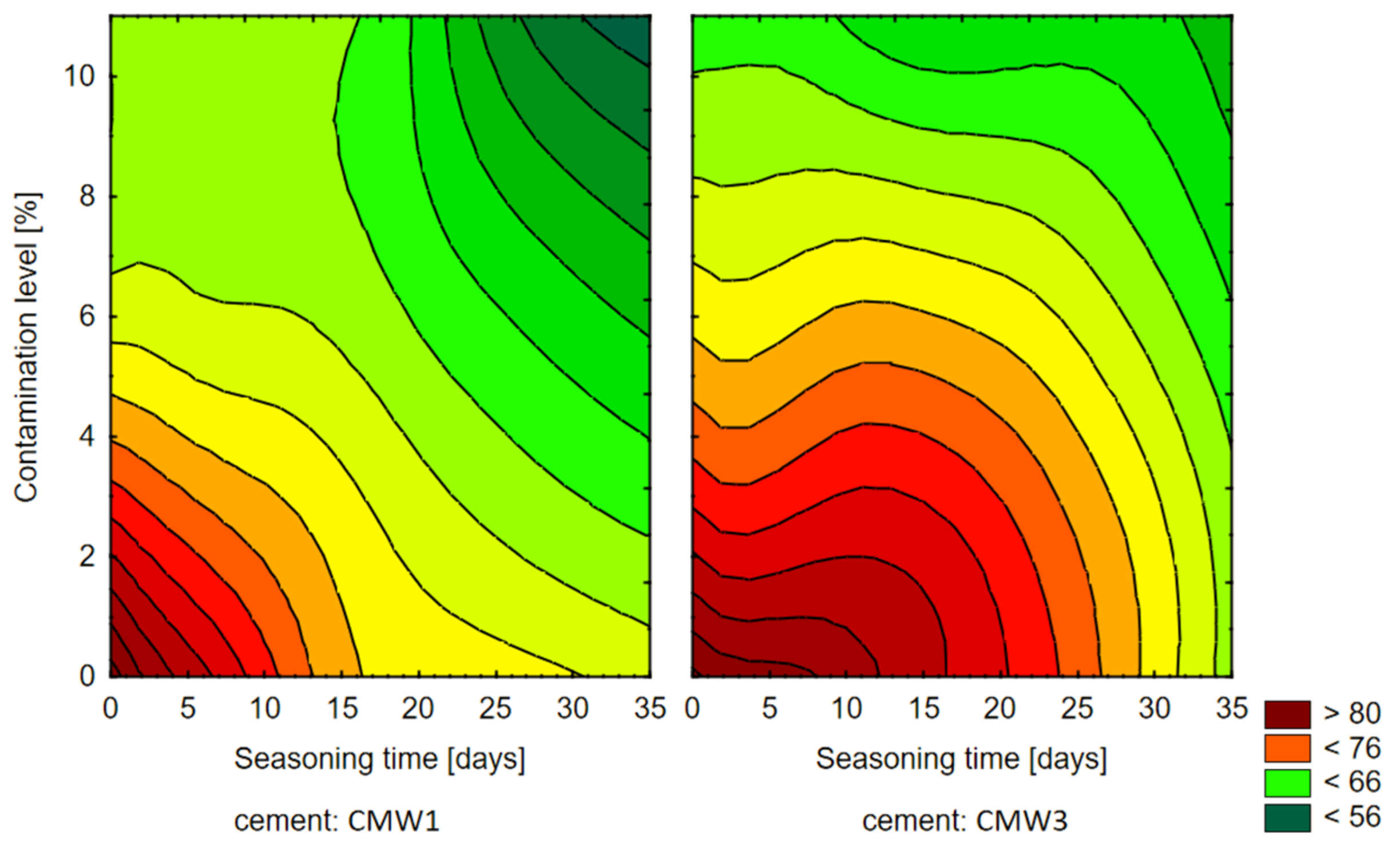
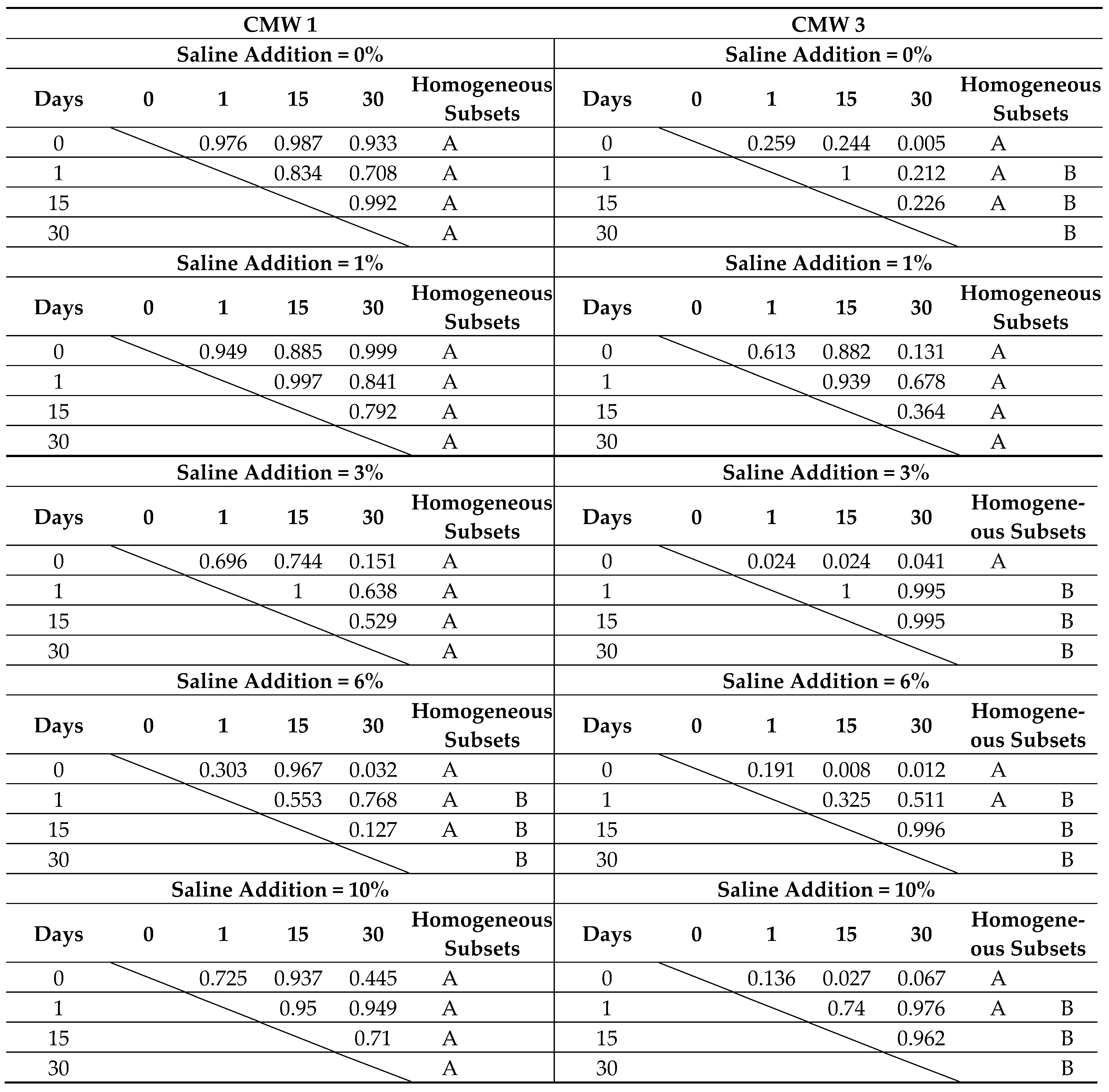
3.1. Change in Strength over Time, Depending on the Degree of Contamination
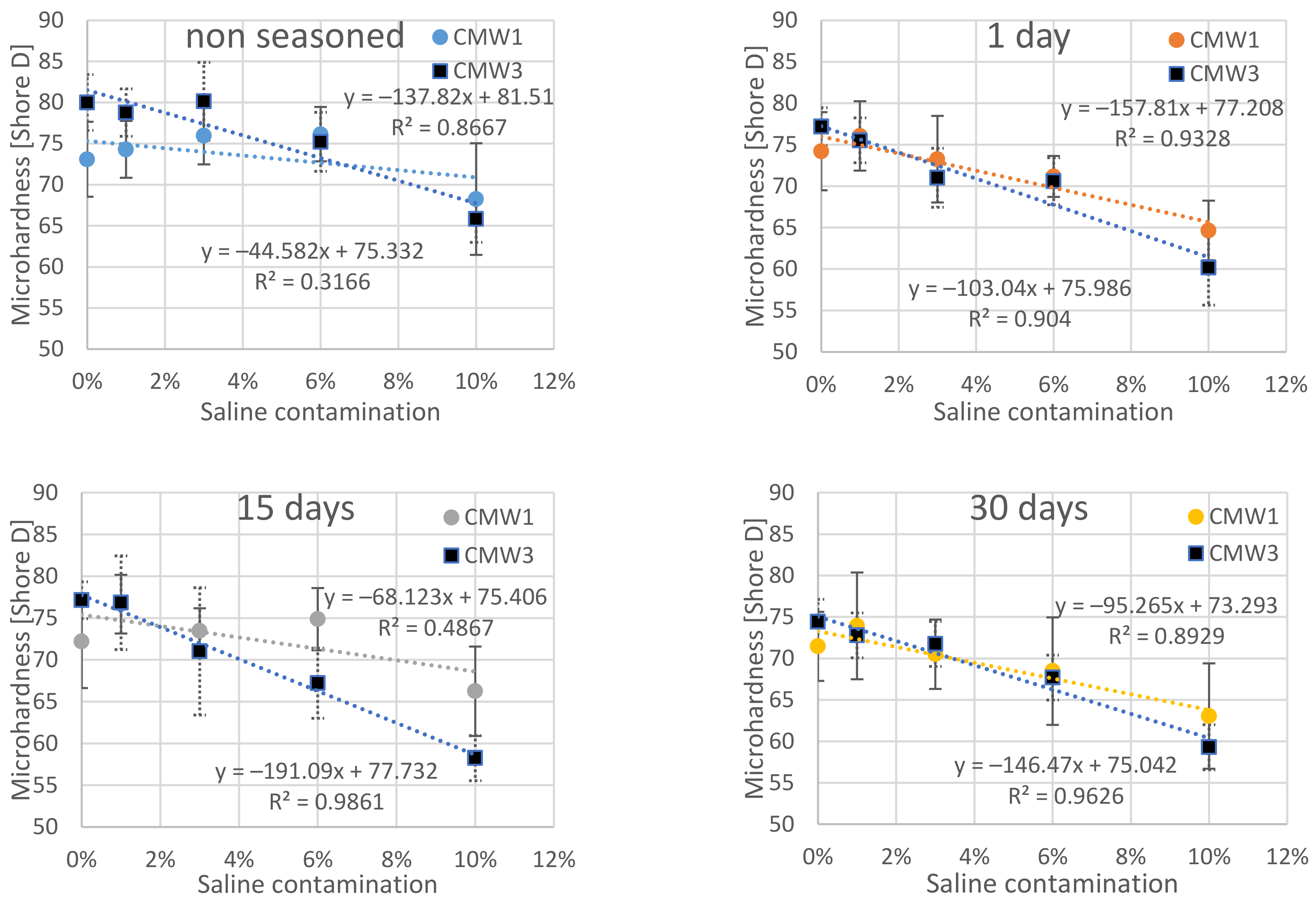
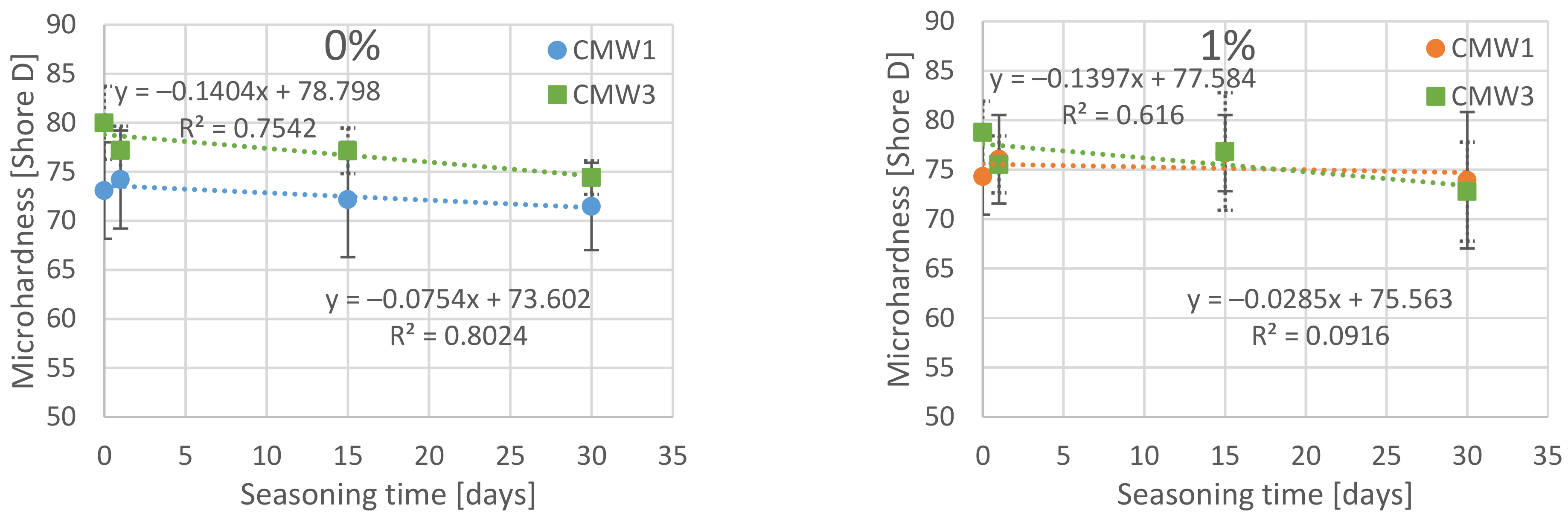
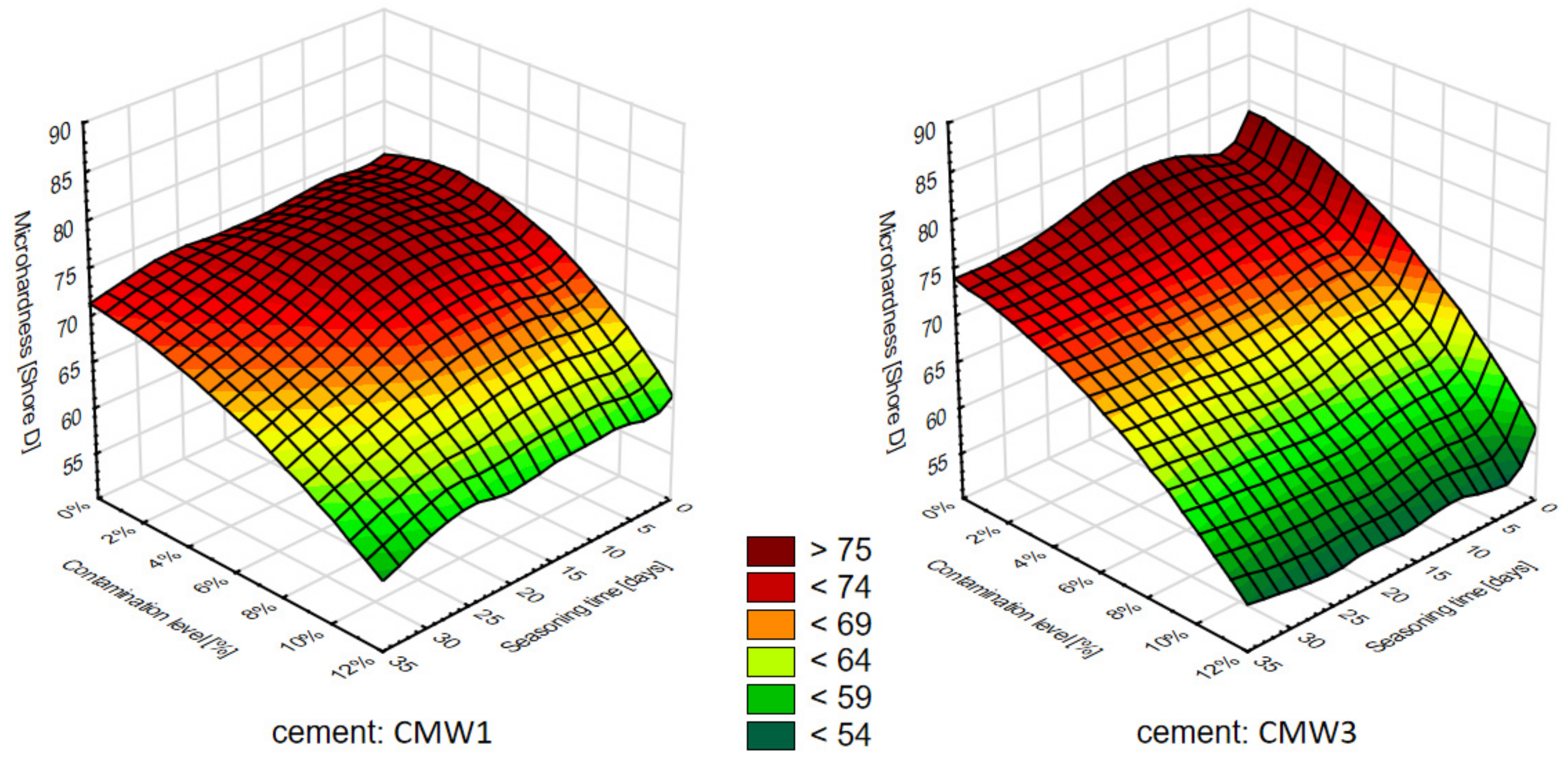
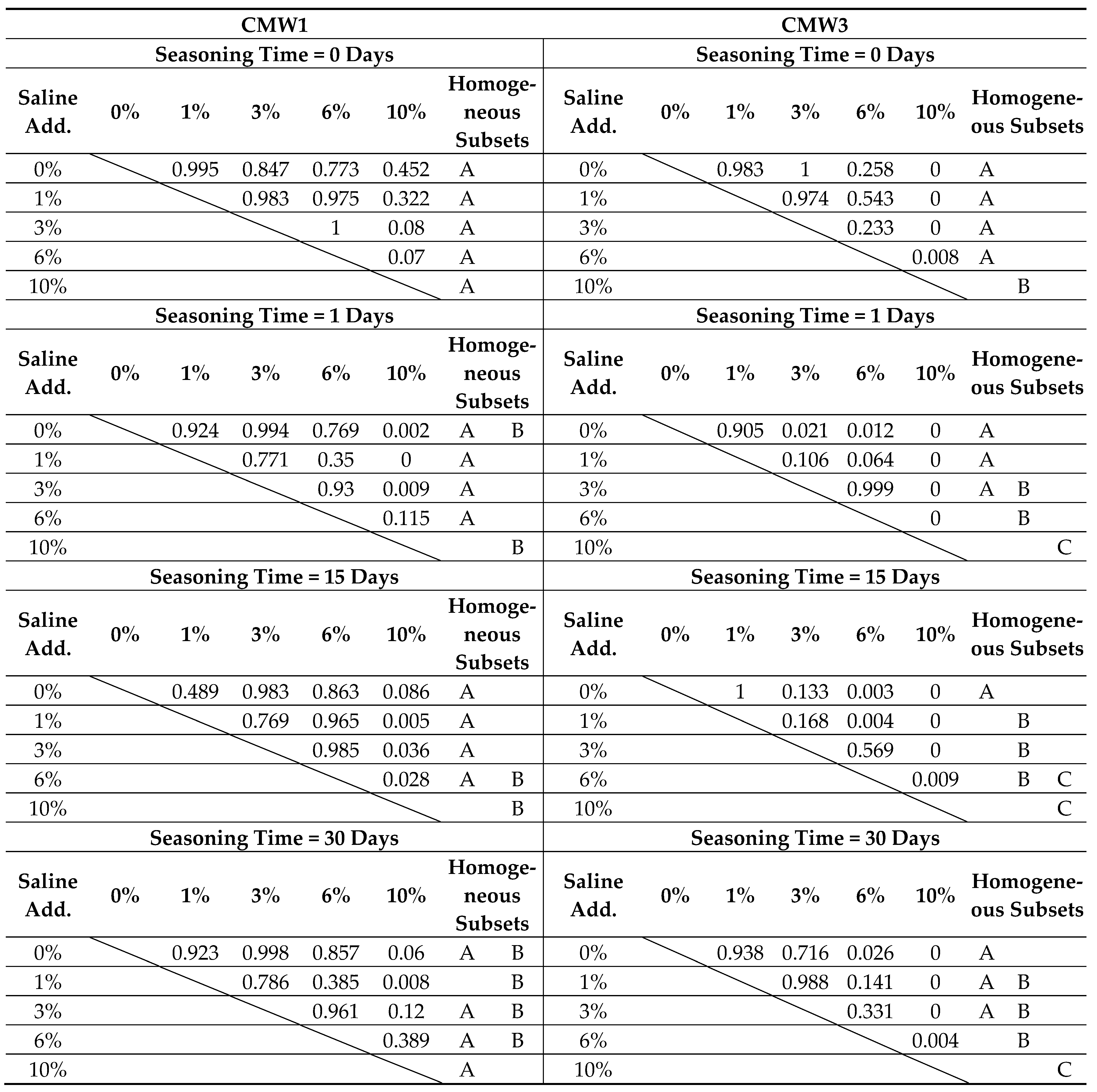
3.2. Change in Microhardness over Time, Depending on the Degree of Contamination
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Learmonth, I.D.; Young, C.; Rorabeck, C. The Operation of the Century: Total Hip Replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J.A. Biomaterials in Orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayeed, Z.; Padela, M.T.; El-Othmani, M.M.; Saleh, K.J. Acrylic bone cements for joint replacement. In Biomedical Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 199–214. ISBN 978-0-08-100752-5. [Google Scholar]
- Miller, A.J.; Stimac, J.D.; Smith, L.S.; Feher, A.W.; Yakkanti, M.R.; Malkani, A.L. Results of Cemented vs Cementless Primary Total Knee Arthroplasty Using the Same Implant Design. J. Arthroplast. 2018, 33, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, Ł.; Olchowik, G.; Mazurkiewicz, T.; Kowalczyk, B.; Zdrojewska, A.; Matuszewska, A.; Ciszewski, A.; Gospo-darek, M.; Morawik, I. Biomechanical Parameters of the BP-Enriched Bone Cement. Eur. J. Orthop. Surg. Traumatol. 2014, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, L.; Chari, J.; Aghyarian, S.; Gindri, I.; Kosmopoulos, V.; Rodrigues, D. Preparation and Characterization of Inject-able Brushite Filled-Poly (Methyl Methacrylate) Bone Cement. Materials 2014, 7, 6779–6795. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.-J.; Xu, J.; Qiu, Z.-Y.; Ma, X.-L.; Zhang, Z.-Q.; Tan, X.-X.; Cui, Y.; Cui, F.-Z. Mechanical Properties and Cytocompati-bility Improvement of Vertebroplasty PMMA Bone Cements by Incorporating Mineralized Collagen. Materials 2015, 8, 2616–2634. [Google Scholar] [CrossRef]
- Liu, H.; Liu, B.; Gao, C.; Meng, B.; Yang, H.; Yu, H.; Yang, L. Injectable, Biomechanically Robust, Biodegradable and Osseoin-tegrative Bone Cement for Percutaneous Kyphoplasty and Vertebroplasty. Int. Orthop. 2018, 42, 125–132. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Z.; He, D.; Shen, K.; Liu, X.; Li, H.; Jin, W. Bioactive Injectable Polymethylmethacrylate/Silicate Bioceramic Hy-brid Cements for Percutaneous Vertebroplasty and Kyphoplasty. J. Mech. Behav. Biomed. Mater. 2019, 96, 125–135. [Google Scholar] [CrossRef]
- Lewis, G. Injectable Bone Cements for Use in Vertebroplasty and Kyphoplasty: State-of-the-Art Review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 76B, 456–468. [Google Scholar] [CrossRef]
- OECD Health Statistics. Available online: http://www.oecd.org/els/health-systems/health-data.htm (accessed on 15 December 2019).
- Neogi, T. The Epidemiology and Impact of Pain in Osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [Green Version]
- Roemer, F.; Jarraya, M.; Niu, J.; Silva, J.; Frobell, R.; Guemrazi, A. Increased Risk for Radiographic Osteoarthritis Features in Young Active Athletes: A Cross-Sectional Matched Case–Control Study. Osteoarthr. Cartil. 2015, 23, 239–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnett, A.J.; Toms, A.D. Revision Total Hip and Knee Replacement. Clin. Geriat. Med. 2012, 28, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, Knee & Shoulder Arthroplasty: 2020 Annual Report. Available online: https://aoanjrr.sahmri.com/documents/10180/689619/Hip%2C+Knee+%26+Shoulder+Arthroplasty+New/6a07a3b8-8767-06cf-9069-d165dc9baca7 (accessed on 29 December 2020).
- Kurtz, S. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780. [Google Scholar] [CrossRef]
- Khan, M.; Osman, K.; Green, G.; Haddad, F.S. The Epidemiology of Failure in Total Knee Arthroplasty: Avoiding Your next Revision. Bone Jt. J. 2016, 98-B, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refsum, A.M.; Nguyen, U.V.; Gjertsen, J.-E.; Espehaug, B.; Fenstad, A.M.; Lein, R.K.; Ellison, P.; Høl, P.J.; Furnes, O. Cement-ing Technique for Primary Knee Arthroplasty: A Scoping Review. Acta Orthop. 2019, 90, 582–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balin, A. Cementy w Chirurgii Kostnej; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2016; ISBN 978-83-7880-351-5. [Google Scholar]
- Balin, A. Materiałowo Uwarunkowane Procesy Adaptacyjne i Trwałość Cementów Stosowanych w Chirurgii Kostnej; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2004. [Google Scholar]
- Machrowska, A.; Karpiński, R.; Jonak, J.; Szabelski, J.; Krakowski, P. Numerical Prediction of the Component-Ratio-Dependent Compressive Strength of Bone Cement. Appl. Comput. Sci. 2020, 87–101. [Google Scholar] [CrossRef]
- Ferraris, S.; Miola, M.; Bistolfi, A.; Fucale, G.; Crova, M.; Massé, A.; Verné, E. In Vitro Comparison between Commercially and Manually Mixed Antibiotic-Loaded Bone Cements. J. Appl. Biomater. Biomech. 2010, 8, 166–174. [Google Scholar] [CrossRef]
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Seasoning Polymethyl Methacrylate (PMMA) Bone Cements with Incorrect Mix Ratio. Materials 2019, 12, 3073. [Google Scholar] [CrossRef] [Green Version]
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Effect of Physiological Fluids Contamination on Selected Mechanical Properties of Acrylate Bone Cement. Materials 2019, 12, 3963. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G.; Janna, S.; Bhattaram, A. Influence of the Method of Blending an Antibiotic Powder with an Acrylic Bone Cement Powder on Physical, Mechanical, and Thermal Properties of the Cured Cement. Biomaterials 2005, 26, 4317–4325. [Google Scholar] [CrossRef]
- Karpinski, R.; Szabelski, J.; Maksymiuk, J. Analysis of the Properties of Bone Cement with Respect to Its Manufacturing and Typical Service Lifetime Conditions. MATEC Web Conf. 2018, 244, 01004. [Google Scholar] [CrossRef]
- Graham, J.; Pruitt, L.; Ries, M.; Gundiah, N. Fracture and Fatigue Properties of Acrylic Bone Cement: The Effects of Mixing Method, Sterilization Treatment, and Molecular Weight. J. Arthroplast. 2000, 15, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Mau, H.; Schelling, K.; Heisel, C.; Wang, J.-S.; Breusch, S. Comparison of Various Vacuum Mixing Systems and Bone Ce-ments as Regards Reliability, Porosity and Bending Strength. Acta Orthop. Scand. 2004, 75, 160–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wlodarski, J.; Szyprowski, J.; Wieckowski, W.; Szarek, A. Fillers Influence on the Mechanical Properties of the Composite Bone Cements. Compos. Theory Pract. 2005, 5, 78-U279. [Google Scholar]
- Santos, J.G.F., Jr.; Pita, V.J.R.R.; Melo, P.A.; Nele, M.; Pinto, J.C. Production of Bone Cement Composites: Effect of Fillers, Co-Monomer and Particles Properties. Braz. J. Chem. Eng. 2011, 28, 28–229. [Google Scholar] [CrossRef] [Green Version]
- Machrowska, A.; Szabelski, J.; Karpiński, R.; Krakowski, P.; Jonak, J.; Jonak, K. Use of Deep Learning Networks and Statisti-cal Modeling to Predict Changes in Mechanical Parameters of Contaminated Bone Cements. Materials 2020, 13, 5419. [Google Scholar] [CrossRef]
- Race, A.; Miller, M.A.; Ayers, D.C.; Mann, K.A. Early Cement Damage around a Femoral Stem Is Concentrated at the Ce-ment/Bone Interface. J. Biomech. 2003, 36, 489–496. [Google Scholar] [CrossRef]
- Yang, D.T.; Zhang, D.; Arola, D.D. Fatigue of the Bone/Cement Interface and Loosening of Total Joint Replacements. Int. J. Fatigue 2010, 32, 1639–1649. [Google Scholar] [CrossRef]
- Kim, D.-G.; Miller, M.A.; Mann, K.A. Creep Dominates Tensile Fatigue Damage of the Cement–Bone Interface. J. Orthop. Res. 2004, 22, 633–640. [Google Scholar] [CrossRef]
- Arola, D.; Stoffel, K.A.; Yang, D.T. Fatigue of the Cement/Bone Interface: The Surface Texture of Bone and Loosening. J. Bio-med. Mat. Res. Part B Appl. Biomater. 2006, 76B, 287–297. [Google Scholar] [CrossRef] [Green Version]
- Bistolfi, A.; Ferracini, R.; Albanese, C.; Vernè, E.; Miola, M. PMMA-Based Bone Cements and the Problem of Joint Arthroplasty Infections: Status and New Perspectives. Materials 2019, 12, 4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wooley, P.; Schwarz, E. Aseptic Loosening. Gene Ther. 2004, 11, 402–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedard, N.A.; Callaghan, J.J.; Stefl, M.D.; Liu, S.S. Systematic Review of Literature of Cemented Femoral Components: What Is the Durability at Minimum 20 Years Followup? Clin. Orthop. Rel. Res. 2015, 473, 563–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.H.; Koh, B.T.; Ramruttun, A.K.; Wang, W. Compression and Flexural Strength of Bone Cement Mixed with Blood. J. Orthop. Surg. 2016, 24, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, A.; Lieb, E.; Berberich, C.; Kühn, K.-D. PMMA Cements in Revision Surgery. In Management of Periprosthetic Joint Infection: A Global Perspective on Diagnosis. Treatment Options, Prevention Strategies and Their Economic Impact; Springer: New York, NY, USA, 2017; Volume 243. [Google Scholar]
- ISO 5833:2002 Implants for Surgery—Acrylic Resin Cements; ISO: Geneva, Switzerland, 2002.
- Rabiej, M. Grupa Wydawnicza Helion Analizy Statystyczne z Programami Statistica i Excel; Wydawnictwo Helion: Gliwice, Wasrsaw, Poland, 2018; ISBN 978-83-283-3922-4. [Google Scholar]
- Sharkey, P.F.; Hozack, W.J.; Rothman, R.H.; Shastri, S.; Jacoby, S.M. Why Are Total Knee Arthroplasties Failing Today? Clin. Orthop. Relat. Res. 2002, 404, 7–13. [Google Scholar] [CrossRef]
- Swedish Knee Arthroplasty Register Annual Report 2019; Media-Tryck: Lund, Sweden, 2019; ISBN 978-91-88017-29-1.
- Jones, C.A.; Pohar, S. Health-Related Quality of Life after Total Joint Arthroplasty: A Scoping Review. Clin. Geriatr. Med. 2012, 28, 395–429. [Google Scholar] [CrossRef]
- Tyson, Y.; Rolfson, O.; Kärrholm, J.; Hailer, N.P.; Mohaddes, M. Uncemented or Cemented Revision Stems? Analysis of 2,296 First-Time Hip Revision Arthroplasties Performed Due to Aseptic Loosening, Reported to the Swedish Hip Arthroplasty Reg-ister. Acta Orthop. 2019, 90, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Paz, E.; Forriol, F.; del Real, J.C.; Dunne, N. Graphene Oxide versus Graphene for Optimisation of PMMA Bone Cement for Orthopaedic Applications. Mater. Sci. Eng. C 2017, 77, 1003–1011. [Google Scholar] [CrossRef]
- Paz, E.; Ballesteros, Y.; Abenojar, J.; del Real, J.C.; Dunne, N.J. Graphene Oxide and Graphene Reinforced PMMA Bone Ce-ments: Evaluation of Thermal Properties and Biocompatibility. Materials 2019, 12, 3146. [Google Scholar] [CrossRef] [Green Version]
- Prokopovich, P.; Perni, S.; Thenault, V.; Abdo, P.; Margulis, K.; Magdassi, S. Antimicrobial Activity of Bone Cements Embed-ded with Organic Nanoparticles. IJN 2015, 6317. [Google Scholar] [CrossRef] [Green Version]
- Deb, S.; Doiron, R.; DiSilvio, L.; Punyani, S.; Singh, H. PMMA Bone Cement Containing a Quaternary Amine Comonomer with Potential Antibacterial Properties. J. Biomed. Mater. Res. 2008, 85B, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.; Nguyen, M.; Kraxenberger, M.; Chevalier, Y.; Melcher, C.; Wegener, B.; Birkenmaier, C. Modification of PMMA Vertebroplasty Cement for Reduced Stiffness by Addition of Normal Saline: A Material Properties Evaluation. Eur. Spine J. 2017, 26, 3209–3215. [Google Scholar] [CrossRef] [PubMed]
- Markus Nottrott Acrylic Bone Cements. Acta Orthop. 2010, 81, 1–27. [CrossRef]
- Nottrott, M.; Mølster, A.O.; Gjerdet, N.R. Time Dependent Mechanical Properties of Bone Cement. Anin Vitro Study over One Year. J. Biomed. Mater. Res. 2007, 83B, 416–421. [Google Scholar] [CrossRef]
- Danesi, V.; Faldini, C.; Cristofolini, L. Methods for the Characterization of the Long-Term Mechanical Performance of Ce-ments for Vertebroplasty and Kyphoplasty: Critical Review and Suggestions for Test Methods. J. Mech. Med. Biol. 2017, 17, 1730002. [Google Scholar] [CrossRef]
- Nottrott, M.; Mølster, A.O.; Moldestad, I.O.; Walsh, W.R.; Gjerdet, N.R. Performance of Bone Cements: Are Current Preclini-cal Specifications Adequate? Acta Orthop. 2008, 79, 826–831. [Google Scholar] [CrossRef]



| Compound Name | CMW 1 | CMW 3 |
|---|---|---|
| Bone Cement Powder Component: | ||
| Polymethyl methacrylate | 88.85 | 83.88 |
| Benzoyl peroxide | 2.05 | 2.00 |
| Barium sulphate | 9.10 | 10.00 |
| Bone Cement Liquid Component: | ||
| Methyl methacrylate | 98.50 | 97.5 |
| N,N-dimethyl-p-toluidine | <2.00 | <2.50 |
| Hydroquinone (ppm) | 75 | 75 |
| Compression Strength [MPa] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Saline Contamination | Seasoning Time > | CMW 1 | CMW 3 | ||||||
| 0 Day | 1 Day | 15 Days | 30 Days | 0 Day | 1 Day | 15 Days | 30 Days | ||
| 0% | MV | 89.23 | 78.85 | 72.07 | 70.26 | 85.45 | 85.15 | 76.02 | 70.38 |
| SD | 5.23 | 7.37 | 7.29 | 6.88 | 2.79 | 3.50 | 8.62 | 3.54 | |
| COV | 5.9% | 9.3% | 10.1% | 9.8% | 3.3% | 4.1% | 11.3% | 5.0% | |
| 1% | MV | 85.35 | 86.63 | 71.65 | 68.78 | 89.01 | 80.68 | 88.91 | 72.67 |
| SD | 8.30 | 7.76 | 7.50 | 13.37 | 6.87 | 3.67 | 6.16 | 6.84 | |
| COV | 9.7% | 9.0% | 10.5% | 19.4% | 7.7% | 4.6% | 6.9% | 9.4% | |
| 3% | MV | 74.46 | 77.38 | 72.97 | 65.20 | 76.67 | 74.50 | 76.68 | 71.22 |
| SD | 3.83 | 4.65 | 2.58 | 3.98 | 2.67 | 2.41 | 4.56 | 3.20 | |
| COV | 5.1% | 6.0% | 3.5% | 6.1% | 3.5% | 3.2% | 5.9% | 4.5% | |
| 6% | MV | 67.96 | 69.57 | 66.48 | 63.46 | 72.89 | 70.95 | 71.86 | 65.91 |
| SD | 4.29 | 5.20 | 5.30 | 5.71 | 8.14 | 3.89 | 2.28 | 3.38 | |
| COV | 6.3% | 7.5% | 8.0% | 9.0% | 11.2% | 5.5% | 3.2% | 5.1% | |
| 10% | MV | 63.89 | 69.11 | 66.37 | 57.50 | 65.50 | 66.52 | 64.02 | 63.41 |
| SD | 3.92 | 7.42 | 5.29 | 2.80 | 4.07 | 3.23 | 3.55 | 4.39 | |
| COV | 6.1% | 10.7% | 8.0% | 4.9% | 6.2% | 4.9% | 5.5% | 6.9% | |
| Surface Microhardness [Shore D] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Saline Contamination | Seasoning Time > | CMW1 | CMW3 | ||||||
| 0 Days | 1 Day | 15 Days | 30 Days | 0 Days | 1 Day | 15 Days | 30 Days | ||
| 0% | MV | 73.09 | 74.21 | 72.18 | 71.46 | 80.00 | 77.19 | 77.13 | 74.41 |
| SD | 4.92 | 5.00 | 5.88 | 4.45 | 3.72 | 2.47 | 2.33 | 1.71 | |
| COV | 6.7% | 6.7% | 8.1% | 6.2% | 4.7% | 3.2% | 3.0% | 2.3% | |
| 1% | MV | 74.30 | 76.05 | 76.67 | 73.93 | 78.77 | 75.53 | 76.83 | 72.79 |
| SD | 3.86 | 4.48 | 3.85 | 6.89 | 3.16 | 2.88 | 5.94 | 5.00 | |
| COV | 5.2% | 5.9% | 5.0% | 9.3% | 4.0% | 3.8% | 7.7% | 6.9% | |
| 3% | MV | 75.97 | 73.24 | 73.45 | 70.50 | 80.14 | 71.01 | 71.01 | 71.74 |
| SD | 3.84 | 5.64 | 2.90 | 4.48 | 5.12 | 3.80 | 8.14 | 3.60 | |
| COV | 5.0% | 7.7% | 3.9% | 6.4% | 6.4% | 5.4% | 11.5% | 5.0% | |
| 6% | MV | 76.14 | 71.17 | 74.87 | 68.48 | 75.22 | 70.59 | 67.21 | 67.69 |
| SD | 3.57 | 2.70 | 4.07 | 6.93 | 3.60 | 3.03 | 4.48 | 3.95 | |
| COV | 4.7% | 3.8% | 5.4% | 10.1% | 4.8% | 4.3% | 6.7% | 5.8% | |
| 10% | MV | 68.25 | 64.65 | 66.24 | 63.05 | 65.84 | 60.17 | 58.25 | 59.29 |
| SD | 7.44 | 3.85 | 5.67 | 6.78 | 3.19 | 4.80 | 2.84 | 4.69 | |
| COV | 10.9% | 6.0% | 8.6% | 10.8% | 4.9% | 8.0% | 4.9% | 7.9% | |
 |
 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpiński, R.; Szabelski, J.; Krakowski, P.; Jonak, J. Effect of Physiological Saline Solution Contamination on Selected Mechanical Properties of Seasoned Acrylic Bone Cements of Medium and High Viscosity. Materials 2021, 14, 110. https://doi.org/10.3390/ma14010110
Karpiński R, Szabelski J, Krakowski P, Jonak J. Effect of Physiological Saline Solution Contamination on Selected Mechanical Properties of Seasoned Acrylic Bone Cements of Medium and High Viscosity. Materials. 2021; 14(1):110. https://doi.org/10.3390/ma14010110
Chicago/Turabian StyleKarpiński, Robert, Jakub Szabelski, Przemysław Krakowski, and Józef Jonak. 2021. "Effect of Physiological Saline Solution Contamination on Selected Mechanical Properties of Seasoned Acrylic Bone Cements of Medium and High Viscosity" Materials 14, no. 1: 110. https://doi.org/10.3390/ma14010110





