Self-Supported Reduced Graphene Oxide Membrane and Its Cu2+ Adsorption Capability
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. GO Synthesis
2.3. Preparation of the rGO Membrane
2.4. Raman Spectrum Analysis
2.5. Morphology Analysis
2.6. Electrical Surface Resistance Analysis
2.7. Adsorption Experiment
3. Results and Discussion
3.1. Reduction of GO Membrane
3.2. Characterization of the rGO Membrane
3.3. Cu2+ Adsorption Performance of the rGO Membrane
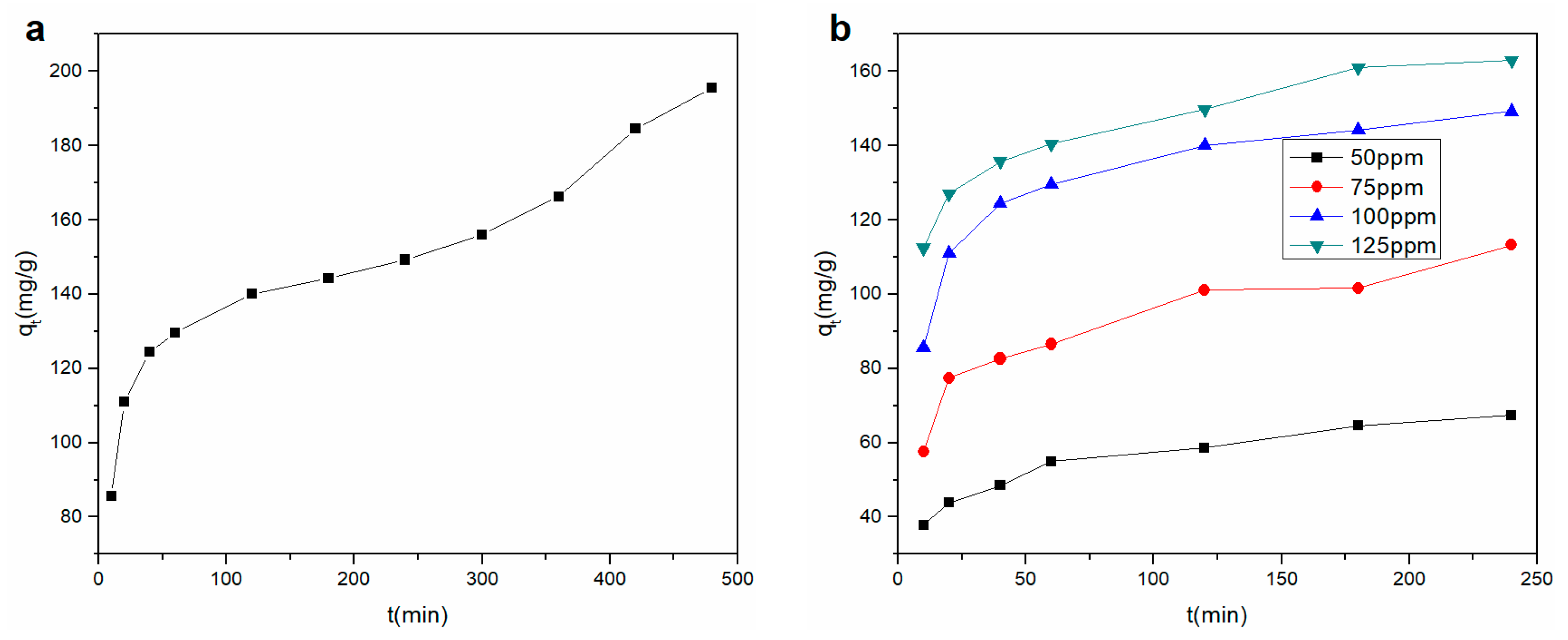
3.3.1. Effect of Contact Time
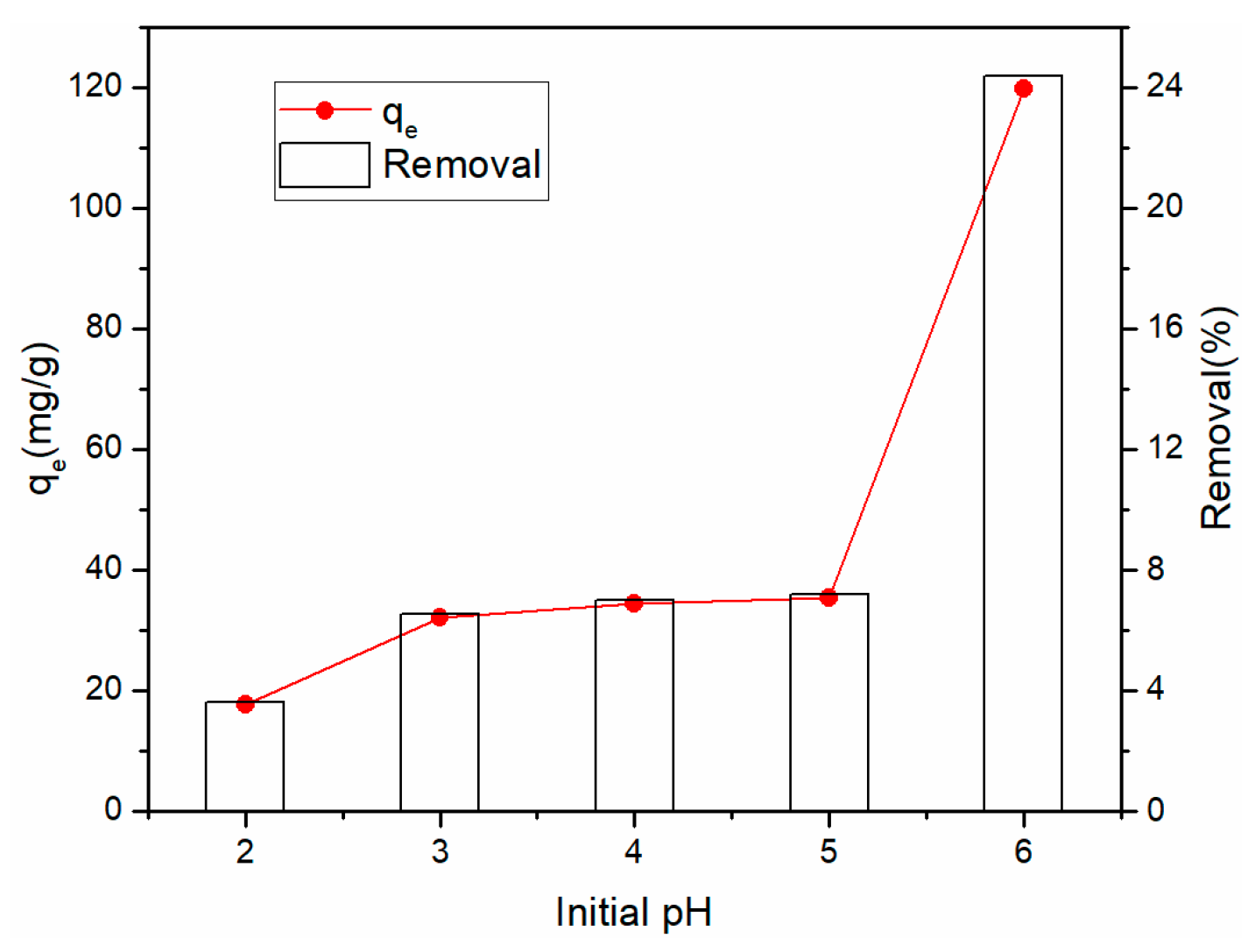
3.3.2. Effect of Initial pH
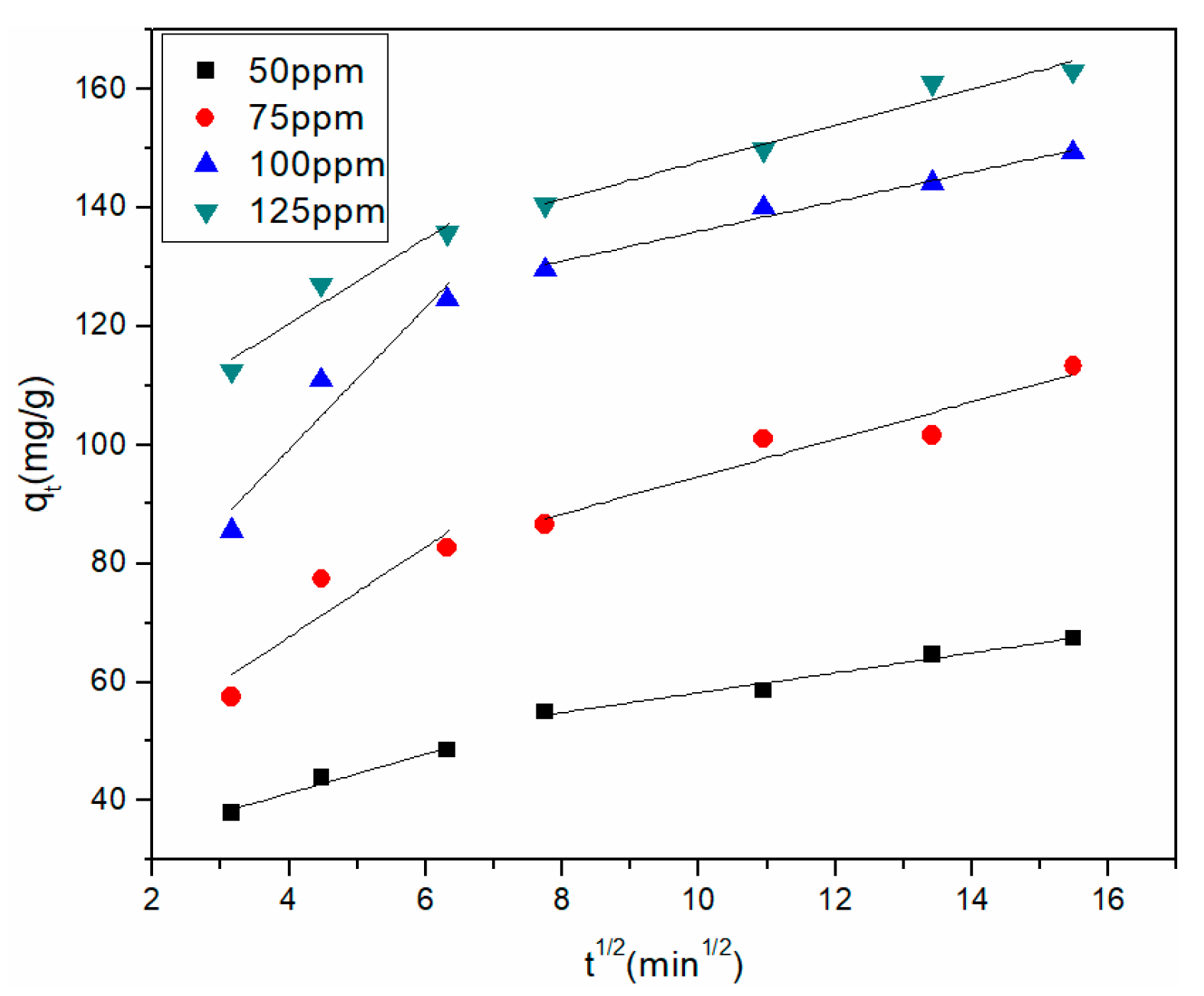
3.3.3. Adsorption Kinetic Study
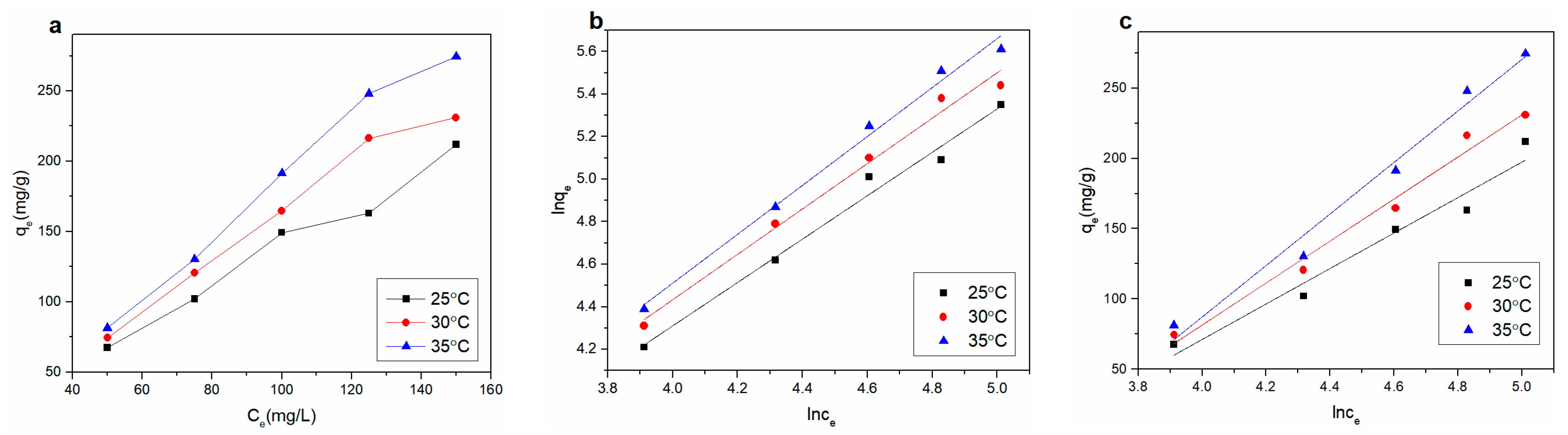
3.3.4. Adsorption Thermodynamic Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.K.; Li, X.F.; Zhang, X.Y.; Wang, W.; Yuan, S.L. Effect of inorganic regenerant properties on pharmaceutical adsorption and desorption performance on polymer anion exchange resin. Chemosphere 2017, 182, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Z.; Nursam, N.M.; Xia, F.; Sani, M.A.; Li, W.; Wang, X.D.; Caruso, R.A. High-performance coral reef-like carbon Nitrides: Synthesis and application in photocatalysis and heavy metal ion adsorption. ACS Appl. Mater. Interfaces 2017, 9, 4540–4547. [Google Scholar] [CrossRef]
- Awual, M.R. A novel facial composite adsorbent for enhanced copper (II) detection and removal from waste water. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Yi, X.F.; Sun, F.L.; Han, Z.H.; Han, F.H.; He, J.R.; Ou, M.R.; Gu, J.; Xu, X. Graphene oxide encapsulated polyvinyl alcohol/sodium alginate hydrogel microspheres for Cu (II) and U (VI) removal. Ecotoxicol. Environ. Saf. 2018, 158, 309–318. [Google Scholar] [CrossRef]
- Rohini, R.; Katti, P.; Bose, S. Tailoring the interface in graphene/thermoset polymer composites: A critical review. Polymer 2015, 70, A17–A34. [Google Scholar] [CrossRef]
- Ersan, G.; Apul, O.G.; Perreault, F.; Karanfil, T. Adsorption of organic contaminants by graphene nanosheets: A review. Water Res. 2017, 126, 385–398. [Google Scholar] [CrossRef]
- Gin, D.L.; Noble, R.D. Designing the next generation of chemical separation membranes. Science 2011, 332, 674–676. [Google Scholar] [CrossRef]
- Logan, B.E.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef]
- Karan, S.; Jiang, Z.; Livingston, A.G. Sub–10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Politano, A.; Drioli, E. The advent of graphene and other two-dimensional materials in membrane science and technology. Curr. Opin. Chem. Eng. 2017, 16, 78–85. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; van der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Miculescu, M.; Thakur, V.K.; Miculescu, F.; Voicu, S.I. Graphene-based polymer nanocomposite membranes: A review. Polym. Adv. Technol. 2016, 27, 844–859. [Google Scholar] [CrossRef]
- Sun, C.Z.; Wen, B.Y.; Bai, B.F. Recent advances in nanoporous graphene membrane for gas separation and water purification. Sci. Bull. 2015, 60, 1807–1823. [Google Scholar] [CrossRef]
- Tan, P.; Sun, J.; Hu, Y.; Fang, Z.; Bi, Q.; Chen, Y.; Cheng, J. Adsorption of Cu2+, Cd2+ and Ni2+ from aqueous single metal solutions on graphene oxide membranes. J. Hazard. Mater. 2015, 297, 251–260. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Ju, X.J.; Xie, R.; Chu, L.Y. Graphene-based membranes for molecular and ionic separations in aqueous environments. Chin. J. Chem. Eng. 2017, 25, 1598–1605. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode= CJFD&filena me =ZHGC201711006 (accessed on 15 November 2017). [CrossRef]
- Nhlane, D.; Richards, H.; Etale, A. Facile and green synthesis of reduced graphene oxide for remediation of Hg(II)-contaminated water. Mater. Today Proc. 2020, in press. [Google Scholar] [CrossRef]
- Ghorbani, M.; Seyedin, O.; Aghamohammadhassan, M. Adsorptive removal of lead (II) ion from water and wastewater media using carbon-based nanomaterials as unique sorbents: A review. J. Environ. Manag. 2020, 254, 109814. [Google Scholar] [CrossRef]
- Tang, X.; Tian, M.; Qu, L.; Zhu, S.; Guo, X.; Han, G.; Sun, K.; Hu, X.; Wang, Y.; Xu, X. Functionalization of cotton fabric with graphene oxide nanosheet and polyaniline for conductive and UV blocking properties. Synth. Met. 2015, 202, 82–88. [Google Scholar] [CrossRef]
- Hussain, S.; Yorucu, C.; Ahmed, I.; Hussain, R.; Chen, B.; Khan, M.B.; Siddique, N.A.; Rehman, I.U. Surface modification of aramid fibres by graphene oxide nano-sheets for multiscale polymer composites. Surf. Coat. Technol. 2014, 258, 458–466. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E. Fabricating electroconductive cotton textiles using graphene. Carbohydr. Polym. 2013, 96, 190–195. [Google Scholar] [CrossRef]
- Molina, J.; Fernández, J.; Fernandes, M.; Souto, A.P.; Esteves, M.; Bonastre, J.; Cases, F. Plasma treatment of polyester fabrics to increase the adhesion of reduced graphene oxide. Synth. Met. 2015, 202, 110–122. [Google Scholar] [CrossRef]
- Mi, X.; Huang, G.B.; Xie, W.S.; Wang, W.; Liu, Y.; Gao, J.P. Preparation of graphene oxide aerogel and its adsorption for Cu2+ ions. Carbon 2012, 50, 4856–4864. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, A.; Khan, S.; Ahmad, S.; Khan, I.; Zada, S.; Fu, P. Algal extracts based biogenic synthesis of reduced graphene oxides(rGO) with enhanced heavy metals adsorption capability. J. Ind. Eng. Chem. 2019, 72, 117–124. [Google Scholar] [CrossRef]
- Fu, W.; Huang, Z. Magnetic dithiocarbamate functionalized reduced graphene oxide for the removal of Cu(II), Cd(II), Pb(II), and Hg(II) ions from aqueous solution: Synthesis, adsorption, and regeneration. Chemosphere 2018, 209, 449–456. [Google Scholar] [CrossRef]
- Zeng, T.; Yu, Y.; Li, Z.; Zuo, J.; Kuai, Z.; Jin, Y.; Wang, Y.; Wu, A.; Peng, C. 3D MnO2 nanotubes@reduced graphene oxide hydrogel as reusable adsorbent for the removal of heavy metal ions. Mater. Chem. Phys. 2019, 231, 105–108. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Manivel, P.; Rajendrakumar, R.T.; Uyar, T. Multifunctional ZnO nanorod-reduced graphene oxide hybrids nanocomposites for effective water remediation: Effective sunlight driven degradation of organic dyes and rapid heavy metal adsorption. Chem. Eng. J. 2017, 325, 588–600. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Figaro, S.; Avril, J.P.; Brouers, F.; Ouensanga, A.; Gaspard, S. Adsorption studies of molasse’s wastewaters on activated carbon: Modelling with a new fractal kinetic equation and evaluation of kinetic models. J. Hazard. Mater. 2009, 161, 649–656. [Google Scholar] [CrossRef]
- Nupearachchi, C.N.; Mahatantila, K.; Vithanage, M. Application of graphene for decontamination of water; Implications for sorptive removal. Groundw. Sustain. Dev. 2017, 5, 206–215. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.G.; Zeng, G.M.; Hu, X.J.; Hu, X. Grafting of β-cyclodextrin to magnetic graphene oxide via ethylenediamine and application for Cr (VI) removal. Carbohydr. Polym. 2014, 113, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Repo, E.; Warchol, J.K.; Kurniawana, T.A.; Sillanpaa, M.E.T. Adsorption of Co (II) and Ni (II) by EDTA- and/or DTPA-modified chitosan: Kinetic and equilibrium modeling. Chem. Eng. J. 2010, 161, 73–82. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.; Choi, I.; Rengaraj, S.; Yi, J. Arsenic Removal Using Mesoporous Alumina Prepared via a Templating Method. Environ. Sci. Technol. 2004, 38, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Temkin, M.J.; Pyzhev, V. Kinetic of ammonia synthesis on promoted iron Catalysts. Acta Physicochim. URSS 1940, 12, 217–222. [Google Scholar]




| Concentration of Na2S2O4 (g∙L−1) | Electrical Surface Resistance (Ω∙cm−1) |
|---|---|
| 10 | 5 × 104 |
| 20 | 480 |
| 30 | 410 |
| 40 | 370 |
| 50 | 560 |
| Adsorbent | Adsorption Capacity (mg·g−1) | Adsorption Conditions | Reference | ||
|---|---|---|---|---|---|
| pH | Time (h) | C0 (ppm) | |||
| N-rGO | 91 | 7 | 9 | 100 | [24] |
| A-rGO | 74 | ||||
| J-rGO | 93 | ||||
| rGO-PDTC/Fe3O4 | 113.64 | 6 | 6 | 100 | [25] |
| MnO2 nanotubes@rGO | 121.5 | 5 | 12 | 200 | [26] |
| ZnO nanorod-rGO | 67.39 | 6 | 2 | 10 | [27] |
| rGO | 149.25 | 6 | 4 | 100 | this work |
| Kinetic Model | Parameter | 50 ppm | 75 ppm | 100 ppm | 125 ppm |
|---|---|---|---|---|---|
| Pseudo-first-order | qe1 (mg·g−1) | 31.31 | 46.98 | 52.06 | 54.93 |
| k1 (min−1) | 1.27 × 10−2 | 0.88 × 10−2 | 1.38 × 10−2 | 1.49 × 10−2 | |
| R2 | 0.9579 | 0.8635 | 0.9443 | 0.9494 | |
| Pseudo-second-order | qe2 (mg·g−1) | 69.98 | 115.47 | 153.14 | 166.94 |
| k2 (g·mg−1·min−1) | 0.98 × 10−3 | 0.60 × 10−3 | 0.71 × 10−3 | 0.70 × 10−3 | |
| R2 | 0.9957 | 0.9929 | 0.9994 | 0.9984 | |
| qe (mg·g−1) | 67.43 | 113.25 | 149.25 | 163.00 |
| Concentration (ppm) | Parameters | |||||
|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | |||||
| kp1 | c1 | (R1)2 | kp2 | c2 | (R2)2 | |
| 50 | 3.30 | 28.04 | 0.9430 | 1.68 | 41.44 | 0.9649 |
| 75 | 7.60 | 37.18 | 0.6641 | 3.14 | 63.18 | 0.8781 |
| 100 | 11.98 | 51.28 | 0.8542 | 2.49 | 111.02 | 0.9736 |
| 125 | 7.18 | 91.68 | 0.8868 | 3.09 | 116.76 | 0.9496 |
| Isotherm Model | Constants | 25 °C | 30 °C | 35 °C |
|---|---|---|---|---|
| Freundlich | kF (mg·g−1 (L·mg−1)−1/n) | 1.69 | 1.46 | 0.82 |
| 1/n | 1.02 | 1.06 | 1.15 | |
| R2 | 0.9809 | 0.9834 | 0.9887 | |
| Temkin | KT (L·mg−1) | 0.0322 | 0.0316 | 0.0295 |
| B | 126.29 | 149.51 | 183.31 | |
| R2 | 0.9494 | 0.9778 | 0.9762 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Wang, Z.; Sun, R.; Chen, Z.; Liu, M.; Zhou, X.; Yao, M.; Wang, G. Self-Supported Reduced Graphene Oxide Membrane and Its Cu2+ Adsorption Capability. Materials 2021, 14, 146. https://doi.org/10.3390/ma14010146
Yu Y, Wang Z, Sun R, Chen Z, Liu M, Zhou X, Yao M, Wang G. Self-Supported Reduced Graphene Oxide Membrane and Its Cu2+ Adsorption Capability. Materials. 2021; 14(1):146. https://doi.org/10.3390/ma14010146
Chicago/Turabian StyleYu, Yangjinghua, Zhong Wang, Runjun Sun, Zhihua Chen, Meicheng Liu, Xiang Zhou, Mu Yao, and Guohe Wang. 2021. "Self-Supported Reduced Graphene Oxide Membrane and Its Cu2+ Adsorption Capability" Materials 14, no. 1: 146. https://doi.org/10.3390/ma14010146
APA StyleYu, Y., Wang, Z., Sun, R., Chen, Z., Liu, M., Zhou, X., Yao, M., & Wang, G. (2021). Self-Supported Reduced Graphene Oxide Membrane and Its Cu2+ Adsorption Capability. Materials, 14(1), 146. https://doi.org/10.3390/ma14010146




