Desulfurization and Denitrification Performance of Modified Rice Husk Ash-Carbide Slag Absorbent
Abstract
:1. Introduction
2. Experiment and Measurement Methods
2.1. Characterization of Materials
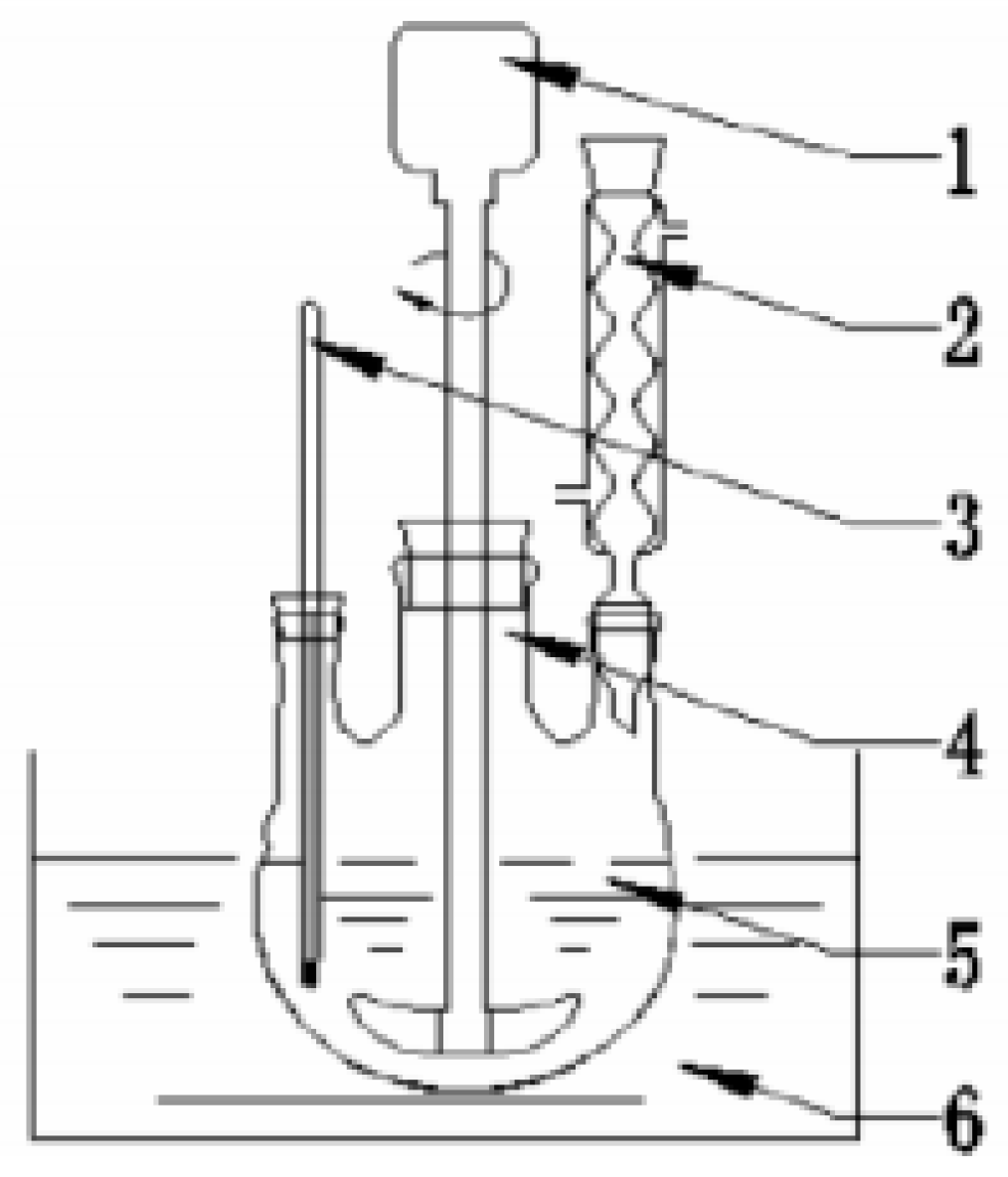
2.2. Desulfurization and Denitrification Performance Test System
2.3. Experimental Method for Modification of Rice Husk Ash-Carbide Slag Sbsorbent
- Rice husk ash and carbide slag with a certain mass ratio were weighed and a certain amount of deionized water was measured.
- After the deionized water is heated to 60–70 °C, put carbide slag into the water and continuously stirred. After the mixture is evenly mixed, the temperature is raised to 70–85 °C, and the rice husk ash is added into the slurry. After that, the temperature is raised to the required hydration temperature and the magnetic stirring is maintained at 300–500 r/min to promote the hydration reaction. The experimental device is shown in Figure 2.
- When the predetermined hydration time is reached, the absorbent slurry is cooled and then pumped for dehydration. Dry the absorbent at 150 °C for 2 h. After drying, grind the fine powder into 100 mesh sieve, and put it into a wide-mouth bottle for airtight storage.
3. Results and Discussion
3.1. Desulfurization and Denitrification Performance of Modified Absorbent
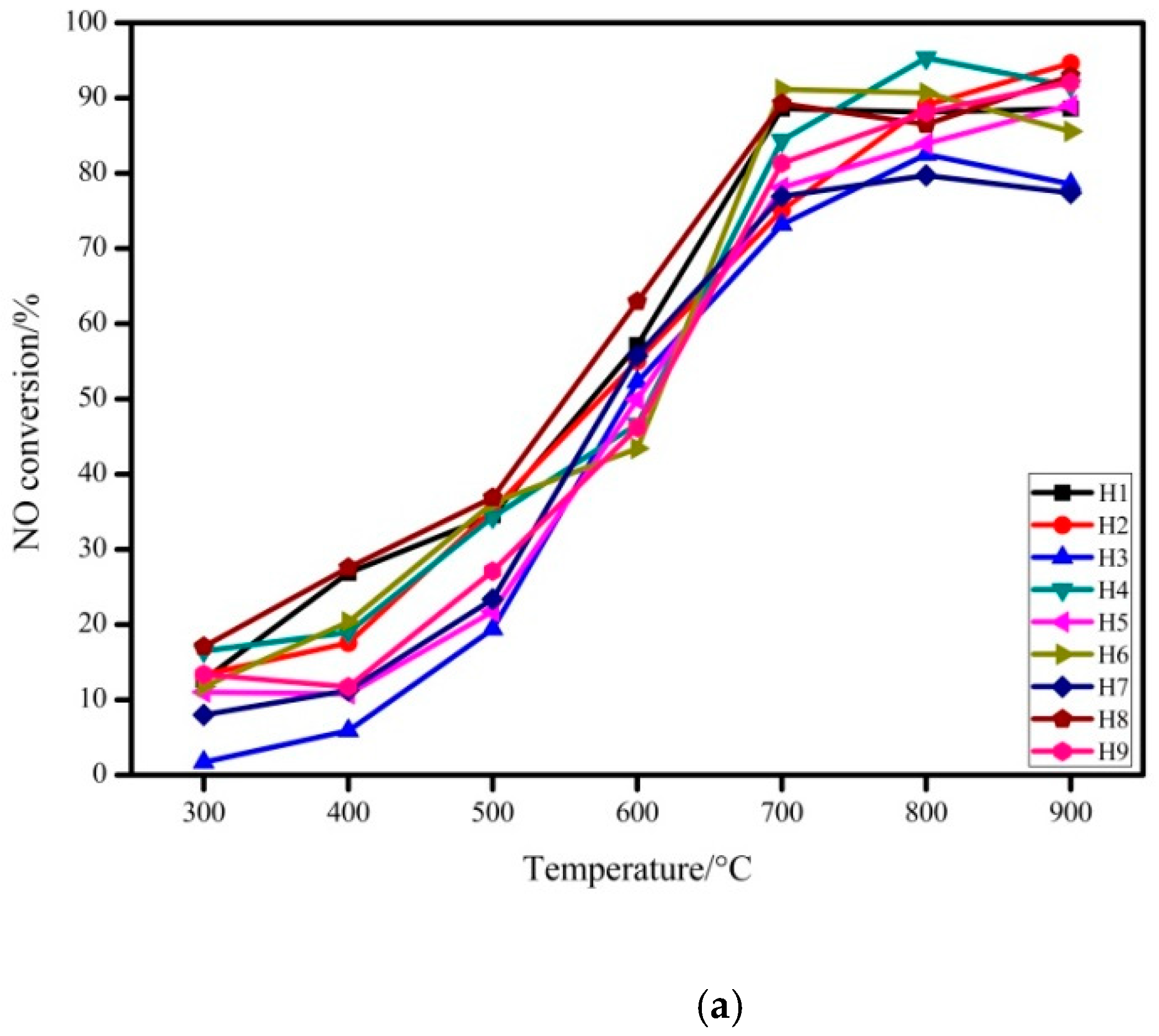
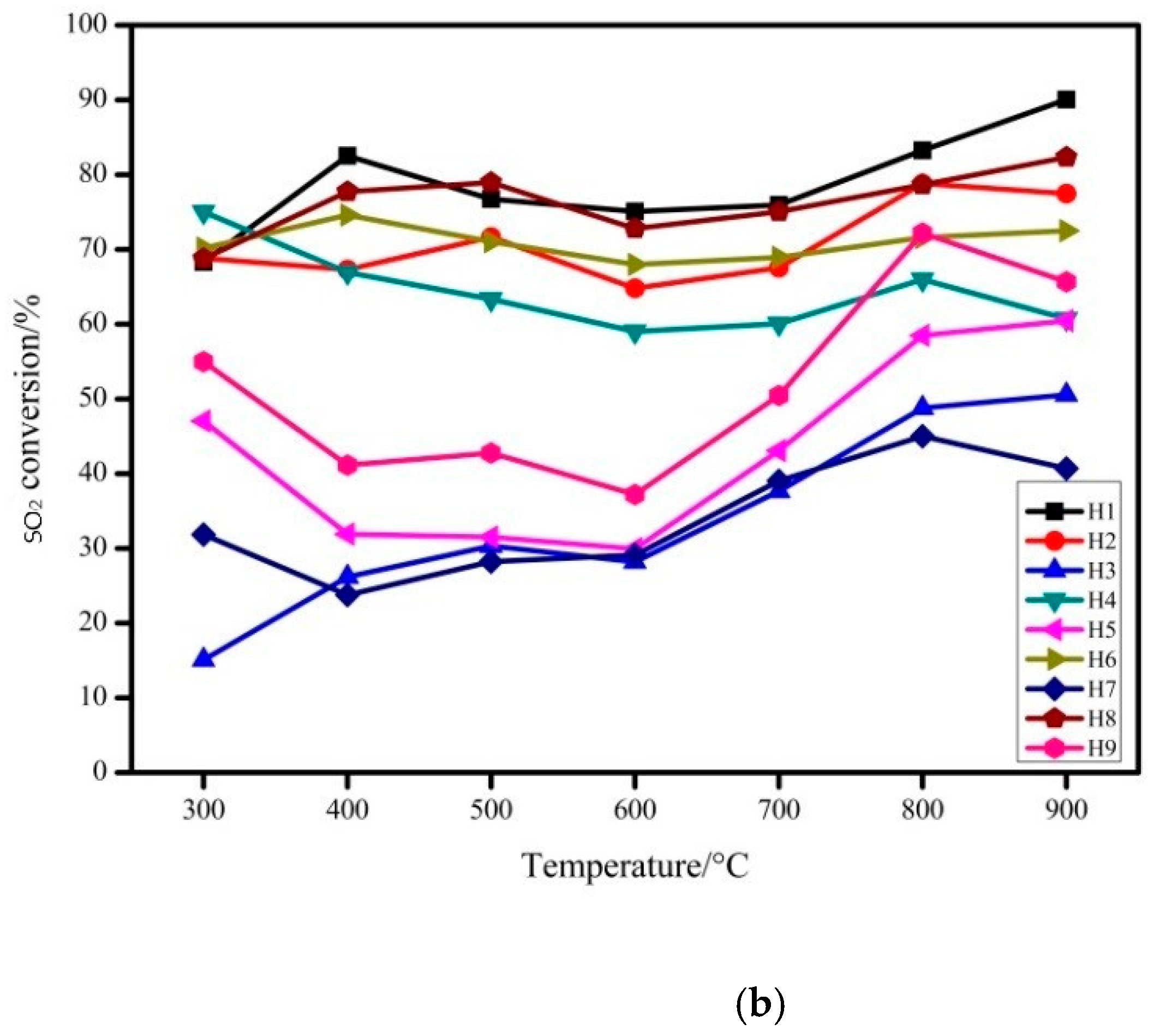
3.1.1. The Effect of Modification Conditions on the Performance of Absorbent
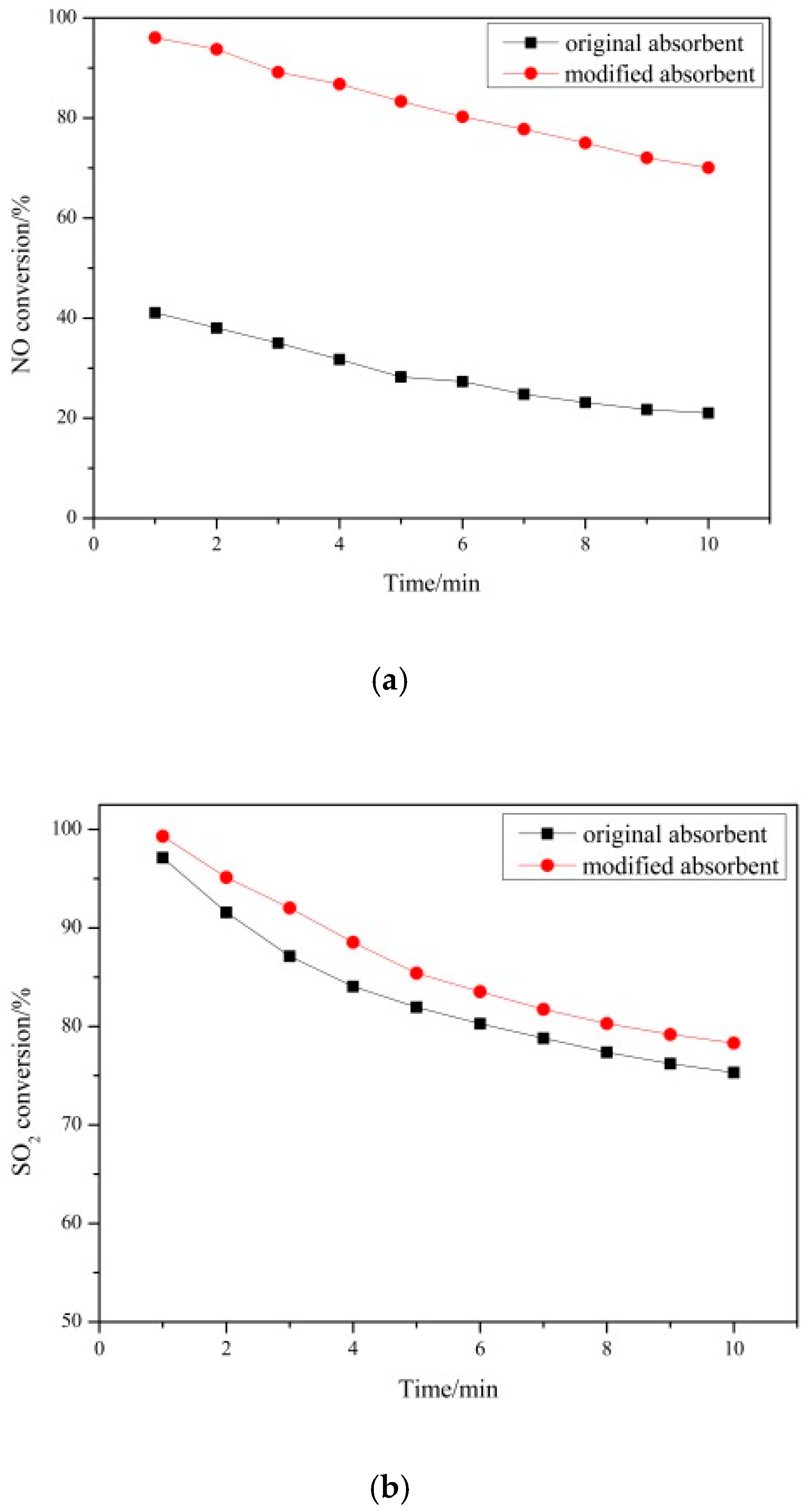
3.1.2. Desulfurization and Denitrification Performance of Absorbent before and after Modification
3.2. Composition and Structure of Modified Absorbent
3.2.1. BET Results
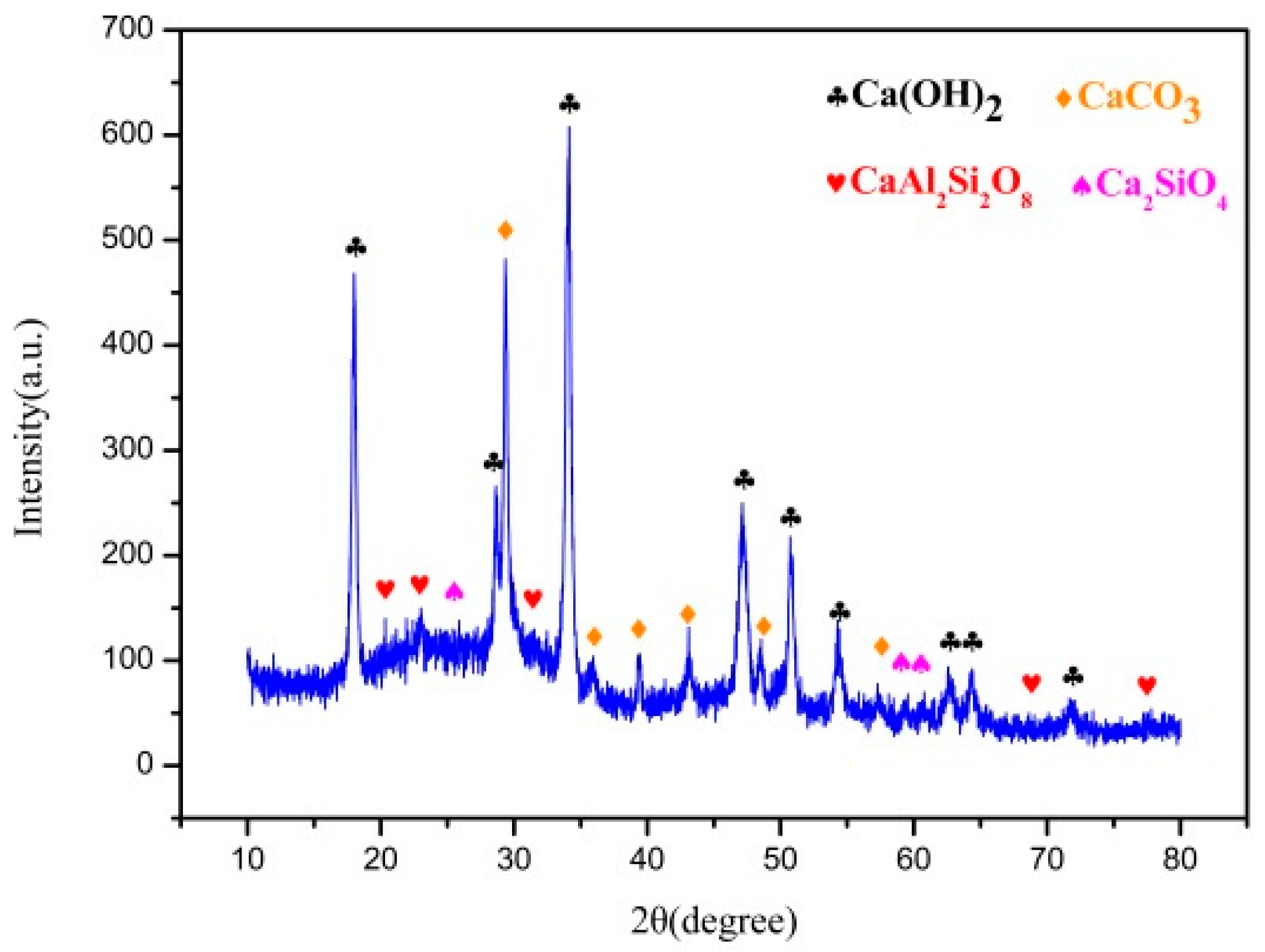
3.2.2. Phase Analysis
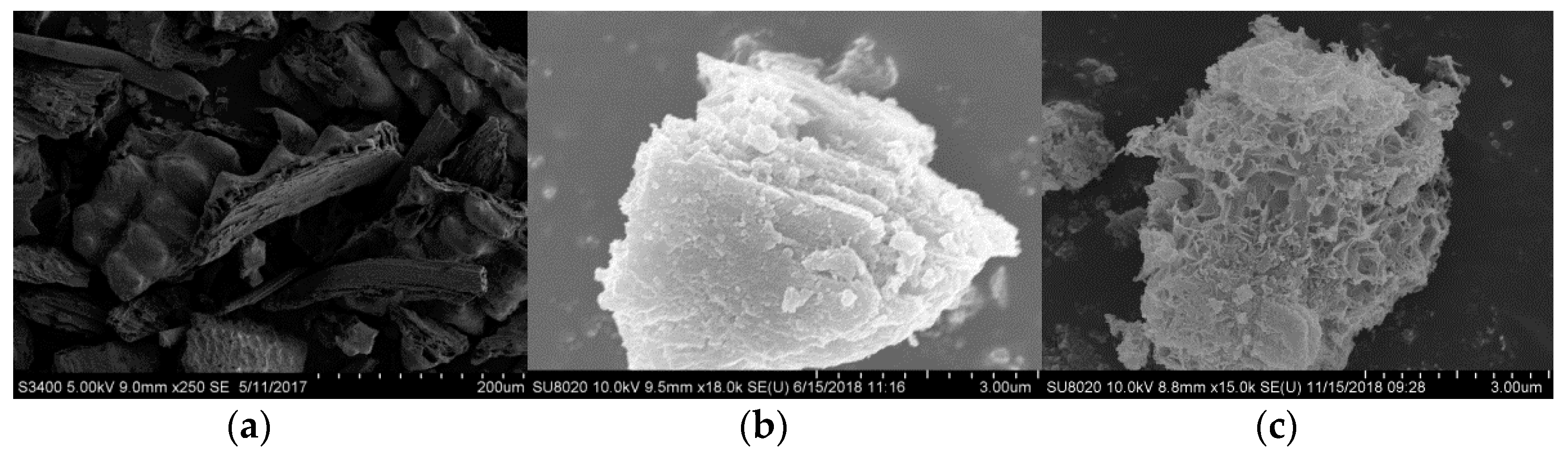
3.2.3. Shape Analysis
3.2.4. XPS Analysis
4. Conclusions
- By changing the hydration temperature, hydration time, mass ratio and solid–liquid ratio of rice husk ash/carbide slag, the optimal preparation conditions for desulfurization and denitrification were determined as follows: the hydration temperature is 130 °C, the hydration time is 8 h, the mass ratio of rice husk ash/ carbide slag is 1:1, and the solid-liquid ratio is 1:20.
- After the absorbent was regulated and surface activated, the reaction temperature window was widened to 700–900 °C. At 700 °C the NO conversion of the modified absorbent is 96% and the SO2 conversion is 99%. Compared with that before modification, NO conversion and SO2 conversion increased by 44% and 2% respectively. An absorbent based on the combination of rice husk ash and carbide slag was proposed for the first time in this paper, which can achieve simultaneous desulfurization and denitrification in the lower temperature stage (about 700 °C) of cement clinker firing.
- During the modification process, hydration products such as fibrous calcium silicate and calcium aluminosilicate are formed. The specific surface area of the absorbent becomes larger, which provides more reactive sites, and the content of oxygen-containing functional groups increases. The decrease of ether CO functional group and the increase of carboxy–COO functional group content are beneficial to the denitrification reaction, solving the problem of the unequal temperature range of SO2 and NOx adsorption and removal in the cement kiln.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, S.C.; Yao, J.J.; Gao, L.; Ma, X.Y.; Zhao, Y. Experimental study on removals of SO2 and NOx using adsorption of activated carbon/microwave desorption. J. Air Waste Manag. Assoc. 2012, 62, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.L. Research on Integrated Technology of Flue Gas Desulfurization and Denitrification(in Chinese). Energy Environ. 2016, 2, 74–75. [Google Scholar]
- Dahlan, I.; Lee, K.T.; Kamaruddin, A.H.; Mohamed, A.R. Selection of metal oxides in the preparation of rice husk ash (RHA)/CaO sorbent for simultaneous SO2 and NO removal. J. Hazard. Mater. 2009, 166, 1556–1559. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Han, Z.; Han, Y.; Yao, J. Applications and New Progress in Simultaneous Desulfurization and Denitrification by Dry Flue Gas Control Technology. Ind. Saf. Environ. Prot. 2009, 35, 4–6. [Google Scholar]
- Ishizuka, T.; Tsuchiai, H.; Murayama, T.; Tanaka, T.; Hattori, H. Preparation of Active Absorbent for Dry-Type Flue Gas Desulfurization from Calcium Oxide, Coal Fly Ash, and Gypsum. Ind. Eng. Chem. Res. 2000, 39, 1390–1396. [Google Scholar] [CrossRef]
- Renedo, M.J.; Fernández, J. Preparation, Characterization, and Calcium Utilization of Fly Ash/Ca(OH)2 Sorbents for Dry Desulfurization at Low Temperature. Ind. Eng. Chem. Res. 2002, 41, 4284–4294. [Google Scholar] [CrossRef]
- Lin, R.-B.; Shih, S.-M. Characterization of Ca(OH)2/Fly Ash Sorbents for Flue Gas Desulfurization. Powder Technol. 2003, 131, 212–222. [Google Scholar] [CrossRef]
- Lee, K.T.; Mohamed, A.R.; Bhatia, S.; Chu, K.H. Removal of Sulfur Dioxide by Fly Ash/CaO/CaSO4 Sorbents. Chem. Eng. J. 2005, 114, 171–177. [Google Scholar] [CrossRef]
- Lee, K.T.; Bhatia, S.; Mohamed, A.R.; Chu, K.H. Optimizing the Specific Surface Area of Fly Ash-based Sorbents for Flue Gas Desulfurization. Chemosphere 2006, 62, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zainudin, N.F.; Lee, K.T.; Kamaruddin, A.H.; Bhatia, S.; Mohamed, A.R. Study of Adsorbent Prepared from Oil Palm Ash (OPA) for Flue Gas Desulfurization. Sep. Purif. Technol. 2005, 45, 50–60. [Google Scholar] [CrossRef]
- Lin, R.B.; Shih, S.-M.; Liu, C.-F. Characteristics and Reactivities of Ca(OH)2/Silica Fume Sorbents for Low-temperature Flue Gas Desulfurization. Chem. Eng. Sci. 2003, 8, 3659–3668. [Google Scholar] [CrossRef]
- Jung, G.-H.; Kin, G.; Kim, S.-G. Utilization of Lime-Silica Solids for Flue Gas Desulfurization. Ind. Eng. Chem. Res. 2000, 39, 5012–5016. [Google Scholar] [CrossRef]
- Dahlan, I.; Lee, K.T.; Kamaruddin, A.H.; Mohamed, A.R. Key Factor in Rice Husk Ash/CaO Sorbent for High Flue Gas Desulfurization Activity. Environ. Sci. Technol. 2006, 40, 6032–6037. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.C.; Lee, K.T.; Mohamed, A.R. Rice Husk Ash Sorbent Doped with Copper for Simultaneous Removal of SO2 and NO: Optimization Study. J. Hazard. Mater. 2010, 183, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Experimental Study on the Effects of Ca-Based Absorbent’s Physical Propertities on Removal of NO2 and SO2. Available online: http://kns-cnki-net-s.libziyuan.bjut.edu.cn:8118/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD2009&filename=2009083646.nh&v=3lc1U0tJcn4rTueQgEqZW6ZFcQtfVVIFXJkRxWhMmfUR8pN8MRdbqeEJfkVKp4Ur (accessed on 23 December 2020).
- Allen, D.; Hayhurst, A.N. The Effect of CaO on Emissions of Nitric Oxide from a Fluidised Bed Combustor. Fuel 2015, 158, 898–907. [Google Scholar] [CrossRef]
- Yang, J.; Ma, L.; Liu, H.; Wei, Y.; Keomounlath, B.; Dai, Q. Thermodynamics and Kinetics Analysis of Ca-looping for CO2 Capture: Application of Carbide slag. Fuel 2019, 242, 1–11. [Google Scholar] [CrossRef]
- Wang, R.J. Application of Waste Calcium Carbide Slag as Desulfurizer in Environmental Pollution Control. Low Carbon World 2017, 1–2. [Google Scholar]
- Wang, Y.L.; Chen, M.N.; Cui, S.P.; Ma, X.Y. Desulfurization and Denitrification Performance of Rice Husk Ash- Carbide-Slag Composite Absorbent. Mater. Rev. 2018, 32, 3995–3999. [Google Scholar]
- Effects of Ca-based Absorbent’s Preparation Conditions on Simultaneous Removal of NO2 and SO2. Available online: http://kns-cnki-net-s.libziyuan.bjut.edu.cn:8118/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2011&filename=ZGDC201135004&v=gAMTYjcYVusY4kK0Xr4My%25mmd2F5O%25mmd2Bdcv1XcBRERl7rDIZdu0Rg5EE7U0VbrBFqZr%25mmd2FtA%25mmd2B (accessed on 23 December 2020).
- Li, Y.H.; Lee, C.W.; Gullett, B.K. Importance of Activated Carbon’s Oxygen Surface Functional Groups on Elemental Mercury Adsorption. Fuel 2003, 82, 451–457. [Google Scholar] [CrossRef]
- Tang, S.; Lu, N.; Li, J.; Shang, K.; Wu, Y. Improved Phenol Decomposition and Simultaneous Regeneration of Granular Activated Carbon by the Addition of a Titanium Dioxide Catalyst under a Dielectric Barrier Discharge Plasma. Carbon 2013, 53, 380–390. [Google Scholar] [CrossRef]



| Sample | CaO | SiO2 | Al2O3 | SO3 | Fe2O3 | MgO | P2O5 | K2O | MnO | Na2O | Cl | Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RHA | 1.60 | 87.60 | 0.50 | 1.03 | 0.35 | 1.34 | 2.86 | 4.36 | 0.36 | - | - | - |
| CS | 69.81 | 2.16 | 1.19 | 0.79 | 0.17 | 0.07 | 0.02 | - | - | 0.44 | 0.27 | 25.08 |
| Sample Number | Hydration Temperature (°C) | Hydration Time (h) | Rice Husk Ash/Carbide Slag Mass Ratio | Solid-Liquid Mass Ratio |
|---|---|---|---|---|
| H1 | 70 | 5 | 1:1 | 1:5 |
| H2 | 70 | 8 | 3:1 | 1:10 |
| H3 | 70 | 11 | 6:1 | 1:20 |
| H4 | 100 | 5 | 3:1 | 1:20 |
| H5 | 100 | 8 | 6:1 | 1:5 |
| H6 | 100 | 11 | 1:1 | 1:10 |
| H7 | 130 | 5 | 6:1 | 1:10 |
| H8 | 130 | 8 | 1:1 | 1:20 |
| H9 | 130 | 11 | 3:1 | 1:5 |
| Sample | BET Surface (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| Rice husk ash | 135.8 | 0.03 | 6.38 |
| Carbide slag | 18.6 | 0.13 | 22.62 |
| Modified absorbent | 142.1 | 0.22 | 12.03 |
| Sample | C | O | N |
|---|---|---|---|
| RHA | 82.62 | 16.80 | 0.58 |
| H8 | 70.09 | 27.86 | 2.05 |
| Functional Group (%) | Binding Energy (ev) | Relative Content (%) | |
|---|---|---|---|
| RHA | H8 | ||
| C–C | 284.2 ± 0.2 | 85.18 | 84.10 |
| C–O | 285.8 ± 0.2 | 9.69 | 5.33 |
| –COO | 288.5 ± 0.4 | 5.14 | 6.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Han, X.; Chen, M.; Cui, S.; Ma, X.; Hao, L. Desulfurization and Denitrification Performance of Modified Rice Husk Ash-Carbide Slag Absorbent. Materials 2021, 14, 68. https://doi.org/10.3390/ma14010068
Wang Y, Han X, Chen M, Cui S, Ma X, Hao L. Desulfurization and Denitrification Performance of Modified Rice Husk Ash-Carbide Slag Absorbent. Materials. 2021; 14(1):68. https://doi.org/10.3390/ma14010068
Chicago/Turabian StyleWang, Yali, Xiaoning Han, Meina Chen, Suping Cui, Xiaoyu Ma, and Liwei Hao. 2021. "Desulfurization and Denitrification Performance of Modified Rice Husk Ash-Carbide Slag Absorbent" Materials 14, no. 1: 68. https://doi.org/10.3390/ma14010068




