A Numerical Model of a Perforated Microcantilever Covered with Cardiomyocytes to Improve the Performance of the Microcantilever Sensor
Abstract
:1. Introduction
2. Results and Discussion
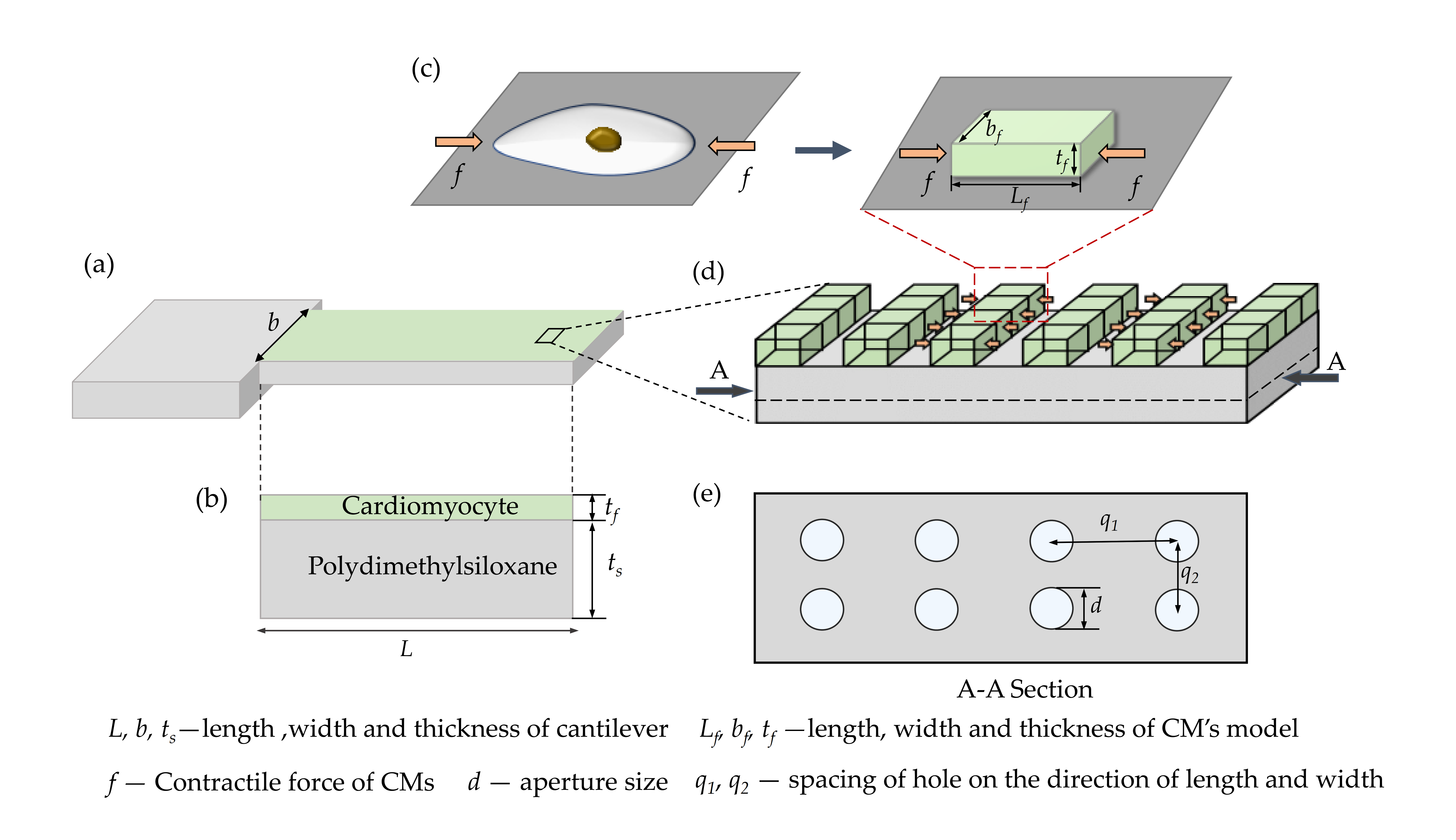
2.1. Numerical Modeling of the Perforated Microcantilever
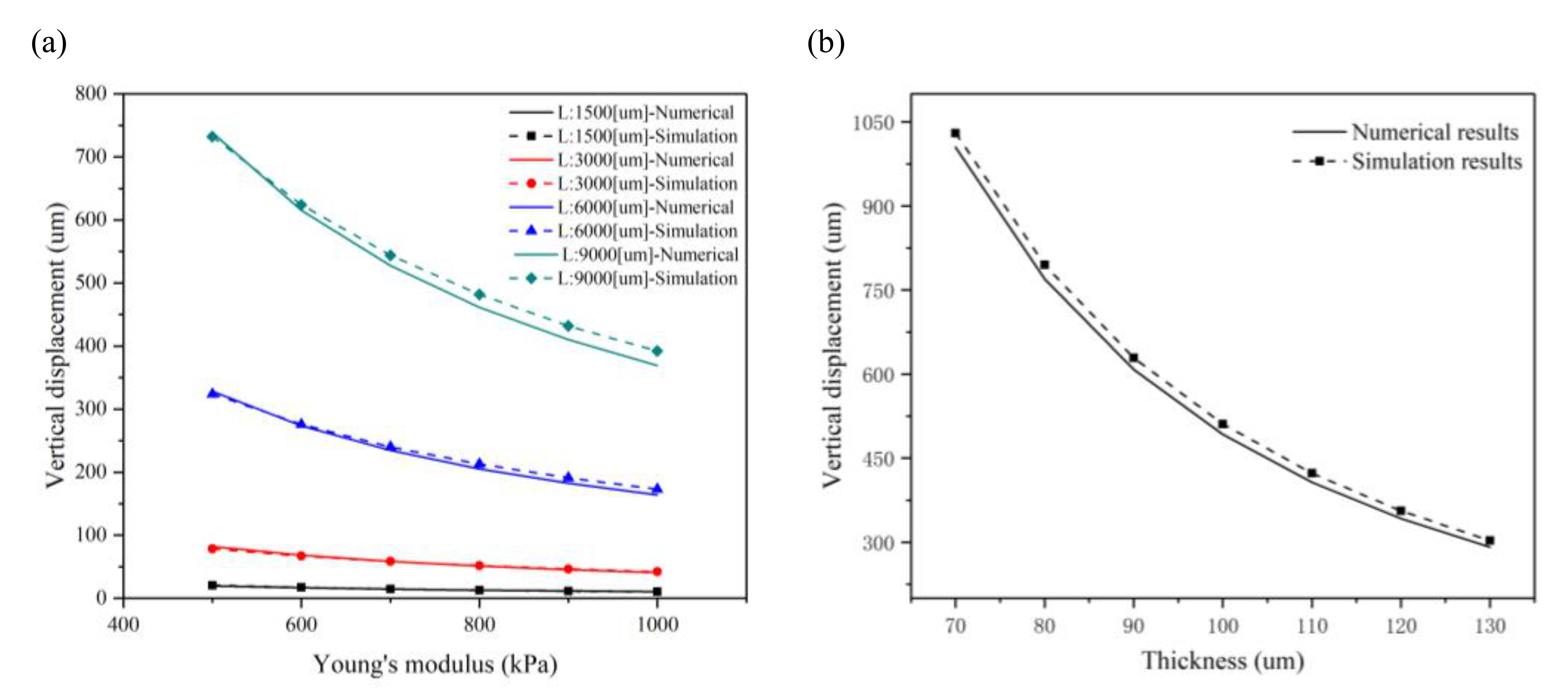
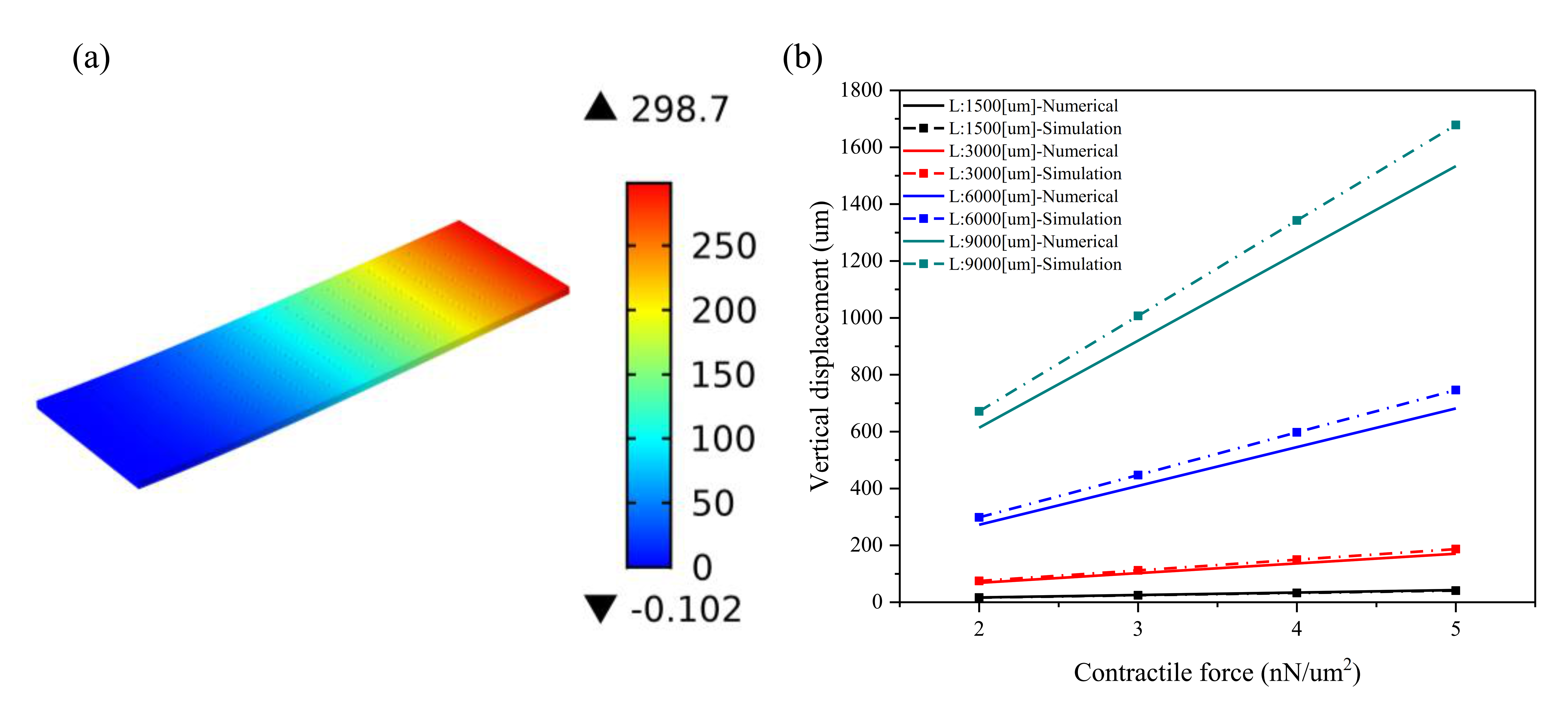
2.2. Plain Microcantilever
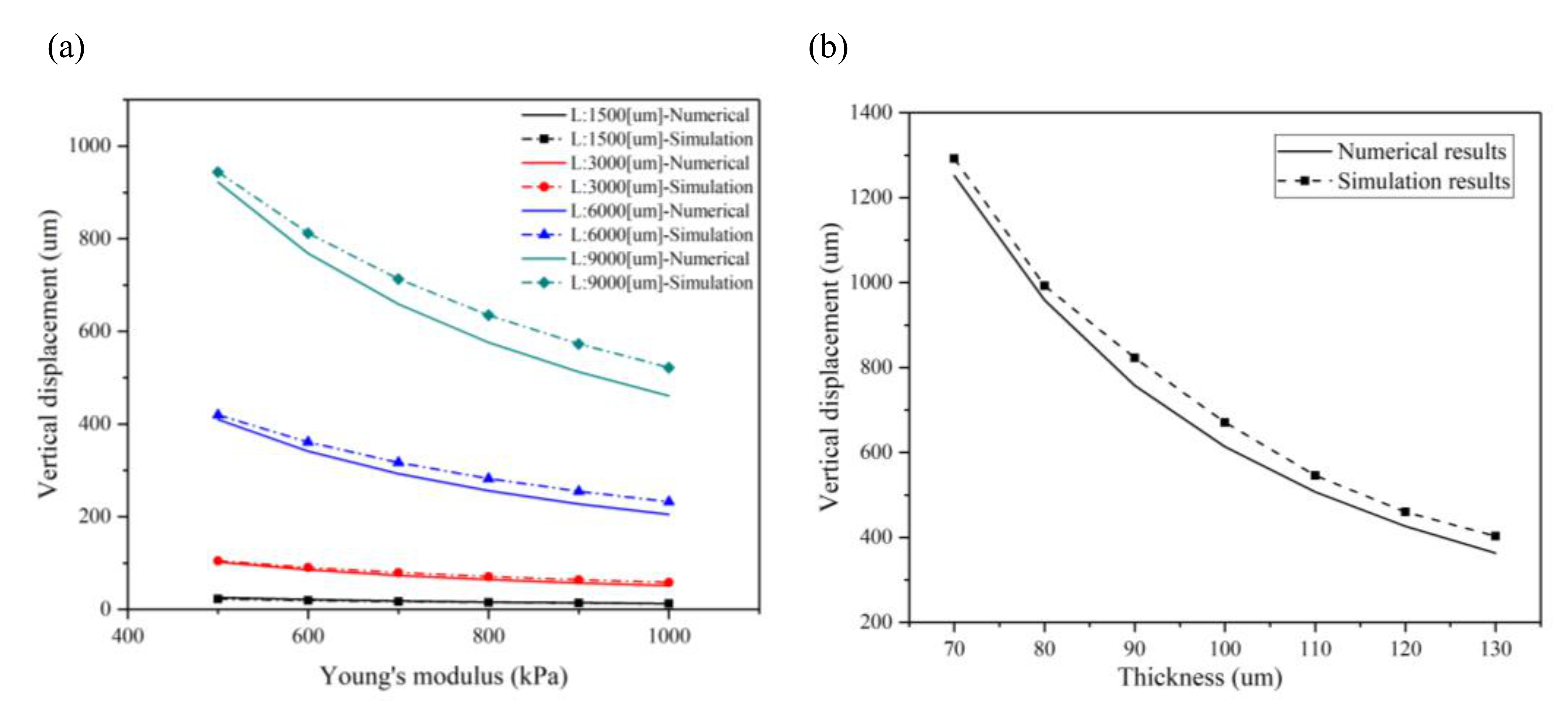
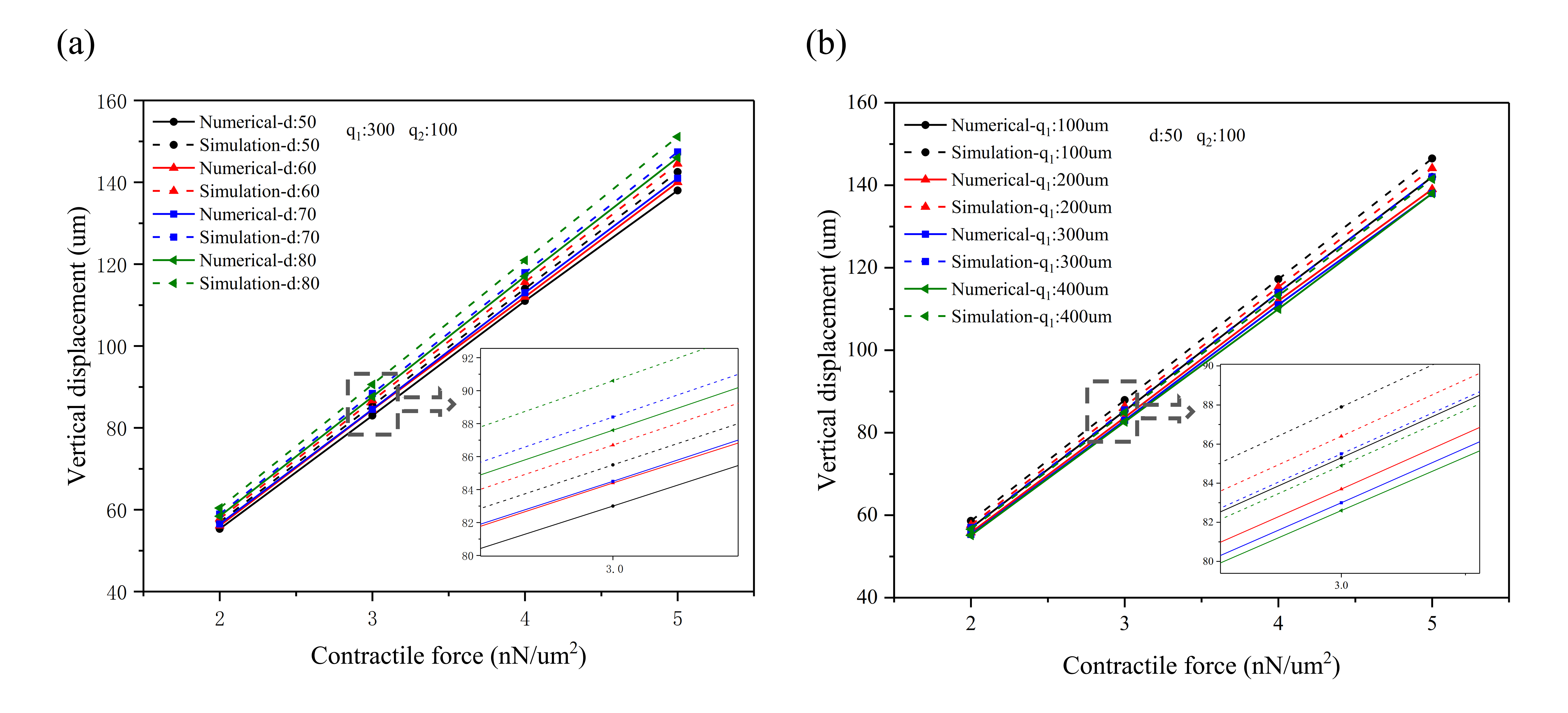
2.3. Perforated Microcantilever
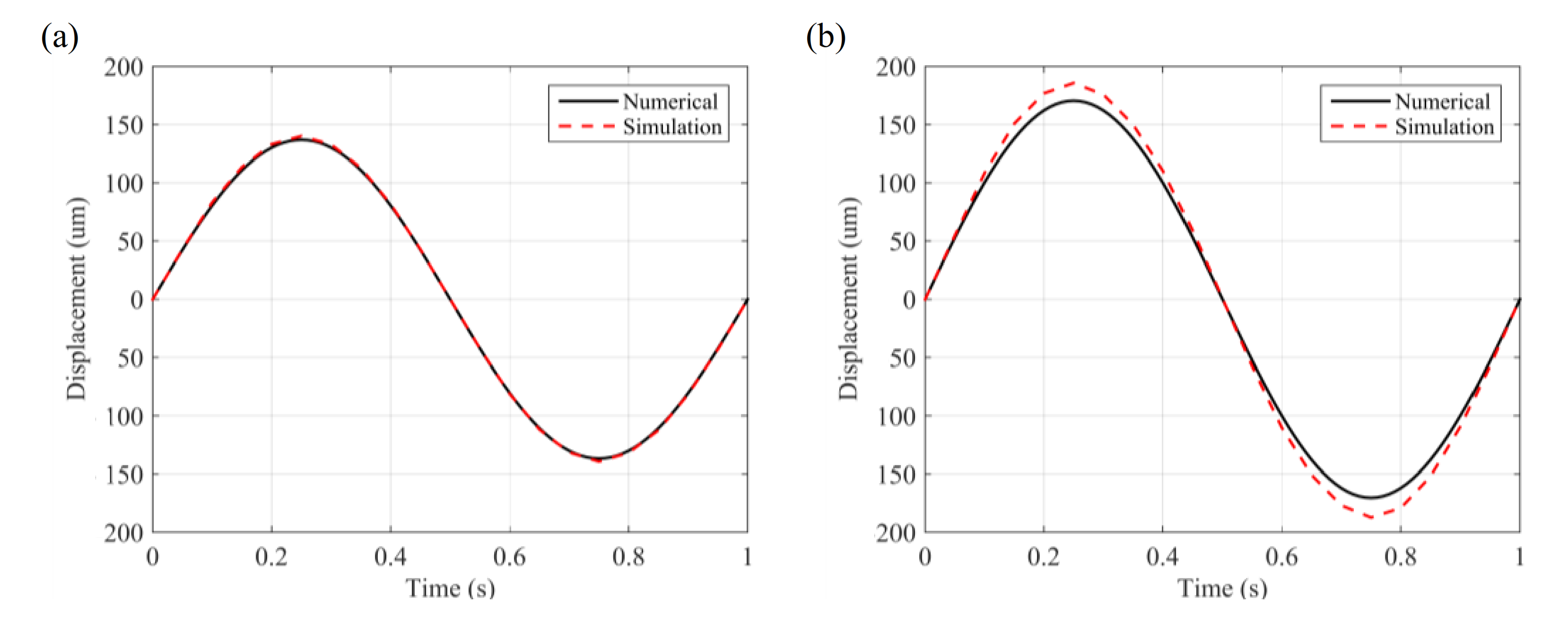
2.4. Transient Analysis for Microcantilever Sensor
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Park, J.; Ryu, J.; Choi, S.K.; Seo, E.; Cha, J.M.; Ryu, S.; Kim, J.; Kim, B.; Lee, S.H. Real-time measurement of the contractile forces of self-organized cardiomyocytes on hybrid biopolymer microcantilevers. Anal. Chem. 2005, 77, 6571. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Takizawa, E.; Nomura, F.; Hamada, T.; Hattori, A.; Yasuda, K. On-Chip Single-Cell-Shape Control Technology for Understanding Contractile Motion of Cardiomyocytes Measured Using Optical Image Analysis System. Jpn. J. Appl. Phys. 2013, 52, 06GK06. [Google Scholar] [CrossRef]
- Nomura, F.; Hattori, A.; Terazono, H.; Kim, H.; Odaka, M.; Sugio, Y.; Yasuda, K. Predictive lethal proarrhythmic risk evaluation using a closed-loop-circuit cell network with human induced pluripotent stem cells derived cardiomyocytes. Jpn. J. Appl. Phys. 2016, 55, 06GN07. [Google Scholar] [CrossRef]
- Tanaka, Y.; Morishima, K.; Shimizu, T.; Kikuchi, A.; Yamato, M.; Okano, T.; Kitamori, T. Demonstration of a PDMS-based bio-microactuator using cultured cardiomyocytes to drive polymer micropillars. Lab. Chip. 2006, 6, 230. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Zhao, H.; Du, Y. Myocardial Cell Pattern on Piezoelectric Nanofiber Mats for Energy Harvesting. J. Phys. Conf. Ser. 2014, 557, 012057. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, M.L.; Graham, B.T.; Pabon, L.M.; Han, S.J.; Murry, C.E.; Sniadecki, N.J. A computational model to describe the regional interlamellar shear of the annulus fibrosus. J. Biomech. Eng. 2014, 136, 051005. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, J.; Na, K.; Yang, S.; Baek, J.; Yoon, E.; Choi, S.; Lee, S.; Chun, K.; Park, J.; et al. Quantitative evaluation of cardiomyocyte contractility in a 3D microenvironment. J. Biomech. 2008, 41, 2396. [Google Scholar] [CrossRef]
- Feinberg, A.W.; Alford, P.W.; Jin, H.; Ripplinger, C.M.; Werdich, A.A.; Sheehy, S.P.; Grosberg, A.; Parker, K.K. Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials 2012, 33, 5732. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.S.; Jeong, Y.J.; Lee, B.K.; Shanmugasundaram, A.; Lee, D.-W. Piezoresistive sensor-integrated PDMS cantilever: A new class of device for measuring the drug-induced changes in the mechanical activity of cardiomyocytes. Sens. Actuators B 2017, 240, 566. [Google Scholar] [CrossRef]
- You, J.; Moon, H.; Lee, B.Y.; Jin, J.Y.; Chang, Z.E.; Kim, S.Y.; Park, J.; Hwang, Y.-S.; Kim, J.J. Cardiomyocyte sensor responsive to changes in physical and chemical environments. J. Biomech. 2014, 47, 400. [Google Scholar] [CrossRef]
- Pesl, M.; Pribyl, J.; Acimovic, I.; Vilotic, A.; Jelinkova, S.; Salykin, A.; Lacampagne, A.; Dvorak, P.; Meli, A.C.; Skladal, P.; et al. Atomic force microscopy combined with human pluripotent stem cell derived cardiomyocytes for biomechanical sensing. Biosens. Bioelectron. 2016, 85, 751. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X. Cellular mechanics study in cardiac myocytes using PDMS pillars array. Sens. Actuators A 2006, 125, 398. [Google Scholar] [CrossRef]
- Cheng, Q.; Sun, Z.; Meininger, G.; Almasri, M. PDMS elastic micropost arrays for studying vascular smooth muscle cells. Sens. Actuators B 2013, 188, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, A.G.; Han, S.J.; Regnier, M.; Sniadecki, N.J. Substrate stiffness increases twitch power of neonatal cardiomyocytes in correlation with changes in myofibril structure and intracellular calcium. Biophys. J. 2011, 101, 2455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Taylor, R.; Sim, J.Y.; Park, J.; Norman, J.; Fajardo, G.; Bernstein, D.; Pruitt, B.L. Calibrated micropost arrays for biomechanical characterisation of cardiomyocytes. Micro Nano Lett. 2011, 6, 317. [Google Scholar] [CrossRef] [Green Version]
- Han, S.J.; Bielawski, K.S.; Ting, L.H.; Rodriguez, M.L.; Sniadecki, N.J. Decoupling substrate stiffness, spread area, and micropost density: A close spatial relationship between traction forces and focal adhesions. Biophys. J. 2012, 103, 640. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Ryu, S.-K.; Kim, J.; Cha, J.; Baek, J.; Park, S.; Kim, B.; Lee, S.H. A three-dimensional model of fluid–structural interactions for quantifying the contractile force for cardiomyocytes on hybrid biopolymer microcantilever. J. Biomech. 2007, 40, 2823. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Lee, D.W.; Lee, B.K. Numerical investigation of perforated polymer microcantilever sensor for contractile behavior of cardiomyocytes. Jpn. J. Appl. Phys. 2017, 56, 06GM01. [Google Scholar] [CrossRef]
- Motlagh, D.; Senyo, S.E.; Desai, T.A.; Russell, B. Microtextured substrata alter gene expression, protein localization and the shape of cardiac myocytes. Biomaterials 2003, 24, 2463. [Google Scholar] [CrossRef]
- Kamgoué, A.; Ohayon, J.; Usson, Y.; Riou, L.; Tracqui, P. Quantification of cardiomyocyte contraction based on image correlation analysis. Cytometry Part A 2009, 75, 298–308. [Google Scholar] [CrossRef]
- Gizzi, A.; Ruiz-Baier, R.; Rossi, S.; Laadhari, A.; Cherubini, C.; Filippi, S. A Three-Dimensional Continuum Model of Active Contraction in Single Cardiomyocytes. In Modeling the Heart and the Circulatory System; Springer: Cham, Germany, 2015; p. 157. [Google Scholar]
- Kim, J.Y.; Choi, Y.-S.; Lee, B.-K.; Lee, D.-W. Surface-patterned SU-8 cantilever arrays for preliminary screening of cardiac toxicity. Biosens. Bioelectron. 2016, 80, 456. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Kim, D.-S.; Kim, E.-S.; Lee, D.-W. Nano-textured polyimide cantilever for enhancing the contractile behavior of cardiomyocytes and its application to cardiac toxicity screening. Sens. Actuators B Chem. 2019, 301, 126995. [Google Scholar] [CrossRef]
- Na, K.; Kim, J.; Yang, S.; Yoon, Y.M.; Yoon, E. New Finite Element Method Modeling for Contractile Forces of Cardiomyocytes on Hybrid Biopolymer Microcantilevers; NSTI-Nanotech: Cambridge, CA, USA, 2008; p. 737. [Google Scholar]
- Balaban, N.Q.; Schwarz, U.S.; Riveline, D.; Goichberg, P.; Tzur, G.; Sabanay, I.; Mahalu, D.; Safran, S.; Bershadsky, A.; Addadi, L.; et al. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001, 3, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Stoney, G.G. The tension of metallic films deposited by electrolysis. Proc. R. Soc. Lond. Ser. A 1909, 82, 172. [Google Scholar]
- Preissig, F.J. Applicability of the classical curvature-stress relation for thin films on plate substrates. J. Appl. Phys. 1989, 66, 4262. [Google Scholar] [CrossRef]
- Berry, B.S.; Pritchet, W.C. Internal stress and internal friction in thin-layer microelectronic materials. Appl. Phys. 1990, 67, 3661. [Google Scholar] [CrossRef]
- Wachtman, J.B., Jr. Elastic Seformation of Ceramics and Other Refractory Materials in Mechanical and Thermal Properties of Ceramics; National Bureau of Standards Spec. Pubi. No 303; NIST: Gaithersburg, MD, USA, 1968; p. 139. [Google Scholar]
- Phani, K.K.; Niyogi, S.K. Elastic modulus-porosity relation in polycrystalline rare-earth oxides. J. Am. Ceram. Soc. 1987, 70, 362. [Google Scholar] [CrossRef]
- Armani, D.; Liu, C.; Aluru, N. Re-configurable Fluid Circuits by PDMS Elastomer Micromachining. In Proceedings of the IEEE Micro Electro Mechanical Systems, Orlando, FL, USA, 17–19 January 1999. [Google Scholar]
- Janz, R.F.; Grimm, A.F. Finite-element model for the mechanical behaviour of the left ventricle: Prediction of deformation in the potassium-arrested rat heart. Circ. Res. 1972, 30, 244. [Google Scholar] [CrossRef] [Green Version]
- Pustoc’h, A.; Boudou, T.; Ohayon, J.; Usson, Y.; Tracqui, P. Finite element modelling of the calcium-induced contraction cardiomyocytes based on time lapse video microscopy. WSEAS Trans. Inf. Sci. Appl. 2004, 1, 376. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, B.; Li, G.; Du, J.; Zhang, A.; Jin, Y. A Numerical Model of a Perforated Microcantilever Covered with Cardiomyocytes to Improve the Performance of the Microcantilever Sensor. Materials 2021, 14, 95. https://doi.org/10.3390/ma14010095
Qiu B, Li G, Du J, Zhang A, Jin Y. A Numerical Model of a Perforated Microcantilever Covered with Cardiomyocytes to Improve the Performance of the Microcantilever Sensor. Materials. 2021; 14(1):95. https://doi.org/10.3390/ma14010095
Chicago/Turabian StyleQiu, Bin, Guangyong Li, Jianke Du, Aibing Zhang, and Yuan Jin. 2021. "A Numerical Model of a Perforated Microcantilever Covered with Cardiomyocytes to Improve the Performance of the Microcantilever Sensor" Materials 14, no. 1: 95. https://doi.org/10.3390/ma14010095
APA StyleQiu, B., Li, G., Du, J., Zhang, A., & Jin, Y. (2021). A Numerical Model of a Perforated Microcantilever Covered with Cardiomyocytes to Improve the Performance of the Microcantilever Sensor. Materials, 14(1), 95. https://doi.org/10.3390/ma14010095




