A Rheological Analysis of Biomaterial Behaviour as a Tool to Detect the Dilution of Heather Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Characterisation of Honey
2.3. Rheological Assays
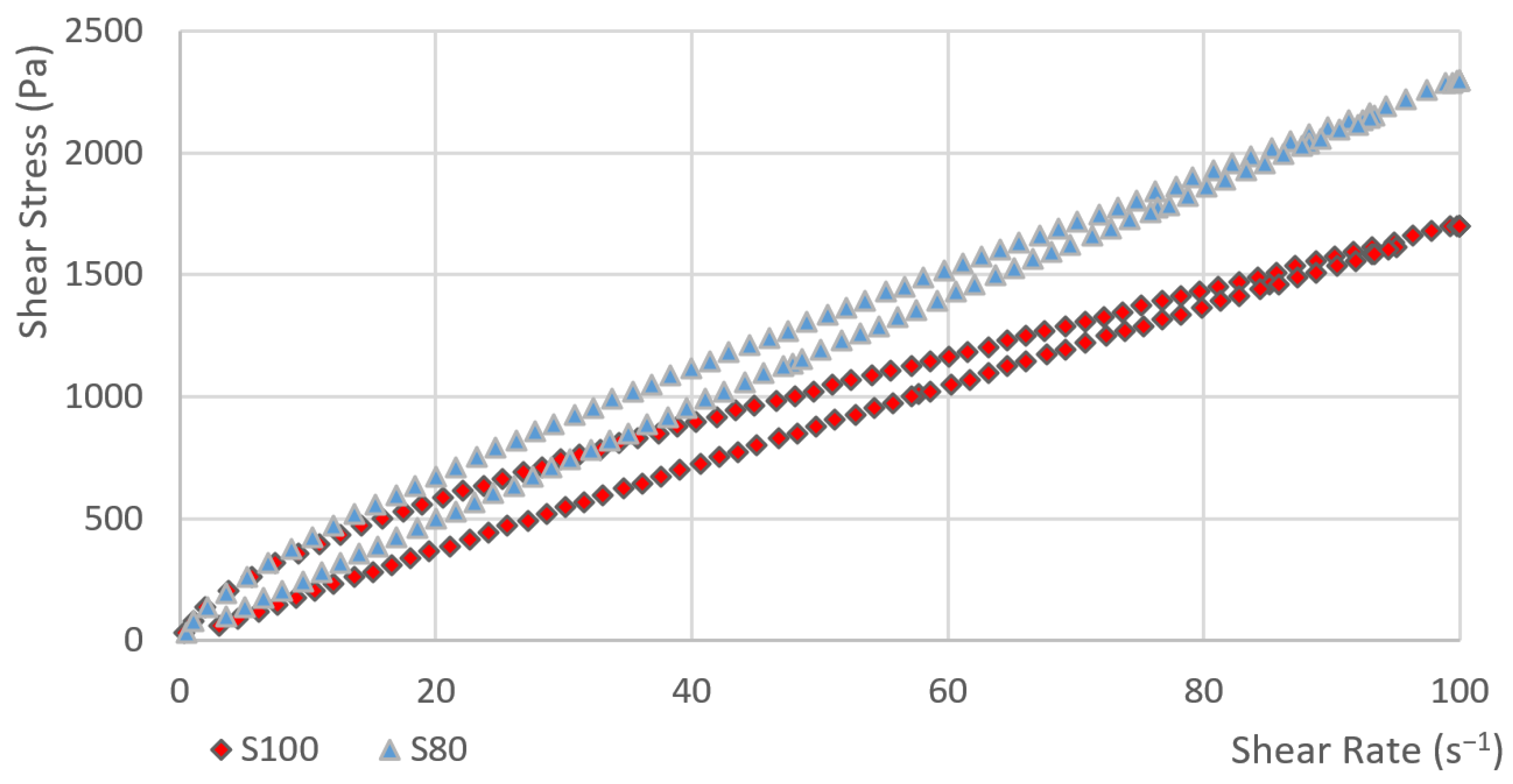
2.3.1. Hysteresis Loop Test
2.3.2. The Determination of Time-Dependent Behaviour
- —shear stress (Pa)
- —value of the shear stress in the first second of the measurement (Pa)
- —time coefficient of the thixotropic breakdown (-)
- —time of shearing (s)
2.3.3. Dynamic Frequency Sweep Test
2.3.4. Mathematical Modelling
- —shear stress (Pa)
- —yield stress (Pa)
- —consistency factor (Pa·sn)
- —shear rate (s−1)
- —flow index (-)
2.4. Statistical Analysis
3. Results
3.1. Hysteresis Area
3.2. Dependence of the Apparent Viscosity versus Time
- —apparent viscosity in the 1st second of the assay (Pa·s)
- —apparent viscosity in the 300th second of the assay (Pa·s)
3.3. Frequency Sweep Test
- —the angular frequency (rad·s−1)
- —the degree of a declining complex viscosity
- —the initial value of the complex viscosity at 1 rad·s−1
4. Discussion
5. Conclusions
- The area value of the hysteresis loop is sensitive to both external and internal factors. This is an unstable parameter and we do not recommend using it as a comparison parameter.
- Due to the low variability of the measured values, the relative comparison n-value parameter and parameter appear to be suitable.
- The dependence of the measured parameters n, B, and C on the degree of the dilution is non-linear and a distinct step change occurs between samples S40 and S60, i.e., the samples that contained 40% (w/w) heather honey and 60% (w/w), respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample | S0 | S10 | S20 | S40 | S60 | S80 | S100 |
|---|---|---|---|---|---|---|---|
| S0 | X | - | - | - | - | - | - |
| S10 | 0.9999 | X | - | - | - | - | - |
| S20 | 0.9950 | 0.9999 | X | - | - | - | - |
| S40 | 0.3652 | 0.5596 | 0.7345 | X | - | - | - |
| S60 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | X | - | - |
| S80 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | 0.0482 | X | - |
| S100 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | 0.0427 | X |
| Sample | S0 | S10 | S20 | S40 | S60 | S80 | S100 |
|---|---|---|---|---|---|---|---|
| S0 | X | - | - | - | - | - | - |
| S10 | 0.27992 | X | - | - | - | - | - |
| S20 | 0.09663 | 0.99876 | X | - | - | - | - |
| S40 | <0.001 | 0.04449 | 0.14767 | X | - | - | - |
| S60 | <0.001 | <0.001 | <0.001 | <0.001 | X | - | - |
| S80 | <0.001 | <0.001 | <0.001 | <0.001 | 0.00133 | X | - |
| S100 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.5623 | X |
| Sample | S0 | S10 | S20 | S40 | S60 | S80 | S100 |
|---|---|---|---|---|---|---|---|
| S0 | X | - | - | - | - | - | - |
| S10 | 0.999368 | X | - | - | - | - | - |
| S20 | 0.999771 | 1.000000 | X | - | - | - | - |
| S40 | 0.927389 | 0.994405 | 0.990261 | X | - | - | - |
| S60 | 0.252295 | 0.472161 | 0.431502 | 0.841039 | X | - | - |
| S80 | 0.022838 | 0.057487 | 0.049694 | 0.194941 | 0.873087 | X | - |
| S100 | 0.000879 | 0.002172 | 0.001869 | 0.008977 | 0.137472 | 0.739904 | X |
References
- Witczak, M.; Juszczak, L.; Gałkowska, D. Non-Newtonian behaviour of heather honey. J. Food Eng. 2011, 104, 532–537. [Google Scholar] [CrossRef]
- Dezmirean, D.S.; Mărghitaș, L.A.; Fiț, N.; Chirilă, F.; Gherman, B.; Mărgăoan, R.; Bobiș, O. Antibacterial Effect of Heather Honey (Calluna vulgaris) against Different Microorganisms of Clinical Importance. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Anim. Sci. Biotechnol. 2015, 72, 72–77. [Google Scholar] [CrossRef][Green Version]
- Lehébel-Péron, A.; Sidawy, P.; Dounias, E.; Schatz, B. Attuning local and scientific knowledge in the context of global change: The case of heather honey production in southern France. J. Rural Stud. 2016, 44, 132–142. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Escuredo, O.; Seijo-Rodríguez, A.; Seijo, M.C. Characterization of the honey produced in heather communities (NW Spain). J. Apic. Res. 2019, 58, 84–91. [Google Scholar] [CrossRef]
- Frydman, G.H.; Olaleye, D.; Annamalai, D.; Layne, K.; Yang, I.; Kaafarani, H.M.A.; Fox, J.G. Manuka honey microneedles for enhanced wound healing and the prevention and/or treatment of Methicillin-resistant Staphylococcus aureus (MRSA) surgical site infection. Sci. Rep. 2020, 10, 13229. [Google Scholar] [CrossRef] [PubMed]
- Le Conte, Y.; Navajas, M. Climate change: Impact on honey bee populations and diseases. Rev. Sci. Tech. Off. Int. Epizoot. 2008, 27, 499–510. [Google Scholar]
- Zábrodská, B.; Vorlová, L. Adulteration of honey and available methods for detection—A review. Acta Vet. Brno 2014, 83, S85–S102. [Google Scholar] [CrossRef]
- Persano Oddo, L.; Piro, R. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35, S38–S81. [Google Scholar] [CrossRef]
- Bogdanov, S.; Martin, P.; Lüllman, C. Harmonised methods of the European Honey Commission. Apidologie 1997, 28, 1–59. [Google Scholar]
- Louveaux, J. Essai de caractérisation des miels de Callune (Calluna vulgaris Salisb.). Annales de L’Abeille 1966, 9, 351–358. [Google Scholar]
- Osés, S.M.; Ruiz, M.O.; Pascual-Maté, A.; Bocos, A.; Fernández-Muiño, M.A.; Sancho, M.T. Ling heather honey authentication by thixotropic parameters. Food Bioprocess Technol. 2017, 10, 973–979. [Google Scholar] [CrossRef]
- Munro, J.A. The viscosity and thixotropy of honey. J. Entomol. 1943, 36, 769–777. [Google Scholar] [CrossRef]
- Waś, E.; Rybak-Chmielewska, H.; Szczęsna, T.; Kachaniuk, K.; Teper, D. Characteristic of polish unifloral honey. III. Heather honey (Calluna vulgaris L.). J. Apic. Sci. 2011, 55, 129–136. [Google Scholar]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Palma, M.; Barbero, G.F. A screening method based on Visible-NIR spectroscopy for the identification and quantification of different adulterants in high-quality honey. Talanta 2019, 203, 235–241. [Google Scholar] [CrossRef]
- Song, Y.Q.; Milne, R.I.; Zhou, H.X.; Ma, X.L.; Fang, J.Y.; Zha, H.G. Floral nectar chitinase is a potential marker for monofloral honey botanical origin authentication: A case study from loquat (Eriobotrya japonica Lindl.). Food Chem. 2019, 282, 76–83. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Zhu, M.; Zhang, J.; Cheng, N.; Cao, W. Method for identifying acacia honey adulterated by resin absorption: HPLC-ECD coupled with chemometrics. LWT Food Sci. Technol. 2020, 118, 108863. [Google Scholar] [CrossRef]
- Geana, E.I.; Ciucure, C.T. Establishing authenticity of honey via comprehensive Romanian honey analysis. Food Chem. 2020, 306, 125595. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Tatlisu, N.B.; Toker, O.S.; Karaman, S.; Dertli, E.; Sagdic, O.; Arici, M. Steady, dynamic and creep rheological analysis as a novel approach to detect honey adulteration by fructose and saccharose syrups: Correlations with HPLC-RID results. Food Res. Int. 2014, 64, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, U.; Mishra, S. Prediction of Adulteration in Honey Using Rheological Parameters. Int. J. Food Prop. 2015, 18, 2056–2063. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S.; Paduret, S.; Todosi, E. Rheological analysis of honeydew honey adulterated with glucose, fructose, inverted sugar, hydrolysed inulin syrup and malt wort. LWT Food Sci. Technol. 2018, 95, 1–8. [Google Scholar] [CrossRef]
- Anidiobu, V.O.; Nwalor, J.U.; Babalola, F.U. Comparative Study of Rheological Characterization and Classification of Honeys with Their Physicochemical Properties. Int. J. Food Eng. 2019, 5, 268–275. [Google Scholar] [CrossRef]
- Nayik, G.A.; Dar, B.N.; Nanda, V. Physico-chemical, rheological and sugar profile of different unifloral honeys from Kashmir valley of India. Arab. J. Chem. 2019, 12, 3151–3162. [Google Scholar] [CrossRef]
- Kurt, A.; Palabiyik, I.; Gunes, R.; Konar, K.; Toker, O.S. Determining Honey Adulteration by Seeding Method: An Initial Study with Sunflower Honey. Food Anal. Methods 2020, 13, 952–961. [Google Scholar] [CrossRef]
- Szczęsna, T.; Rybak-Chmielewska, H. The temperature correction factor for electrical conductivity of honey. J. Apic. Sci. 2004, 48, 97–102. [Google Scholar]
- Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Maurizio, A.; Louveaux, J. Union des Groupements Apicoles Français, 1st ed.; Pollens de Plantes Melliféres d’Europe: Paris, France, 1965; p. 148. [Google Scholar]
- Sawyer, R. Honey Identification, 1st ed.; Cardiff Academic Press: Cardiff, UK, 1988; p. 115. [Google Scholar]
- Ohe, K.; Ohe, W. LAVES—Institut für Bienenkunde, 3rd ed.; Celle’s Melissopalynological Collection: Celle, Germany, 2007; p. 236. [Google Scholar]
- Dapčević, T.; Dokić, P.; Hadnađev, M.; Krstonošić, V. An approach in numerical evaluation of thixotropy. Food Process. Qual. Saf. 2008, 35, 33–39. [Google Scholar]
- Tovar, C.; Rodríguez-Flores, M.S.; Escuredo, O.; Del Carmen Seijo, M. Rheology of Honey-Advances in Rheology Research, 1st ed.; Nova Science Publisher: New York, NY, USA, 2017; pp. 175–191. [Google Scholar]
- Karasu, S.; Toker, O.S.; Yilmaz, M.T.; Karaman, S.; Dertli, E. Thermal loop test to determine structural changes and thermal stability of creamed honey: Rheological characterization. J. Food Eng. 2015, 150, 90–98. [Google Scholar] [CrossRef]
- Kumbár, V.; Strnková, J.; Nedomová, Š.; Buchar, J. Fluid dynamics of liquid egg products. J. Biol. Phys. 2015, 41, 303–311. [Google Scholar] [CrossRef]
- Kumbár, V.; Nedomová, Š.; Trnka, J.; Buchar, J.; Pytel, R. Effect of storage duration on the rheological properties of goose liquid egg products and eggshell membranes. Poult. Sci. 2016, 95, 1693–1701. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: http://www.R-project.org/ (accessed on 23 March 2020).
- Barnes, H.A. Thixotropy—A review. J. Non Newton. Fluid Mech. 1997, 70, 1–33. [Google Scholar] [CrossRef]
- Mewis, J.; Wagner, N.J. Thixotropy. Adv. Colloid Interface Sci. 2009, 147–148, 214–227. [Google Scholar] [CrossRef]
- Adamczyk, G.; Krystyjan, M.; Dobosz, A.; Sikora, M. Thixotropic properties of starch. Food Sci. Technol. Qual. 2013, 20, 16–31. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Behrouzian, F.; Alghooneh, A. Temperature dependency of the interaction between xanthan gum and sage seed gum: An interpretation of dynamic rheology and thixotropy based on creep test. J. Texture Stud. 2017, 48, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Stelmakienė, A.; Ramanauskiene, K.; Briedis, V.; Leskauskaite, D. Examination of rheological and physicochemical characteristics in Lithuanian honey. Afr. J. Biotechnol. 2012, 11, 12406–12414. [Google Scholar] [CrossRef]
- Samanalieva, J.; Senge, B. Analytical and rheological investigations into selected unifloral German honey. Eur. Food Res. Technol. 2009, 229, 107–113. [Google Scholar] [CrossRef]
- Afonso, M.J.; Magalhães, M.; Fernandes, L.; Castro, M.; Ramalhosa, E.C.D. Temperature Effect on Rheological Behavior of Portuguese Honeys. Pol. J. Food Nutr. Sci. 2018, 68, 217–222. [Google Scholar] [CrossRef]
- Nedomova, S.; Kumbar, V.; Pytel, R.; Buchar, J. Mechanical properties of sugar beet root during storage. Int. Agrophys. 2017, 31, 507–513. [Google Scholar] [CrossRef]









| Sample | Botanical Origin | Geographical Origin | Pollen Grains in 1 g of Honey | Percentage of Important Pollen Grains | Important Pollen Grains in 1 g of Honey | Honeydew Elements | Water Content (%) | Electrolytic Conductivity (mS·cm−1) |
|---|---|---|---|---|---|---|---|---|
| PA/386 | heather nectar honey | Norway | 8886 ± 467 | 28.1 ± 1.2 | 1786 ± 21 | sporadic clusters, no bodies | 16.9 ± 0.2 | 86.6 ± 4.2 |
| PA/416 | lime nectar honey | Czech Republic | 2252 ± 139 | 9.6 ± 0.5 | 216 ± 1 | sporadic clusters, no bodies | 17.8 ± 0.1 | 50.4 ± 1.9 |
| Sample | Mean (Pa·s−1) | Median (Pa·s−1) | SD (Pa·s−1) | VC (%) | IQR (Pa·s−1) | S-W (-) |
|---|---|---|---|---|---|---|
| S0 | −493.87 | −532.96 | 240.619 | −48.7208 | 320.631 | 0.72 |
| S10 | −217.28 | −245.97 | 69.333 | −31.9095 | 82.403 | 0.05 |
| S20 | 20.39 | 0.67 | 81.724 | 400.7579 | 112.616 | 0.54 |
| S40 | 1254.58 | 1476.30 | 629.889 | 50.2071 | 769.839 | 0.14 |
| S60 | 7742.53 | 7386.65 | 1342.529 | 17.3397 | 2020.350 | 0.39 |
| S80 | 10,409.83 | 10,577.50 | 2469.510 | 23.7229 | 3373.350 | 0.91 |
| S100 | 13,124.50 | 12,859.00 | 974.223 | 7.4229 | 1473.000 | 0.35 |
| Ostwald-De Waele Model | Herschel-Bulkley Model | ||||||
|---|---|---|---|---|---|---|---|
| Sample | K (Pa·sn) | n (-) | R2 (-) | K (Pa·sn) | n (-) | R2 | |
| S0 | 7.91 ± 0.3 | 0.9924 ± 0.007 | 1.0000 | −1.15 ± 2.2 | 8.0 ± 0.4 | 0.9891 ± 0.011 | 1.0000 |
| S10 | 8.79 ± 0.3 | 0.9864 ± 0.003 | 1.0000 | 0.29 ± 1.7 | 8.8 ± 0.5 | 0.9872 ± 0.008 | 1.0000 |
| S20 | 10.35 ± 0.1 | 0.9768 ± 0.003 | 1.0000 | −0.02 ± 1.2 | 10.4 ± 0.2 | 0.9768 ± 0.003 | 1.0000 |
| S40 | 17.39 ± 1.1 | 0.9265 ± 0.003 | 1.0000 | −2.86 ± 2.8 | 17.8 ± 1.4 | 0.9220 ± 0.007 | 1.0000 |
| S60 | 43.80 ± 2.1 | 0.7970 ± 0.006 | 0.9998 | 17.28 ± 7.8 | 40.2 ± 2.3 | 0.8141 ± 0.014 | 0.9998 |
| S80 | 67.22 ± 6.1 | 0.7634 ± 0.017 | 0.9995 | 36.45 ± 6.7 | 58.8 ± 5.5 | 0.7898 ± 0.019 | 0.9997 |
| S100 | 74.50 ± 6.2 | 0.6745 ± 0.019 | 0.9986 | 44.94 ± 22.9 | 61.0 ± 6.4 | 0.7134 ± 0.016 | 0.9990 |
| Sample | Mean (-) | Median (-) | SD (-) | VC (%) | IQR (-) | S-W (-) |
|---|---|---|---|---|---|---|
| S0 | 1.0534 | 1.0260 | 0.0610 | 0.0338 | 5.7936 | 0.01092 |
| S10 | 1.0108 | 1.0110 | 0.0043 | 0.0062 | 0.4218 | 0.48 |
| S20 | 1.0005 | 1.0049 | 0.0131 | 0.0143 | 1.3060 | 0.1875 |
| S40 | 0.9514 | 0.9522 | 0.0143 | 0.0233 | 1.4984 | 0.1185 |
| S60 | 0.8251 | 0.8258 | 0.0209 | 0.0302 | 2.5322 | 0.5364 |
| S80 | 0.7390 | 0.7390 | 0.0184 | 0.0297 | 2.4838 | 0.1605 |
| S100 | 0.7054 | 0.7074 | 0.0075 | 0.0075 | 1.0650 | 0.4843 |
| Sample | A (Pa) | B (-) | R2 |
|---|---|---|---|
| S0 | 368.5 ± 14.2 | −4.5 ± 2.8 | 0.83 ± 0.13 |
| S10 | 441.3 ± 9.4 | −1.7 ± 1.3 | 0.68 ± 0.17 |
| S20 | 515.9 ± 27.0 | −2.2 ± 1.6 | 0.55 ± 0.34 |
| S40 | 673.7 ± 22.7 | 2.3 ± 2.2 | 0.76 ± 0.19 |
| S60 | 864.5 ± 111.4 | 10.6 ± 7.0 | 0.75 ± 0.17 |
| S80 | 1284.8 ± 72.2 | 18.4 ± 12.4 | 0.73 ± 0.21 |
| S100 | 1064.2 ± 75.6 | 28.0 ± 18.5 | 0.84 ± 0.19 |
| Sample | C (-) | R2 |
|---|---|---|
| S0 | −1.93 ± 1.34 | 0.81 ± 0.2 |
| S10 | −0.91 ± 0.85 | 0.66 ± 0.5 |
| S20 | −0.61 ± 1.05 | 0.31 ± 0.4 |
| S40 | −2.93 ± 3.22 | 0.92 ± 0.1 |
| S60 | −5.05 ± 1.43 | 0.95 ± 0.01 |
| S80 | −18.6 ± 3.90 | 0.95 ± 0.002 |
| S100 | −15.7 ± 2.25 | 0.95 ± 0.01 |
| Herschel-Bulkley | Ostwald-de Waele | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | n (-) | K (Pa·sn) | n (-) | K (Pa·sn) | t (°C) | ϕ (%) | HA (Pa·s−1) | ||
| (A) | 0.70 ± 0.01 | 50.7 ± 1.2 | 50.2 ± 1.3 | - | - | 20 | 18.0 | 15,000 | 100 |
| 0.77 ± 0.01 | 29.1 ± 0.3 | 50.2 ± 1.9 | - | - | 20 | 18.2 | 7000 | ||
| 0.88 ± 0.01 | 10.8 ± 0.6 | 3.8 ± 0.2 | - | - | 20 | 20 | 2000 | ||
| (B) | 0.901 | 13.39 | 0.15 | - | - | 20 | 18.7 | - | 50 |
| (C) | 0.988 | 112 | −0.64 | 0.996 | 4.71 | 30 | 24.0 | - | 5 |
| (D) | - | - | - | 0.88 ± 0.1 | 23.67 ± 8.0 | 25 | 17.5 | 6994 ± 1945 | 100 |
| (A) | 0.70 ± 0.01 | 50.7 ± 1.2 | 50.2 ± 1.3 | - | - | 20 | 18.0 | 15,000 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Přidal, A.; Trávníček, P.; Kudělka, J.; Nedomová, Š.; Ondrušíková, S.; Trost, D.; Kumbár, V. A Rheological Analysis of Biomaterial Behaviour as a Tool to Detect the Dilution of Heather Honey. Materials 2021, 14, 2472. https://doi.org/10.3390/ma14102472
Přidal A, Trávníček P, Kudělka J, Nedomová Š, Ondrušíková S, Trost D, Kumbár V. A Rheological Analysis of Biomaterial Behaviour as a Tool to Detect the Dilution of Heather Honey. Materials. 2021; 14(10):2472. https://doi.org/10.3390/ma14102472
Chicago/Turabian StylePřidal, Antonín, Petr Trávníček, Jan Kudělka, Šárka Nedomová, Sylvie Ondrušíková, Daniel Trost, and Vojtěch Kumbár. 2021. "A Rheological Analysis of Biomaterial Behaviour as a Tool to Detect the Dilution of Heather Honey" Materials 14, no. 10: 2472. https://doi.org/10.3390/ma14102472
APA StylePřidal, A., Trávníček, P., Kudělka, J., Nedomová, Š., Ondrušíková, S., Trost, D., & Kumbár, V. (2021). A Rheological Analysis of Biomaterial Behaviour as a Tool to Detect the Dilution of Heather Honey. Materials, 14(10), 2472. https://doi.org/10.3390/ma14102472





